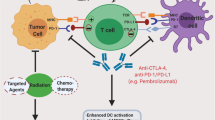
Abstract
The emergence of immune checkpoint inhibitors (ICIs) has revolutionized the field of oncology. For many cancer types, treatment paradigms have changed, as immunotherapy is increasingly being integrated into frontline standard-of-care treatments and producing meaningful and prolonged responses. This has inspired an avalanche of clinical trials studying ICIs in all types of malignancies, including gynecological cancers. Ovarian and endometrial cancers are characterized by DNA damage repair defects, either via disruption of the homologous recombination DNA repair mechanism in the former or via defects in the mismatch repair (MMR) pathway in the latter, which lead to a high load of neoantigens in both. Cervical cancer is dependent on the expression of human papillomavirus (HPV) proteins, which induce an immune response. Regardless, clinical trials testing ICIs in gynecological malignancies have initially led to disappointing results. Despite durable responses in some patients, overall response rates have been dismal. Nevertheless, in recent years, with the development of better predictive tumor biomarkers, such as microsatellite instability for endometrial cancer and programmed death ligand 1 for cervical cancer, ICIs have found their way into routine treatments for patients with advanced-stage disease. ICI-based combinations, although adding toxicity, have further improved response rates, and new combinations are currently being tested in clinical trials, as are other immunotherapy modalities, such as adoptive cell transfer and HPV-based vaccines. This review summarizes current clinical evidence supporting the use of immunotherapy in gynecological malignancies and describes studies in progress, with a focus on ICIs and predictive response biomarkers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Implementation of immune checkpoint inhibitors (ICIs) as a standard-of-care treatment in gynecological malignancies is currently lagging behind other tumor types. |
ICIs have become standard-of-care treatment in biomarker-selected patient populations, such as patients with programmed death ligand 1-positive previously treated cervical cancer and microsatellite instability-high endometrial cancer. |
ICI combinations represent the next evolutionary phase for the incorporation of ICIs into standard-of-care treatments. The first such combination, pembrolizumab + lenvatinib, was recently approved by the US FDA for microsatellite-stable endometrial cancers. |
Characterization of better biomarkers is key to tailoring ICI treatments to specific patients, thus avoiding unnecessary side effects and financial burdens. |
1 Background
The immunotherapy revolution represents the most significant progress for oncology in this century. After years of limited success using different immunotherapy modalities, the emergence of immune checkpoint inhibitors (ICIs) has led to the approval of a number of new drugs in a variety of cancer fields [1]. Currently, ICIs—mostly programmed death-1 (PD-1)-blocking drugs (nivolumab, pembrolizumab, cemiplimab), programmed death ligand-1 (PD-L1)-blocking drugs (atezolizumab, avelumab, durvalumab), and cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) inhibitors (ipilimumab)—are approved and available for a number of indications, such as advanced lines of therapy for metastatic disease [2,3,4], first-line treatment for metastatic disease [5,6,7,8], and even maintenance and adjuvant treatments for earlier-stage cancers [9, 10]. For several tumor types, the emergence of ICIs has led to a significant change in treatment paradigms. For example, in the past 3 decades, no new medications had significantly improved treatment outcomes for small-cell lung cancer (SCLC) until ICIs were used [11, 12].
For decades, physicians and scientists have been interested in the question of how cancerous cells that carry many foreign neoantigens are able to evade immune destruction. Although the first well-documented attempts to treat cancer by manipulating the immune system took place as early as the second half of the nineteenth century [13], these efforts did not evolve to become widely accepted and administered treatments. The discovery of the first immune checkpoint proteins (ICPs) and, later, at the dawn of the twenty-first century, the ability to inhibit their activity, led to the first widespread and meaningful use of immune modulation to treat a significant array of cancers [13]. ICPs are a variety of receptors expressed on the surface of immune cells that interact with ligands on other immune cells or on tumor cells. This interaction between the ICPs and their ligands modulates the immune response and, as determined by the type of receptor activated, can have either a stimulatory or an inhibitory effect on immune cells. Cancer cells and immune cells express ligands that bind ICPs on the surface of T cells, thereby effectively shutting down the immune system and thwarting its protective role [13]. The main inhibitory checkpoint receptors that have entered into clinical use are CTLA-4 and PD-1.
CTLA-4 was the first inhibitory receptor to be targeted in patients. Cancer-emergent neoantigens appear on the surface of antigen-presenting cells (APCs) and are recognized by T-cell receptors, which typically elicit an antitumor immune response. However, shortly afterward T cells express CTLA-4, which binds ligands that are expressed on the surface of APCs, namely B7-1 and B7-2. CD28 is a costimulatory molecule on the surface of T cells, which also binds B7-1 and B7-2, and has a stimulatory effect on immune activation. The expression and binding of CTLA-4 has not only an inhibitory effect on T cell activation, but also competes with CD-28’s stimulatory effect, which results in immune evasion. Therefore, CTLA-4-blocking antibodies, such as ipilimumab [2], tremelimumab [14] and MK-1308 [15], lead to T cell activation and subsequent anti-tumor immune responses. In clinical trials, ipilimumab, the first FDA-approved anti-CTLA-4 antibody for the treatment of melanoma, produced a response in only 11% of patients. However, for those patients that did respond, there were markedly prolonged remissions, which were especially meaningful as their prognoses without this treatment were particularly grim [2].
Another significant clinical improvement was achieved by the emergence of anti-PD-1 antibodies. PD-1 is another inhibitory molecule expressed on the surface of T cells that can bind its ligands, PD-L1 and PD-L2, leading to T-cell inhibition. Yet, while the ligands of CTLA-4 are expressed on the surface of APCs that are mainly found in lymph nodes, PD-L1 and PD-L2 are expressed on non-lymphoid cells. Therefore, the interaction of PD-1 with its ligands facilitates T-cell inhibition in peripheral organs [16], including the cancer microenvironment, and PD-1 inhibitory antibodies act in the tumor microenvironment to facilitate T-cell antitumor responses. The first trials that tested the activity of anti-PD-1 antibodies showed better response rates than previously described for anti-CTLA-4 antibodies, and similarly prolonged and meaningful responses (Table 1), paving the way for the approval of several anti-PD-1 antibodies for clinical use. The different modes of immune evasion activated by anti-CTLA-4 and anti-PD-1 antibodies were the rationale for testing combinations of both types of antibodies in clinical trials. The combinations resulted in higher response rates than monotherapies and gradually received regulatory approval for many indications, including melanoma and renal cell carcinoma (Table 1).
