Abstract
Background
There has been a significant increase in the number and efficacy of therapies for advanced melanoma. Immunotherapies, such as anti-cytotoxic T-lymphocyte antigen-4 and programmed cell death-1 inhibitors, have improved the prognosis for patients with advanced melanoma. While spontaneous melanoma-associated vitiligo is a known phenomenon, the occurrence of melanoma-associated vitiligo following melanoma therapy is now recognized to associate with favorable outcomes.
Objective
The objective of this article is to provide a comprehensive literature review of melanoma-associated vitiligo and explore the insights these findings provide about the pathobiology of vitiligo and mechanisms underlying melanoma therapies.
Methods
PubMed and Science Direct databases were searched for studies pertaining to melanoma-associated vitiligo. The 36 studies reviewed included meta-analyses (n = 2), prospective cohort studies (n = 4), prospective observational studies (n = 3), retrospective studies (n = 12), case series (n = 2), and case reports (n = 13).
Results
The basic mechanisms underlying melanoma-associated vitiligo and vitiligo may be shared. Characterization of these mechanisms will identify new biomarkers and therapeutic targets for both melanoma and vitiligo.
Conclusions
Co-opting the immune system to target tumor antigens highlights the potential overlap between anti-tumor immunity and autoimmunity. The development of vitiligo-like depigmentation in association with immunotherapy for melanoma may provide insights into both the immune response against melanoma as well as the pathogenesis of vitiligo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Melanoma-associated vitiligo (MAV) can occur spontaneously or in response to a number of treatment modalities. MAV is associated with improved treatment outcomes for advanced melanoma patients treated with various immunotherapies. |
There is a growing understanding regarding the clinical and pathophysiologic similarities and differences between MAV and vitiligo vulgaris. |
The phenomenon of MAV provides insight into the anti-tumor immune response against melanoma, as well as the pathogenesis of vitiligo vulgaris. |
1 Introduction
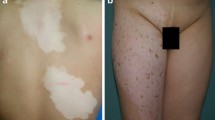
Immunotherapies represent a significant breakthrough in the management of advanced melanoma. Melanomas express multiple antigens that can be therapeutically targeted via immunotherapy; however, these antigens may also be expressed on normal cells, thus increasing the risk of autoimmunity. Autoimmune-mediated melanocyte destruction results in vitiligo, characterized by depigmented skin patches. Melanoma-associated vitiligo (MAV), also called melanoma-associated depigmentation or leukoderma, occurs in 2–16% of patients with melanoma overall [1], and may reflect an immune response against antigens shared by melanoma cells and normal melanocytes [1,2,3,4]. For example, antibodies from sera of patients with vitiligo can target melanoma cells for destruction [5]. Importantly, both spontaneous and therapy-induced MAV have been associated with improved prognosis in all stages of melanoma [2, 6, 7].
This article provides a review addressing melanoma-associated depigmentation including its clinical features, incidence, association with immunotherapies, prognostic implications, underlying mechanisms relating to anti-tumor responses, as well as an examination of MAV etiology that may provide insights into vitiligo pathogenesis. PubMed search terms included “melanoma” AND “vitiligo” OR “leucoderma” OR “depigmentation” OR “hypopigmentation,” as well as “melanoma” AND “immunotherapy” (Fig. 1, Table 1).
2 Melanoma-Associated Vitiligo
Spontaneous vitiligo in individuals with melanoma is significantly more common than in the general population [6, 8]. Melanoma-associated vitiligo and vitiligo are indistinguishable based on histology [9]. A prospective study of 2954 patients with melanoma of all stages found the prevalence of vitiligo was 2.8%, compared with 0.4–2.0% in the greater population [7]. Three-quarters of MAV occurred spontaneously in the absence of treatment. Vitiligo preceded melanoma diagnosis in 20.5% of patients by a range of 2–45 years and was an independent favorable predictor of overall survival in stage III and IV patients. Five-year survival of stage III patients with MAV was 65.0% compared to 42.5% among patients without MAV. For stage IV patients, mean survival time was 14.4 months for patients with MAV vs 9.6 months for those without MAV [7].
3 Immunotherapy and Melanoma-Associated Vitiligo
Teulings et al. conducted a systematic review of 137 studies comprising 5737 Stage III–IV patients with melanoma treated with various immunotherapies. The overall cumulative MAV incidence was 3.4% (2.0–6.3%) [10]. Vitiligo was associated with a 50% reduction in the risk of disease progression and a four times reduced risk of death [10].
The most commonly studied classes of immunotherapies include general stimulation with interferon-α (IFN-α) or interleukin-2 (IL-2), use of a modified oncolytic virus to enhance anti-tumor responses, and immune checkpoint inhibitors, namely anti-cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death (PD)-1 inhibitors.
3.1 Interferon-α- and Interleukin-2-Based Treatments
Rosenberg and White reported vitiligo in 26% of patients with metastatic melanoma responding to high-dose IL-2, while vitiligo was not observed among non-responders [11]. In another study, 49 patients with metastatic melanoma were treated with a regimen of dacarbazine, cisplatin, vinblastine, IL-2, and IFN-α, followed by sustained IL-2 and granulocyte macrophage-colony stimulating factor therapy. Vitiligo developed in 43% of patients and was associated with a significant improvement in median overall survival compared with patients who did not develop vitiligo (18.2 vs 8.5 months) [2]. Interleukin-2 may promote CD8+ T-cell responses against melanoma-associated antigens, which are also shared by melanocytes [6, 12]. Notably, higher IL-2 and IL-2 receptor levels have been reported in sera of patients with non-melanoma-associated vitiligo, compared with controls [13]. In addition, soluble IL-2 receptor serum levels correlated with body surface area in vitiligo [14], suggesting that responses to immunotherapy and autoimmunity may involve the same cytokine pathways.
3.2 Talimogene Laherparepvec
Talimogene laherparepvec is a genetically modified, attenuated herpes simplex virus that replicates within melanoma cells and stimulates an anti-tumor immune response. In a phase II study of talimogene laherparepvec for metastatic melanoma, vitiligo was described in 6.0% (3/50) of patients, two of whom achieved complete remission [15]. Furthermore, Iglesias et al. reported on two patients with advanced melanoma treated with talimogene laherparepvec injections into cutaneous melanoma nodules resulting in depigmentation in areas distinct from the injection site. Both patients achieved complete remission [16]. Development of vitiligo at distant sites and favorable treatment response in these patients suggest that a systemic immune response against common melanocyte antigens may develop even with localized immunotherapy [16].
3.3 Anti-Cytotoxic T-Lymphocyte Antigen-4 Inhibitor
Immune checkpoint inhibitors aim to disinhibit the immune response against tumor-related antigens and are associated with a number of immune-related adverse events (irAEs) [17]. However, irAEs are generally associated with more favorable responses to therapy and may reflect stimulation of a robust immune response [18,19,20]. Ipilimumab is a CTLA-4 inhibitor approved for use in advanced melanoma. Cytotoxic T-lymphocyte antigen-4 is a receptor expressed on effector and regulatory T cells, and CTLA-4 blockage disinhibits the cell-mediated response against melanoma. The overall incidence of ipilimumab-induced MAV is 2.0–14.3%. [19, 20]. Vitiligo is generally persistent after treatment cessation [18], suggesting that a durable immune response is induced.
3.4 Programmed Cell Death-1 Inhibitors
Anti-PD-1 agents approved for advanced melanoma include nivolumab and pembrolizumab. Programmed cell death-1, a receptor expressed by activated T cells, dampens T-cell activation and promotes T-cell “exhaustion” to prevent autoimmunity [17]. Programmed cell death-1 inhibition prevents down-regulation of T-cell responses and produces a survival benefit in advanced melanoma [21]. Melanoma-associated vitiligo generally occurs at higher rates after anti-PD-1 therapy compared with CTLA-4 inhibition [22,23,24]. The incidence of MAV in patients treated with pembrolizumab ranges between 9.6 and 25% [8, 25,26,27]. In a randomized controlled trial of nivolumab for metastatic melanoma involving 418 patients, 10.7% (22/206) of nivolumab-treated patients developed vitiligo, compared with 0.5% (1/205) of dacarbazine-treated controls [28]. Belum and colleagues performed a meta-analysis of dermatologic adverse events associated with pembrolizumab and nivolumab used for lung cancer, renal cancer, and advanced melanoma [22]. For pembrolizumab, the cumulative incidence of vitiligo was 8.3% (9/111, relative risk of vitiligo = 17.5 as compared to chemotherapy controls). For nivolumab, vitiligo incidence was 7.5% (62/878, relative risk = 14.6) [22].