Improvements in response rates are also occurring through treatments that combine ICIs with chemotherapy. In some clinical scenarios, such combinations have resulted in high response rates that are characteristic of chemotherapy treatments, along with prolonged responses that often characterize ICI use (Table 1). One example that highlights the advantage of this strategy is the IMpower133 study, which combined the PD-L1 inhibitor atezolizumab with standard chemotherapy in the treatment of extensive disease SCLC. While the combination resulted in the same response rate as chemotherapy alone (overall response rate ≈ 60%), overall survival was significantly longer in the ICI-containing arm [11]. Chemotherapy combinations have received regulatory approval for the treatment of SCLC and non-small-cell lung cancer (NSCLC), as well as triple-negative breast cancer (TNBC) [8, 11, 17]. For bladder cancer, it is noteworthy that the combination of pembrolizumab (which has been approved for platinum-ineligible patients) and platinum-containing chemotherapy has not shown any advantage over chemotherapy alone, suggesting caution is required in the use of ICI/chemotherapy combinations [18, 19]. Another way to combine the high response rates from chemotherapy with the prolonged responses with ICIs is to employ ICIs as maintenance therapy following chemotherapy. One such example, which has received regulatory approval, arose from the PACIFIC trial, which used Durvalumab as maintenance therapy following chemoradiotherapy for stage III NSCLC [10].
However, in other types of cancers—such as prostate, colon, and gastric, as well as hepatocellular carcinomas –the response rates to ICIs were much lower, leading to a reduced hope for routine clinical implementation of ICIs for these diseases [25,26,27,28]. Nevertheless, even in diseases where response rates were low, the rare responses were prolonged and durable, which raises enthusiasm for incorporating ICIs into treatment paradigms [29]. Furthermore, some patients that did not show a significant reduction in tumor size and, therefore, were not included in the number of responding patients, did evidence a prolonged stabilization of tumor size, which demonstrates clinical benefit[29]. On the other hand, the combination of the very high costs of these new drugs, together with their immune-related side effects, have led to significant efforts to identify predictive biomarkers for ICI response. Such biomarkers can be used to cherry-pick those patients who would be considered more likely to respond to ICI treatments in comparison to the general patient population. One example is the use of tumor mutational burden (TMB) to select patients who are most likely to respond to ICI treatment. As a result of the KEYNOTE-158 study, which evaluated the use of pembrolizumab against multiple diseases, the FDA recently approved the drug for the treatment of patients with unresectable or metastatic solid tumors with a TMB ≥10 that have progressed following prior treatment, and for which no satisfactory alternative treatment options exist [30].
Another biomarker which has been heavily studied as a predictor for ICI response is PD-L1 staining of tumor tissues. These stains vary with use of different antibodies for different ICIs, namely SP142 for atezolizumab [8, 31] 22C3 for pembrolizumab [21, 32], and 28-8 for nivolumab and ipilimumab [33]. What is still open for debate is how to calculate a PD-L1 percentage (i.e., whether to score PDL-1 positive vs negative tumor cells, immune cells, or both). One such score that is commonly used in NSCLC is the tumor proportion score (TPS), which represents the percentage of viable tumor cells showing partial or complete membrane staining at any intensity [21]. More widely used in gynecological malignancies is the combined positive score (CPS), which evaluates the number of PD-L1 staining cells (tumor cells, lymphocytes, and macrophages) divided by the total number of viable tumor cells, multiplied by 100 [32]. Better standardization of PD-L1 staining and scoring is essential for improved tailoring of ICIs to patients being treated for gynecological and other malignancies.
As regards gynecological malignancies, several biomarkers for response have already been suggested and are discussed in detail in the following sections. Briefly, currently available biomarkers are aimed at identifying tumors with high neoantigen load, evaluating the extent of immune response and inflammation in the tumor microenvironment, or detecting activation of the in-tumor PD-1 signaling system.
Compared with other more immune-responsive diseases, gynecological tumors are lagging behind other cancer types in the implementation of ICI therapy. However, some ICIs have been approved for use in gynecologic malignancies, specifically in cervical and endometrial cancers [32, 34]. In clinical trials, tests are currently being carried out on different therapeutic combinations that include ICIs and other immunotherapy modalities, such as adoptive cell transfers (ACTs) and a variety of vaccines against human papillomavirus (HPVs) or other gynecological cancer-related epitopes. In this review, we summarize the current and anticipated near future evidence for immunotherapy in gynecological malignancies, with a focus on ICIs and predictive response biomarkers.
2 Epithelial Ovarian Cancer
Ovarian cancer is the deadliest gynecological malignancy in the western world [35] with a 5-year survival rate of less than 50% [36]. Discussed herein will be epithelial ovarian cancer (EOC), a subtype which represents the vast majority (90%) of ovarian cancers. EOC has been shown to be an immunogenic tumor that can induce a broad immune response [37], and studies have shown a correlation between the extent of immunologic response and overall survival [38,39,40,41,42], suggesting that the extent of immune response has a significant clinical impact on this disease. Although EOC is a heterogeneous disease that presents different histologies that assume different clinical courses, the most common subtype is high-grade serous ovarian carcinoma (HGSOC)
About half of HGSOC tumors have a deficiency in the homologous recombination (HR) DNA damage repair pathway, either through germline mutation in BRCA1/2 genes or through somatic changes [43]. As compared to HR-proficient tumors, Strickland et al. showed that HR-deficient HGSOC tumors have an elevated mutational load, more neoantigens, and a higher amount of tumor-infiltrating lymphocytes (TILs). These characteristics correlate with improved immune response and higher rates of survival [44, 45], which implies that immunotherapy may be effective in EOC, especially in HR-deficient tumors.
The first ICI study in EOC tested the effect of the CTLA-4 inhibitor ipilimumab in patients who had previously received an infusion of GVAX, irradiated autologous tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor. Only one of the nine patients treated in this trial showed a response, but the response was very prolonged, lasting 4 years [29]. Despite this impressively lengthy response, low response rates in general and a difficult side effect profile have led to an emphasis on other types of ICIs, specifically PD-1 and PD-L1 inhibitors.