Hua et al. conducted a prospective study specifically evaluating for vitiligo in 67 patients with metastatic melanoma treated with pembrolizumab [25]. The incidence of vitiligo was 25% (17/67 patients: generalized vitiligo in 82%, localized in 12%, and mixed in 1%). Development of vitiligo was significantly associated with higher rates of objective treatment response [25]. Nakamura and colleagues retrospectively examined the correlation between vitiligo and nivolumab treatment response in 35 patients with stage III and IV disease [27]. Vitiligo occurred in 25.7% of patients and was predominantly localized, affecting less than 10% body surface area. The objective response rate was higher in patients who developed vitiligo compared with those who did not (44.4% vs 7.7%, p = 0.027). Onset of vitiligo within 5 months of treatment initiation was a positive indication of treatment response [27].
Indini and colleagues assessed 173 patients treated with nivolumab or pembrolizumab for metastatic melanoma for adverse events and survival [29]. In this study, 59% of patients developed irAEs, which included vitiligo, dermatitis (rash, pruritus, or lichenoid reactions), diarrhea, hepatitis, and hypothyroidism. Immune-related adverse effects generally correlated with improved progression-free survival and overall survival. When vitiligo was separately compared with other irAEs, there was a non-significant trend towards improved overall survival (p = 0.06). Meanwhile, when patients who developed vitiligo were compared to patients without irAEs, there was a significantly improved overall survival. Median overall survival was undefined for patients with vitiligo, compared to 9.7 months for patients who did not develop irAEs [29]. Nardin and colleagues retrospectively reviewed 111 patients with advanced melanoma treated with a PD-1 inhibitor [30]. In this study, 29% of patients developed a cutaneous adverse reaction, including 13.5% of patients developing vitiligo. The development of vitiligo was associated with greater overall survival and progression-free survival, compared with the development of other cutaneous adverse reactions. This was confirmed in landmark analyses to avoid lead-time bias [30].
Several studies compared MAV rates between CTLA-4 and PD-1 inhibitor treatment for advanced melanoma. In a phase III trial of 834 patients randomized to receive pembrolizumab 10 mg/kg every 2 or 3 weeks, or ipilimumab 3 mg/kg every 3 weeks [21], vitiligo occurred in 9% of patients treated with pembrolizumab every 2 weeks, 11.2% treated with pembrolizumab every 3 weeks, and 1.6% of patients treated with ipilimumab. Pembrolizumab use led to longer progression-free survival and overall survival.[21]. Hwang et al. compared a 25-patient cohort with metastatic melanoma who received combination ipilimumab and pembrolizumab therapy with 82 patients treated with pembrolizumab alone [31]. There was no significant difference in vitiligo rates between therapies, although combination treatment was associated with a shorter time to vitiligo development (3.2 vs 10.3 months) [31]. Of note, vitiligo incidence increased at an approximately constant rate after treatment initiation, with no plateau, unlike the incidence of lichenoid reactions or pruritus [31]. This constant rate is in concordance with other studies of anti-PD-1 therapy [23]. Quach and colleagues retrospectively reviewed 318 patients with advanced melanoma treated with PD-1 monotherapy or combination ipilimumab–nivolumab therapy at a single center [32]. The authors reported a significantly increased rate of cutaneous toxic effects in patients treated with combination therapy compared with PD-1 monotherapy. In addition, a multivariate analysis confirmed significantly superior response rates associated with the development of vitiligo (odds ratio 7.05) or other cutaneous reactions (odds ratio 4.37), compared with patients without cutaneous toxicity [32].