Since then, a plethora of studies have tested the role of PD-1 and PD-L1 inhibitors in EOC (Table 2). All of these showed modest response rates of 10–15% [31, 46,47,48]. A promising improvement on the results of PD-1 immunotherapy in EOC was the combination of anti-PD-1 and anti-CTLA-4. In a study that compared nivolumab alone and in combination with ipilimumab, the latter produced an impressive 31.4% response rate. Furthermore, 15.7% of patients in the combination arm had a prolonged response that lasted more than 6 months. However, the overall number of those treated (51 patients) was small and the toxicity was significant, with nearly 50% of patients suffering from significant (mostly grade 3) treatment-related adverse events [49], highlighting the need for ICI treatment optimization.
To achieve this, one of the most important tools is the predictive biomarker response characterization. The most widely assessed biomarker in PD-1/PD-L1 inhibitor studies in EOC has been PD-L1 immunohistochemical (IHC) stain. Distinct antibodies, different staining evaluation methods (intensity vs. number of positive cells), and a variety of cutoffs were used. Some of these studies demonstrated some correlation between PD-L1 staining and response [46, 48], but none of the staining methods could identify a population that would have a significant chance of meaningful, long-term response. Other biomarkers that have been tested in other malignancies, such as tumor mutational burden (TMB) [50] and T-cell inflamed gene expression profile [51], have not been properly evaluated in any clinical trial for their ability to predict response in ovarian cancer. A biomarker that is likely to be predictive for ICI response in ovarian cancer is mismatch repair deficiency (dMMR), but this is a rare event in ovarian cancers, accounting for less than 10% of cases in most epithelial histologies (other than the endometrioid histology subtype). The response of dMMR tumors to ICI will be detailed in the endometrial cancer section.
The low response rates for ovarian cancer treatments have led to many attempts to combine ICIs with other drugs. The most promising and widely used partners are poly (ADP-ribose) polymerase inhibitors (PARPi) [52, 53], which are small molecules that show promising activity in EOC. Their effect is most pronounced in germline BRCA mutation carriers and then, in a diminishing order of effectivity, women with HR defects and women with HR-proficient tumors [54]. An interesting, early phase investigation that is producing promising results is the TOPACIO study. This investigation enrolled triple-negative breast cancer patients and chemotherapy (platinum) resistant ovarian cancer patients; treated them with a combination of the PARPi, niraparib, and an anti-PD-1 antibody, pembrolizumab; and achieved a response rate of 18% [52]. Although only a single-arm study without a comparator arm, this was a remarkable response rate considering the lack of response to PARPi monotherapy in this population [55, 56]. Testimonial evidence for the promise of this combination in EOC is the fact that there are currently ten ongoing clinical trials combining PARPi and anti-PD-1 or PD-L1[57].
The low response rates for ovarian cancer treatments have led to many attempts to combine ICIs with other drugs. The most promising and widely used partners are poly (ADP-ribose) polymerase inhibitors (PARPi) [53, 54], which are small molecules that show promising activity in EOC. Their effect is most pronounced in germline BRCA mutation carriers and then, in a diminishing order of effectivity, women with HR defects and women with HR-proficient tumors [55]. The TOPACIO study is an interesting early-phase investigation producing promising results. This investigation enrolled patients with TNBC and those with chemotherapy (platinum)-resistant ovarian cancer and treated them with a combination of the PARPi niraparib and an anti-PD-1 antibody, pembrolizumab, for a response rate of 18% [53]. Although this was only a single-arm study without a comparator arm, the response rate was remarkable considering the usual lack of response to PARPi monotherapy in this population [56, 57]. Testimonial evidence for the promise of this combination in EOC is the fact that ten ongoing clinical trials are currently combining PARPi and anti-PD-1 or PD-L1 [58].
Other anti-PD-1-based combinations are also undergoing testing. For example, ICI/chemotherapy combinations include sintilimab, a novel anti-PD-1, with manganese; nab-paclitaxel and platinum (NCT03989336); and pembrolizumab with paclitaxel and platinum (NCT02766582) [58]. Other trials are testing ICIs in combination with vascular endothelial growth factor inhibitors [59] and ICIs in combination with other ICIs.
Additional immunomodulatory methods for treating EOC are being evaluated in clinical trials. One of the most widely studied non-ICI immunotherapies in EOC are dendritic cell vaccines because of strong preclinical evidence supporting the approach, although success to date has been very limited [60]. Other studies examining ACT in EOC and vaccines are ongoing [58].
3 Cervical Cancer
Cervical cancer shows great promise for immunotherapy treatment because nearly 100% of cases are associated with HPV infections. This disease’s HPV-dependent nature has led to the development of anti-HPV vaccines, which have reduced the incidence of cervical cancer in the Western world [61] and allowed efficient early detection methods that rely on viral detection [61, 62]. However, although the incidence of cervical cancer is declining in the West, it remains the fourth most common cancer in women worldwide and the leading cause of cancer death in women in 42 countries. This reflects the still-bleak prognosis for far too many with advanced cervical cancer [63].
HPV-positive tumors rely on the constant expression of two viral proteins, E6 and E7. These proteins are foreign to the human immune system and therefore should elicit an anti-tumoral immune response [64]. Nevertheless, HPV-positive tumors often successfully evade immune response. Understanding how and why this is can shed light on possible new routes for immunotherapy development
Several pre-clinical and clinical attempts have been made to generate anti-HPV vaccines that could have a therapeutic effect in cervical cancer patients [64,65,66]. A phase 1 study treated patients presenting preinvasive, cervical cancer precursor lesions with a DNA-based vaccine. Administering a DNA plasmid that targets the HPV E7 protein led to a 30% regression of the pre-invasive lesions [66]. Another study used a live-attenuated Listeria-based vaccine expressing a fusion protein between a Listeria protein and an HPV E7 protein, and reached a 17% response rate in a phase II trial [67]. ISA-101 is a third anti-HPV vaccine, which consists of peptides covering the sequence of E6 and E7 proteins. This vaccine was tested in a phase II trial in combination with nivolumab in patients with HPV-positive cancers, and showed an overall response rate of 33% [68]. However, the combination with nivolumab makes it difficult to ascertain the efficacy of this vaccine from the efficacy of nivolumab.