In a retrospective cohort study using the French Pharmacovigilance Database, Babai and colleagues described the clinical characteristics of 96 patients who developed vitiligo-like lesions associated with checkpoint inhibitors [33]. Patients treated with pembrolizumab (n = 78), nivolumab (n = 14), ipilimumab (n = 6), and combined ipilimumab plus nivolumab (n = 2) were included in the discussion. The median time to onset of depigmentation was shorter for patients treated with ipilimumab (3.8 months), than for pembrolizumab (5.4 months) or nivolumab (5 months). Six patients (6.25%) in the study demonstrated repigmentation after discontinuation of immunotherapy, which the authors highlight was associated with disease progression [33]. Of note, the latter finding of repigmentation heralding disease progression or recurrence has been supported by several other reports [6, 34].
In addition to inducing vitiligo, PD-1 therapy for metastatic melanoma has been associated with the development of halo nevi [35], as well as regression of melanocytic nevi and lentigines [24, 36]. Similar to vitiligo, disappearance of pigmented lesions may result from an immune response against antigens shared by melanocytes and melanoma cells [36]. Additionally, there have been several reports of patients developing a Vogt–Koyanagi–Harada (VKH)-like condition in association with checkpoint inhibitor therapy for melanoma [4, 37, 38]. For example, Obata and colleagues reported a case of patient who developed VKH-like uveitis in association with nivolumab treatment for metastatic melanoma [38]. Similarly, Mihailovic et al. reported the case of patient who developed VKH-like uveitis, along with vitiligo and poliosis in a patient with metastatic melanoma treated with nivolumab [37]. The development of VKH in the setting of immunotherapy for melanoma suggests that a T-cell response against normal melanocytes in extra-cutaneous sites may also be triggered by immunotherapy [38].
It was previously thought that MAV exclusively occurs when immunotherapy is employed to treat melanoma, not other malignancies [22, 25, 27, 39]. This idea was supported by a retrospective study of 83 patients treated with pembrolizumab for melanoma (n = 66), lung (n = 15), prostate (n = 1), and Merkel cell (n = 1) cancers, in which pigment loss was only observed in patients with melanoma [40]. However, more recently, there have been several reports of patients developing vitiligo in association with checkpoint inhibitor treatment for non-melanoma cancers [4], including renal cell carcinoma [41], cholangiocarcinoma [42], acute myeloid leukemia [43], non-small cell lung cancer [44], and squamous cell carcinoma [42]. In a case series by Liu and colleagues, the authors note that depigmented patches were photo-distributed and proposed that one possible explanation for depigmentation may be the induction of a de novo immune response against previously damaged melanocytes in the skin [42].
4 Proposed Mechanisms of Melanoma-Associated Vitiligo
Melanoma cells and melanocytes share a number of immunogenic antigens including, Melan-A/MART-1, tyrosinase-related protein (TYRP)-1/gp75, TYRP-2, tyrosinase, and gp100 [1,2,3, 6, 36]. Overexpression of these antigens by malignant cells and/or a breakdown in immune tolerance due to therapeutics may promote an immunogenic response [25, 27]. Anti-tumor immunity targets neo-antigens produced by mutations within malignant cells [45, 46]. The concurrent recognition of antigens on melanocytes may be due to epitope spreading, when an initial immune response against one epitope extends to similar antigens [45, 46]. These antigens can be recognized by HLA-A2-restricted cytotoxic T cells on both melanomas and melanocytes [8]. The same cytotoxic T-cell clone attacks both melanoma cells and bystander melanocytes due to expression of shared antigens [25, 27, 47].
Animal studies have previously demonstrated vitiligo induction after immunization to melanoma antigens TYRP-1 and g100 [48]. In a another study, a patients with metastatic melanoma was infused with MART-1-specific CD8 + T cells, and infused clones localized to both the tumor and melanocytes resulting in a loss of MART-1 expression and an absence of melanocytes in depigmented skin lesions [48]. Hua and colleagues investigated skin biopsies from two patients who developed vitiligo while receiving pembrolizumab. A dermal inflammatory infiltrate composed of T cells was observed concordant with the disappearance of melanocytes [25]. As for increased rates of MAV associated with treatment with checkpoint inhibitors, it has been proposed that PD-1 mediates tolerance to melanosomal proteins, such as tyrosinase and TYRP-2, and with inhibition of PD-1 activity, autoimmune vitiligo results [17].