Despite any of the promising results from early phase trials, currently no HPV-based vaccine has been shown to be better than the standard of care. Therefore, further research is needed before HPV vaccine therapies can become standard treatment. Accordingly, there are several ongoing trials studying various vaccine strategies against HPV-positive cervical dysplasia or cervical cancer [57].
The success of ICI therapies in the treatment of other malignancies, and the immune response that HPV-associated cancers invoke, have generated great interest in the potential applications of ICIs in the treatment of cervical cancer. KEYNOTE-028 [69] and KEYNOTE-158 [32] tested the efficacy of the PD-1 inhibitor pembrolizumab in cervical cancer patients. Both studies treated a similar cervical cancer patient population—previously treated metastatic or recurrent patients. However, there was a difference in patient selection based on a biomarker assay. KEYNOTE-028 enrolled patients with PD-L1 in over 1% of immune cells divided by number of tumor cells (termed modified proportion score, MPS) [69]. KEYNOTE-158 did not select by biomarker, but did analyze PD-L1 expression in all patients enrolled using a different score, the CPS, which accounted for PD-L1-positive immune cells and tumor cells [32].
KEYNOTE-028 had a 17% response rate to pembrolizumab [69], while KEYNOTE-158 showed a slightly lower response rate of 12.2% [32]. A biomarker analysis of KEYNOTE-158 showed that there were no responses in PD-L1-negative patients. This suggests that although PD-L1 is not sufficient to predict which cervical cancer patients will respond, this biomarker can rule out some patients who are unlikely to respond. These results have led to approval of pembrolizumab for patients with recurrent or metastatic cervical cancer with at least one previous line of chemotherapy, and a PD-L1 CPS≥ 1%.
In KEYNOTE-158, about 84% of patients were PD-L1-positive, making this therapy relevant for the majority of metastatic cervical cancer patients. As in other ICI treatments, pembrolizumab therapy is important because although a minority of patients respond, the duration of response is long and meaningful, with over 80% of responding patients having a duration of response that is over six months [32].
CheckMate-358 is a study that evaluated the efficacy of a different PD-1 inhibitor, nivolumab, in HPV-positive cancers, including cervical, vulvar, and vaginal cancers. The study accepted patients who were HPV-positive or whose HPV status was unknown, but not HPV-negative patients. The results for twenty-six patients with metastatic or recurrent cervical cancer treated with nivolumab produced an impressive response rate of 26.3%, albeit this was a relatively small group of patients [70]. On the other hand, a very similar trial testing nivolumab on a similar patient population showed a very different result—only one confirmed response among twenty-five treated patients [71]. This variability in efficacy highlights the caution needed when interpreting small, early-phase trials.
Another PD-1 inhibitor is cemiplimab, which has been FDA approved for metastatic cutaneous squamous cell carcinoma [72]. Following promising results of a phase 1 trial that included cervical cancer patients [73], cemiplimab is currently being tested versus investigator choice chemotherapy in recurrent or metastatic platinum refractory cervical carcinoma (EMPOWER, NCT03257267) [57].
In an effort to improve the efficacy of ICIs in cervical cancer, the CheckMate-358 trial included another arm testing an anti-PD-1 (nivolumab) and anti-CTLA-4 (ipilimumab) combination in patients with HPV-positive or HPV-undetermined cervical cancer. Although the results of this arm are yet to be formally published, conference presentations showed a response rate of 31.6% compared with an insignificant response with an anti-CTLA-4 agent alone (overall response rate < 5%). When analyzing patients with previously untreated cervical cancer, the response rate for the ICI combination was a remarkable 45.8% [74]. In total, 91 patients were treated in this trial, raising the reliability of these results in comparison with the previously described nivolumab studies (Table 3).
The promise of ICIs in cervical cancer has led to multiple trials testing combinations of PD-1 inhibitors and chemotherapy, chemoradiotherapy, or novel immunotherapies in different settings of cervical cancer treatment [57]. Another promising methodology in immune therapy for cervical cancer is the use of ACT. A phase II study treated 18 patients with cervical cancer with TILs, which were cultured from metastatic tumor samples. Lymphocytes that were reactive towards the HPV E6 and E7 proteins were selected for infusion. Five of these patients (28%) responded to this treatment and two had an ongoing response after 53 and 67 months, respectively [75]. Despite those impressive, prolonged responses, the response rates were low. Additionally, the use of ACT in the treatment of epithelial cancers involves many technical challenges, such as the need for surgery in every patient, long T-cell culturing and selection times, and low prevalence of HPV-positive cells [75]. With the intent to overcome some of these issues, an ongoing trial is using T cells engineered to target HPV proteins in different HPV-positive cancers, including cervical cancer (NCT02858310) [57].
4 Endometrial Cancer
Endometrial cancer (EC) is the most common gynecologic malignancy in the Western world and generally carries a relatively good prognosis compared with other gynecological malignancies. It is estimated that, in 2020, there will be 65,620 new EC cases in the USA but only 12,590 deaths [35]. At present, most cases are cured through treatment either by surgery alone or by surgery and irradiation [77]. Historically, based on histology, ECs were divided into type I or type II cancers. Type I tumors are mostly of endometrioid adenocarcinoma histology. They represent the majority of EC cases and are largely responsible for the good prognosis of this disease. Type II tumors include less common histologies, of which uterine papillary serous cancers (UPSCs) are most the frequent, accounting for 10–20% of this segment. UPSCs carry a less favorable prognosis and are therefore overrepresented in stage IV and recurrent tumors [78]. In the last decade, a new EC classification emerged as a result of The Cancer Genome Atlas Project (TCGA) [79], dividing endometrial tumors into four molecular subtypes: POLE (DNA polymerase epsilon) ultramutated, microsatellite instability (MSI) hypermutated, copy number variants (CNV) low, and CNV high. These subtypes partially overlap with the traditional morphologic classification of tumor. Traditional type I tumors are divided among the first three molecular groups, whereas type II tumors are mostly seen in the CNV-high molecular group. The new molecular classification correlates well with prognosis and response to immunotherapy [79].