Le Gal and colleagues described an infiltrate of CD8 + T cells located at the basal layer of the epidermis within MAV patches. These T cells had a clonal or oligoclonal T-cell response, reflecting antigenic stimulation, as well as expression of cutaneous lymphocyte antigen, a skin homing receptor [47]. Furthermore, CD8 + T cells from patients with vitiligo are capable of destroying melanoma cells ex vivo; conversely, cytotoxic T cells from patients with melanoma attack melanocytes [12, 49].
Circulating antibodies against melanoma-associated antigens, namely gp100, tyrosinase, TYRP-1, and TYRP-2 and B16 melanoma-cell line antigens (presumed to be a heterogenous group of antibodies) have been detected in patients with MAV [1, 3, 24, 45]. Development of antibodies against melanocyte-specific antigens may occur secondarily to T-cell destruction of melanoma cells and release of antigens, to which antibodies are generated [3, 24]. Merimsky and colleagues measured anti-tyrosinase antibody levels in patients with melanoma, vitiligo, MAV, and healthy controls [3]. Anti-tyrosinase antibodies were detected in patients with melanoma and MAV, but at lower levels than in vitiligo [3]. Furthermore, high levels of serum antibodies to TYRP-2 have been demonstrated in both patients with vitiligo and melanoma [50]. In a study of an IL-2 therapy for advanced melanoma, 29% of patients who developed MAV had IgG titers to TYRP-2, compared with 14% among patients who did not develop vitiligo, which the authors suggest represents a break in immune tolerance to TYRP-2 after immunotherapy [2].
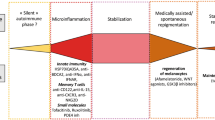
Melanoma-associated vitiligo persists beyond completion of immunotherapy in patients with advanced melanoma [51], and may be a key factor in achieving a long-lasting anti-melanoma immune response [26, 49]. Bryne and Turk proposed that an immune response against bystander melanocytes might actively maintain a T-cell response against melanoma. Furthermore, if a T-cell response against shared melanoma and melanocyte antigens is produced efficiently, these T cells may develop memory properties to maintain a durable anti-melanoma response [49]. Thus, maintenance of these memory cells may rely on melanocyte destruction [49].
While the vast majority of data regarding treatment-associated MAV is available after immunotherapy, it is notable that there are emerging reports of MAV occurring after treatment with BRAF/MEK inhibitors [52] and an oral tyrosine kinase inhibitor in c-kit-mutated mucosal melanoma [53]. In the case of BRAF/MEK inhibition, Ramondetta et al. hypothesized that MAV may relate to increased lymphocyte infiltration of tumor cells after targeted therapy [52].
5 Vitiligo vs Melanoma-Associated Vitiligo
While an autoimmune mechanism underlies vitiligo progression, other factors, including oxidative stress [54, 55], endoplasmic reticulum stress [56], dysregulation of signaling between epidermal and dermal components [57], and neurogenic disturbances may also contribute to pathogenesis [58]. Several studies sought to clarify if vitiligo and MAV are distinct clinical entities (Table 2). Lommerts et al. investigated whether MAV and vitiligo could be distinguished clinically [9]. Four expert evaluators blindly examined photographs of 33 patients with vitiligo and 11 patients with MAV. In the study, 72.7% of MAV cases were misdiagnosed, with 80% labeled as vitiligo. Both MAV and vitiligo presented primarily as bilateral, symmetric, well-demarcated depigmented patches and both were associated with “confetti-like” lesions. Overall, no differentiating clinical factors were identified except for a later mean age of MAV presentation (55 vs 34 years) [9]. Hartmann and colleagues compared depigmented lesions in 15 patients with melanoma and 30 patients with vitiligo by digital photography [59]. Depigmented patches had a similar morphology and distribution between groups. Histologic and immunohistologic features of depigmented lesions were also similar [59].
However, other studies suggest MAV and vitiligo are clinically distinct. Larsabal et al. compared eight patients with metastatic melanoma and anti-PD-1-induced MAV with 30 subjects with vitiligo [26]. In contrast to vitiligo, depigmented lesions in MAV subjects presented as numerous small macules progressing into a larger patch in a photo-distributed pattern, associated with preexisting solar lentigines, and without evidence of Koebnerization [26]. Similarly, in the study by Babai et al., nearly half of patients with MAV demonstrated depigmented lesions on photo-exposed areas such as the face and hands [33]. A predominantly photo-exposed pattern was also noted by Ramondetta and colleagues [52].