The first molecular subtype, POLE ultramutated tumors, represent only 7% of ECs but carry a remarkably good prognosis. It has been suggested that the exceptionally high TMB induces a strong natural immune response, which is responsible for the positive prognosis and, accordingly, this subtype is theorized to have a high response rate to immunotherapy [80, 81]. However, no published, prospective clinical trial has yet tested the use of ICIs in POLE-mutated tumors.
The second molecular subtype, MSI-high (MSI-H) tumors, are more frequent, comprising 13–30% of endometrial tumors [79, 82, 83]. The MSI-H phenotype is a result of either a germinal or a somatic loss of one or more of the MMR genes, and can therefore be diagnosed based on the lack of expression of one or more of the MMR proteins (dMMR).
One trial that tested the effect of ICIs in MSI-H/dMMR tumors was the KEYNOTE-158 trial, which recruited 233 previously treated patients with non-colorectal MSI-H tumors and treated them with pembrolizumab. In this trial, 49 patients had MSI-H EC; of these, 28 (57.1%) responded to treatment [84]. Of note, the median duration of response in this patient population was not reached, with one patient still responding after 27 months of follow-up. This highlights the prolonged responses that characterize responding patients in this population. A combined analysis of four studies treating various non-colorectal MSI-H tumor types with pembrolizumab resulted in a 39.6% overall response rate, leading to an accelerated FDA approval of pembrolizumab for this patient population [34]. Dostarlimab (TSR-042) is a novel anti-PD-1 antibody that was tested in a phase II trial for patients with previously treated MSI-H and microsatellite stable (MSS) EC. The MSI-H population in this trial had a 50% response rate, similar to that with pembrolizumab [85]. Table 4 summarizes the response rates for all PD-1 and PD-L1 inhibitors that were tested in MSI-H patients.
For EC patients within the last two molecular subgroups, CNV high and CNV low, collectively referred to clinically as MSS [or MMR proficient (pMMR)] tumors, the response rates to immunotherapy were poor. Most of the trials studying the efficacy of ICIs in MSS EC were small, eliciting only occasional responses [86,87,88,89], which is especially troubling since these two subtypes combined comprise the majority of patients with EC [79, 82, 83]. One exception to this was a recent trial (the largest of its kind) studying the efficacy of the novel PD-1 inhibitor, dostarlimab, in EC. In a total population of 110 patients with MSI-H and MSS EC, the latter cohort had a 20.3% response rate [85].
To improve response rates to ICIs in patients with MSS EC, ongoing studies are testing ICI combinations with the intent of overcoming primary ICI resistance. One such promising method is the combination of pembrolizumab with lenvatinib, a multikinase inhibitor targeting vascular endothelial growth factor receptor (VEGFR)-1, VEGFR2, and VEGFR3. Recently, in a phase II single-arm study, this combination was tested in patients with previously treated EC. In 94 patients with pMMR/MSS EC, 38.3% of patients experienced a response [90, 91], leading to accelerated FDA approval of the pembrolizumab–lenvatinib combination for this patient population. Nevertheless, it is important to note that, despite the encouraging findings, this combined treatment also resulted in significant toxicity: about two-thirds of the patients in this trial experienced a significant treatment-related adverse event, and about 18% of the patients in the trial discontinued at least one of the study drugs because of adverse events. Currently, additional ongoing studies are testing this combination in a phase III randomized trial in a similar patient population (KEYNOTE-775, NCT03517449) and in patients with earlier phase disease (MK-7902-001, NCT03884101) [57].
Another way to improve ICI response rates is through improved biomarker selection. PD-L1 has been tested as a biomarker for ICI treatment, with some showing no correlation with response and others showing minor correlation [86, 89, 92]. However, none of the trials found PD-L1 to be a clear and useful marker for selecting patients who are anticipated to achieve prolonged responses. TILs have also been tested as a biomarker in a phase I study testing atezolizumab in patients with EC, but the number of responding patients was too small to reach any definite conclusions [92].
To summarize, the use of ICIs is constantly growing in both MSI-H and MSS EC patient populations, but a significant need still exists to better tailor ICI treatments for specific patients through the use of improved biomarkers and optimized drug combinations.
5 Future Directions
Despite the acceptance of ICIs in the treatment of a small subset of gynecological malignancies, for most tumor types and in the majority of therapeutic settings, the efficacy of single-agent ICIs is minimal and insufficient to be accepted as a standard of care. Therefore, most current clinical trials are exploring the use of ICIs in combination with chemotherapy or other biological agents. Table 5 offers a list of ongoing phase III clinical trials that are investigating ICI/chemotherapy combinations, along with several approved targeted therapies.
For gynecological malignancies, we expect to see ICI combinations featuring heavily in upcoming treatment option approvals. PD-1 and PDL-1 combinations with anti-CTLA-4 antibodies are showing great promise, which justifies its additional toxicity compared with a single-agent ICI. The added value of combining ICIs with chemotherapy remains to be seen, as it is unclear whether the efficacy of this approach is greater than the sequential use of chemotherapy and ICIs. We are also encouraged by preclinical which is evidence suggesting efficacy in the use of irradiation therapy in conjunction with ICIs. Although the utility of such combinations still needs to be shown in randomized, prospective clinical trials, it seems that irradiation leads to immunogenic cell death, which, in turn, synergizes ICI activity [95, 96].
Additional progress in the use of ICIs for gynecological malignancies will be achieved when treatments for specific patients can be tailored as a result of deploying improved biomarkers for patient selection. Currently, different biomarkers are being used in different fields for different ICIs. However, we anticipate that prioritization and standardization of biomarkers will eventually occur. Finally, we believe that the discovery of new targets for checkpoint inhibition and the development of novel methods to target them can lead to a significant change in the treatment of gynecological malignancies, substantial and durable responses that will prolong patients’ lives, and potential cures for advanced diseases.
6 Summary
The use of ICIs is gradually becoming a treatment option for advanced and recurrent gynecological malignancies, mostly because of the meaningful and prolonged responses seen in a small fraction of patients. As a result, the adverse event profile of ICIs in gynecological patients is coming into focus, as is the need for skillful treatment of toxicities. This is particularly so because adverse events can be severe, especially with the use of ICI combinations that include CTLA-4-targeting antibodies. For example, the NRG-GY003 trial investigated the single-agent anti-PD-1 nivolumab compared with an anti-PD-1/anti-CTLA-4 combination in the treatment of ovarian cancer. In this study, 33% of patients experienced grade 3 or higher treatment-related adverse events with PD-1 monotherapy, compared with 49% in the combination group [49]. Although the frequency of adverse events is similar to adverse event profiles associated with ICI combinations used in the treatment of other malignancies [6, 7], this might be reduced by using a lower-dose intensity of anti-CTLA-4 antibodies, as has been the case in certain cervical cancer and NSCLC trials [33, 75]. While the side effect profile seen in gynecological malignancies using ICI combinations [49] is similar to the adverse event frequency of chemotherapy combinations frequently used against these malignancies [97,98,99], the toxicity profile of ICIs is very different from that of chemotherapy. ICI toxicity mostly comprises immune-mediated adverse events, with high-grade toxicities requiring steroid treatments for symptom mitigation [100]. It is likely that the growing use of ICIs in gynecological malignancies will lead to better choices for ICI dosages and better treatment of adverse events, which will result in improved tolerability of these agents.
References
Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–86. https://doi.org/10.1158/2159-8290.CD-18-0367.
Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. https://doi.org/10.1056/NEJMoa1003466.
Motzer RJ, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–13. https://doi.org/10.1056/NEJMoa1510665.
Bellmunt J, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–26. https://doi.org/10.1056/NEJMoa1613683.
Socinski MA, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–301. https://doi.org/10.1056/NEJMoa1716948.
Hodi FS, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(11):1480–92. https://doi.org/10.1016/S1470-2045(18)30700-9.
Motzer RJ, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–90. https://doi.org/10.1056/NEJMoa1712126.
Schmid P, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–21. https://doi.org/10.1056/NEJMoa1809615.
Eggermont AMM, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378(19):1789–801. https://doi.org/10.1056/NEJMoa1802357.
Antonia SJ, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–29. https://doi.org/10.1056/NEJMoa1709937.
Oiseth SJ, Aziz MS. Cancer immunotherapy: a brief review of the history, possibilities, and challenges ahead. JCMT. 2017;3(10):250. https://doi.org/10.20517/2394-4722.2017.41.
Ribas A, et al. Tremelimumab (CP-675,206), a cytotoxic T lymphocyte associated antigen 4 blocking monoclonal antibody in clinical development for patients with cancer. Oncologist. 2007;12(7):873–83. https://doi.org/10.1634/theoncologist.12-7-873.
Perets R, et al. Antitumor activity and safety of MK-1308 (anti-CTLA-4) plus pembrolizumab (pembro) in patients (pts) with non-small cell lung cancer (NSCLC): updated interim results from a phase I study. JCO. 2019;37(15_suppl):2558. https://doi.org/10.1200/JCO.2019.37.15_suppl.2558.
Keir ME, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203(4):883–95. https://doi.org/10.1084/jem.20051776.
Horn L, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–9. https://doi.org/10.1056/NEJMoa1809064.
Gandhi L, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92. https://doi.org/10.1056/NEJMoa1801005.
Stenger M. First-line atezolizumab plus platinum-based chemotherapy vs chemotherapy alone in advanced urothelial cancer—the ASCO post; 2020. https://ascopost.com/issues/june-25-2020/first-line-atezolizumab-plus-platinum-based-chemotherapy-vs-chemotherapy-alone-in-advanced-urothelial-cancer/. Accessed 13 Sep 2020.
Slater H. Phase III KEYNOTE-361 trial fails to meet primary end points. Cancer Network; 2020. https://www.cancernetwork.com/view/phase-iii-keynote-361-trial-fails-to-meet-primary-end-points. Accessed 13 Sep 2020.
Rini BI, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–27. https://doi.org/10.1056/NEJMoa1816714.
Reck M, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33. https://doi.org/10.1056/NEJMoa1606774.
Langer CJ, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–508. https://doi.org/10.1016/S1470-2045(16)30498-3.
Mok TSK, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–30. https://doi.org/10.1016/S0140-6736(18)32409-7.
Paz-Ares L, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–39. https://doi.org/10.1016/S0140-6736(19)32222-6.
Schachter J, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017;390(10105):1853–62. https://doi.org/10.1016/S0140-6736(17)31601-X.
Fuchs CS, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013. https://doi.org/10.1001/jamaoncol.2018.0013.
El-Khoueiry AB, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–502. https://doi.org/10.1016/S0140-6736(17)31046-2.
O’Neil BH, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS ONE. 2017;12(12):e0189848. https://doi.org/10.1371/journal.pone.0189848.
Antonarakis ES, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. JCO. 2020;38(5):395–405. https://doi.org/10.1200/JCO.19.01638.
Hodi FS, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci. 2008;105(8):3005–10. https://doi.org/10.1073/pnas.0712237105.
Marabelle A, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. JCO. 2020;38(1):1–10. https://doi.org/10.1200/JCO.19.02105.
Liu JF, et al. Safety, clinical activity and biomarker assessments of atezolizumab from a phase I study in advanced/recurrent ovarian and uterine cancers. Gynecol Oncol. 2019a;154(2):314–22. https://doi.org/10.1016/j.ygyno.2019.05.021.
Chung HC, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2019;37(17):1470–8. https://doi.org/10.1200/JCO.18.01265.
Hellmann MD, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020–31. https://doi.org/10.1056/NEJMoa1910231.
Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res. 2019;25(13):3753–8. https://doi.org/10.1158/1078-0432.CCR-18-4070.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. https://doi.org/10.3322/caac.21590.
Bast RC, et al. Critical questions in ovarian cancer research and treatment: report of an American Association for Cancer Research Special Conference. Cancer. 2019;125(12):1963–72. https://doi.org/10.1002/cncr.32004.
Schlienger K, et al. TRANCE- and CD40 ligand-matured dendritic cells reveal MHC class I-restricted T cells specific for autologous tumor in late-stage ovarian cancer patients. Clin Cancer Res. 2003;9(4):1517–27.
Zhang L, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–13. https://doi.org/10.1056/NEJMoa020177.
Hwang W-T, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012;124(2):192–8. https://doi.org/10.1016/j.ygyno.2011.09.039.
Bachmayr-Heyda A, et al. Prognostic impact of tumor infiltrating CD8+ T cells in association with cell proliferation in ovarian cancer patients—a study of the OVCAD consortium. BMC Cancer. 2013;13:422. https://doi.org/10.1186/1471-2407-13-422.
Adams SF, et al. Intraepithelial T cells and tumor proliferation: impact on the benefit from surgical cytoreduction in advanced serous ovarian cancer. Cancer. 2009;115(13):2891–902. https://doi.org/10.1002/cncr.24317.
Sato E, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102(51):18538–43. https://doi.org/10.1073/pnas.0509182102.
Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–15. https://doi.org/10.1038/nature10166.
Strickland KC, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;7(12):13587–98. https://doi.org/10.18632/oncotarget.7277.
Morse CB, et al. Tumor infiltrating lymphocytes and homologous recombination deficiency are independently associated with improved survival in ovarian carcinoma. Gynecol Oncol. 2019;153(2):217–22. https://doi.org/10.1016/j.ygyno.2019.02.011.
Matulonis UA, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol. 2019;30(7):1080–7. https://doi.org/10.1093/annonc/mdz135.
Hamanishi J, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. JCO. 2015;33(34):4015–22. https://doi.org/10.1200/JCO.2015.62.3397.
Disis ML, et al. Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol. 2019;5(3):393. https://doi.org/10.1001/jamaoncol.2018.6258.
Zamarin D, et al. Randomized phase II trial of nivolumab versus nivolumab and ipilimumab for recurrent or persistent ovarian cancer: an NRG oncology study. JCO. 2020. https://doi.org/10.1200/JCO.19.02059.
Alexandrov LB, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–21. https://doi.org/10.1038/nature12477.
Cristescu R, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. 2018;362(6411):eaar3593. https://doi.org/10.1126/science.aar3593.
Feinberg J, Elvin JA, Bellone S, Santin AD. Identification of ovarian cancer patients for immunotherapy by concurrent assessment of tumor mutation burden (TMB), microsatellite instability (MSI) status, and targetable genomic alterations (GA). Gynecol Oncol. 2018;149:36. https://doi.org/10.1016/j.ygyno.2018.04.081.
Konstantinopoulos PA, et al. Single-arm phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol. 2019a;5(8):1141. https://doi.org/10.1001/jamaoncol.2019.1048.
Drew Y, et al. An open-label, phase II basket study of olaparib and durvalumab (MEDIOLA): Results in germline BRCA-mutated (gBRCA m) platinum-sensitive relapsed (PSR) ovarian cancer (OC). Gynecol Oncol. 2018;149:246–7. https://doi.org/10.1016/j.ygyno.2018.04.555.
Mirza MR, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154–64. https://doi.org/10.1056/NEJMoa1611310.
Gelmon KA, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12(9):852–61. https://doi.org/10.1016/S1470-2045(11)70214-5.
Sandhu SK, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;14(9):882–92. https://doi.org/10.1016/S1470-2045(13)70240-7.
Home—ClinicalTrials.gov. https://clinicaltrials.gov/. Accessed 27 Apr 2020.
Liu JF, et al. Assessment of combined nivolumab and bevacizumab in relapsed ovarian cancer: a phase 2 clinical trial. JAMA Oncol. 2019b. https://doi.org/10.1001/jamaoncol.2019.3343.
Martin-Lluesma S, Graciotti M, Grimm AJ, Boudousquié C, Chiang CL, Kandalaft LE. Are dendritic cells the most appropriate therapeutic vaccine for patients with ovarian cancer? Curr Opin Biotechnol. 2020;65:190–6. https://doi.org/10.1016/j.copbio.2020.03.003.
Phase II study of ipilimumab monotherapy in recurrent platinum-sensitive ovarian cancer—study results—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/results/NCT01611558. Accessed 25 Apr 2020.
Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. https://doi.org/10.1016/S0140-6736(07)61416-0.
Zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2(5):342–50. https://doi.org/10.1038/nrc798.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Borysiewicz LK, et al. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996;347(9014):1523–7. https://doi.org/10.1016/s0140-6736(96)90674-1.
Sharma RK, et al. Costimulation as a platform for the development of vaccines: a peptide-based vaccine containing a novel form of 4–1BB ligand eradicates established tumors. Cancer Res. 2009;69(10):4319–26. https://doi.org/10.1158/0008-5472.CAN-08-3141.
Alvarez RD, et al. A pilot study of pNGVL4a-CRT/E7(detox) for the treatment of patients with HPV16+ cervical intraepithelial neoplasia 2/3 (CIN2/3). Gynecol Oncol. 2016;140(2):245–52. https://doi.org/10.1016/j.ygyno.2015.11.026.
Basu P, et al. A randomized phase 2 study of ADXS11-001 listeria monocytogenes–listeriolysin O immunotherapy with or without cisplatin in treatment of advanced cervical cancer. Int J Gynecol Cancer. 2018;28(4):764–72. https://doi.org/10.1097/IGC.0000000000001235.
Massarelli E, et al. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16-related cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5(1):67–73. https://doi.org/10.1001/jamaoncol.2018.4051.
Frenel J-S, et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the phase Ib KEYNOTE-028 trial. J Clin Oncol. 2017;35(36):4035–41. https://doi.org/10.1200/JCO.2017.74.5471.
Naumann RW, et al. Safety and efficacy of nivolumab monotherapy in recurrent or metastatic cervical, vaginal, or vulvar carcinoma: results from the phase I/II CheckMate 358 trial. JCO. 2019a;37(31):2825–34. https://doi.org/10.1200/JCO.19.00739.
Santin AD, et al. Phase II evaluation of nivolumab in the treatment of persistent or recurrent cervical cancer (NCT02257528/NRG-GY002). Gynecol Oncol. 2020;157(1):161–6. https://doi.org/10.1016/j.ygyno.2019.12.034.
Migden MR, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379(4):341–51. https://doi.org/10.1056/NEJMoa1805131.
Papadopoulos KP, et al. First-in-human study of cemiplimab alone or in combination with radiotherapy and/or low-dose cyclophosphamide in patients with advanced malignancies. Clin Cancer Res. 2020;26(5):1025–33. https://doi.org/10.1158/1078-0432.CCR-19-2609.
Naumann RW, et al. Efficacy and safety of nivolumab (Nivo) + ipilimumab (Ipi) in patients (pts) with recurrent/metastatic (R/M) cervical cancer: results from CheckMate 358. Ann Oncol. 2019b;30:v898–9. https://doi.org/10.1093/annonc/mdz394.059.
Stevanović S, et al. A phase II study of tumor-infiltrating lymphocyte therapy for human papillomavirus-associated epithelial cancers. Clin Cancer Res. 2019;25(5):1486–93. https://doi.org/10.1158/1078-0432.CCR-18-2722.
Lheureux S, et al. Association of ipilimumab with safety and antitumor activity in women with metastatic or recurrent human papillomavirus-related cervical carcinoma. JAMA Oncol. 2018;4(7):e173776. https://doi.org/10.1001/jamaoncol.2017.3776.
Charo LM, Plaxe SC. Recent advances in endometrial cancer: a review of key clinical trials from 2015 to 2019. F1000Res. 2019;8:849. https://doi.org/10.12688/f1000research.17408.1.
Suarez AA, Felix AS, Cohn DE. Bokhman Redux: endometrial cancer ‘types’ in the 21st century. Gynecol Oncol. 2017;144(2):243–9. https://doi.org/10.1016/j.ygyno.2016.12.010.
Cancer Genome Atlas Research Network, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. https://doi.org/10.1038/nature12113.
Rayner E, et al. A panoply of errors: polymerase proofreading domain mutations in cancer. Nat Rev Cancer. 2016;16(2):71–81. https://doi.org/10.1038/nrc.2015.12.
Wang F, et al. Evaluation of POLE and POLD1 mutations as biomarkers for immunotherapy outcomes across multiple cancer types. JAMA Oncol. 2019. https://doi.org/10.1001/jamaoncol.2019.2963.
Prendergast EN, et al. Comprehensive genomic profiling of recurrent endometrial cancer: implications for selection of systemic therapy. Gynecol Oncol. 2019;154(3):461–6. https://doi.org/10.1016/j.ygyno.2019.06.016.
Soumerai TE, et al. Clinical utility of prospective molecular characterization in advanced endometrial cancer. Clin Cancer Res. 2018;24(23):5939–47. https://doi.org/10.1158/1078-0432.CCR-18-0412.
Oaknin A, et al. Preliminary safety, efficacy, and pharmacokinetic/pharmacodynamic characterization from GARNET, a phase I/II clinical trial of the anti-PD-1 monoclonal antibody, TSR-042, in patients with recurrent or advanced MSI-h and MSS endometrial cancer. Gynecol Oncol. 2019;154:17. https://doi.org/10.1016/j.ygyno.2019.04.044.
Hasegawa K, et al. “Efficacy and safety of nivolumab (Nivo) in patients (pts) with advanced or recurrent uterine cervical or corpus cancers. JCO. 2018;36(15_suppl):5594–5594. https://doi.org/10.1200/JCO.2018.36.15_suppl.5594.
Konstantinopoulos PA, et al. Phase II study of avelumab in patients with mismatch repair deficient and mismatch repair proficient recurrent/persistent endometrial cancer. J Clin Oncol. 2019b;37(30):2786–94. https://doi.org/10.1200/JCO.19.01021.
Antill YC, et al. “Activity of durvalumab in advanced endometrial cancer (AEC) according to mismatch repair (MMR) status: the phase II PHAEDRA trial (ANZGOG1601). JCO. 2019;37(15_suppl):5501–5501. https://doi.org/10.1200/JCO.2019.37.15_suppl.5501.
Ott PA, et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: results from the KEYNOTE-028 study. J Clin Oncol. 2017a;35(22):2535–41. https://doi.org/10.1200/JCO.2017.72.5952.
Makker V, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20(5):711–8. https://doi.org/10.1016/S1470-2045(19)30020-8.
Makker V, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020. https://doi.org/10.1200/JCO.19.02627.
Fleming GF, et al. Clinical activity, safety and biomarker results from a phase Ia study of atezolizumab (atezo) in advanced/recurrent endometrial cancer (rEC). JCO. 2017;35(15_suppl):5585–5585. https://doi.org/10.1200/JCO.2017.35.15_suppl.5585.
Ott PA, et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1–positive endometrial cancer: results from the KEYNOTE-028 study. JCO. 2017b;35(22):2535–41. https://doi.org/10.1200/JCO.2017.72.5952.
Fader AN, et al. Preliminary results of a phase II study: PD-1 blockade in mismatch repair–deficient, recurrent or persistent endometrial cancer. Gynecol Oncol. 2016;141:206–7. https://doi.org/10.1016/j.ygyno.2016.04.532.
Mollica V, et al. Immunotherapy and radiation therapy in renal cell carcinoma. CDT. 2020. https://doi.org/10.2174/1389450121666200311121540.
Fife K, Bang A. Combined radiotherapy and new systemic therapies—have we moved beyond palliation? Clin Oncol. 2020. https://doi.org/10.1016/j.clon.2020.07.021.
Neijt JP, et al. Exploratory phase III study of paclitaxel and cisplatin versus paclitaxel and carboplatin in advanced ovarian cancer. JCO. 2000;18(17):3084–92. https://doi.org/10.1200/JCO.2000.18.17.3084.
Pectasides D, et al. Carboplatin and paclitaxel in advanced or metastatic endometrial cancer. Gynecol Oncol. 2008;109(2):250–4. https://doi.org/10.1016/j.ygyno.2008.01.028.
Miller D, et al. Late-breaking abstract 1: randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: a gynecologic oncology group study. Gynecol Oncol. 2012;125(3):771. https://doi.org/10.1016/j.ygyno.2012.03.034.
Haanen JBAG, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119–42. https://doi.org/10.1093/annonc/mdx225.
Acknowledgements
The authors thank Steve Spencer for English editing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This manuscript was funded by the women’s health grant at Rambam.
Conflict of interest
AR served on an advisory board for MSD, the manufacturer of pembrolizumab, and has no other conflicts of interest. TT, AA, and RP have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
TT and RP wrote the first draft of the manuscript. All of the authors participated in the conceptual design and writing of the manuscript, as well as critical revisions of important intellectual content, while RP supervised the writing of the manuscript.
Rights and permissions
About this article
Cite this article
Taha, T., Reiss, A., Amit, A. et al. Checkpoint Inhibitors in Gynecological Malignancies: Are we There Yet?. BioDrugs 34, 749–762 (2020). https://doi.org/10.1007/s40259-020-00450-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-020-00450-x