Quaglino and colleagues studied 2954 patients with melanoma and reported that MAV was less well demarcated, less progressive and generalized, and less likely to be associated with autoimmune conditions, than is typically observed in vitiligo vulgaris [7]. Of note, in this study, three quarters of MAV occurred in the absence of treatment, and among treated patients, management included IFN, IL-2, and/or chemotherapy. However, in the study by Lommerts et al., no clinical differences could be identified between spontaneous and immunotherapy-induced MAV cases [9]. Further studies directly comparing the differences in clinical features between spontaneous and treatment-associated MAV are thus warranted.
There are conflicting reports about the humoral response in MAV and vitiligo. Several studies have suggested similar mechanisms in vitiligo and MAV. For example, high levels of antibodies against TYRP2, gp100, and tyrosinase have been reported in both vitiligo and MAV [1, 17]. In contrast, Teulings et al. studied seven patients with MAV and 27 patients with vitiligo and found that antibodies against MART-1 were only present in MAV and not vitiligo [1], although T cells specific for MART-1, gp100, and tyrosinase were present in the blood of both patients with MAV and vitiligo [1]. Larsabal et al. reported a CD8+ T-cell skewed response, characterized by CXCR3 expression and increased CXCL10 (a ligand of CXCR3) serum levels, in patients with MAV after anti-PD-1 therapy, but not in vitiligo. This may implicate different T-cell subsets in MAV compared to vitiligo [26]. However, other studies have shown CXCL10 and CD8+ CXCR3+ T-cell expression in patients with vitiligo [60]. Nakashima and colleagues examined the composition of the T-cell infiltrate present in vitiligo-like lesions induced by nivolumab therapy in a patient with metastatic melanoma [61]. The presence of CD49a + CD103 + CD8 + tissue-resident memory T cells was detected within the biopsied lesion. The authors discuss that this same tissue-resident T-cell population has been reported to be present in lesions of classic vitiligo vulgaris, suggesting overlapping mechanisms in MAV and vitiligo vulgaris [61].
Genome-wide association studies for vitiligo highlighted two genes, HLA-A and TYR (encodes tyrosinase), associated with an inverse risk of vitiligo and melanoma [62]. Tyrosinase serves as a key auto-antigen in vitiligo and polymorphisms are associated with differing susceptibilities to both vitiligo and melanoma [62]. Furthermore, tyrosinase, expressed in melanoma and melanocytes, is presented to the immune system by HLA-A*0201, which has been associated with a risk of vitiligo. Therefore both HLA-A*0201 and TYR genetic variations interact to determine the robustness of immune surveillance and susceptibility to vitiligo and melanoma [62]. These same factors may also promote MAV, in which increased immune surveillance due to melanomagenesis results in vitiligo and a more favorable prognosis [62].
Given the development of MAV has been associated with a more favorable prognosis for advanced melanoma, this raises the question of the best approach to treating cutaneous lesions in MAV. Belum and colleagues proposed a treatment algorithm for anti-PD-1-associated dermatologic adverse effects based on the National Cancer Institute Common Terminology Criteria for Adverse Events grading scale [22]. The authors propose that for grade 1 vitiligo, topical corticosteroids may be attempted, for grade 2, the addition of phototherapy can be considered, and if depigmentation does not improve or worsens, the authors proposed encouraging patients to continue anti-melanoma therapy despite the presence of depigmentation [22]. Considering the psychosocial implications of vitiligo, further study specifically examining the optimal approach of treatment relating to improvement of MAV lesions and overall melanoma outcomes is warranted.
6 Conclusions
The mechanistic association between vitiligo and melanoma thus spans disease predisposition, onset, progression, and susceptibility to therapy. Areas of future study are numerous and necessary to better elucidate the clinical presentation of MAV. These include implications of MAV occurring spontaneously vs in the context of immunotherapy, long-term prognostic implications of MAV, and the mechanistic overlap between MAV and vitiligo. Additionally, rare situations, such as MAV presenting during treatment with targeted molecular therapies for melanoma and vitiligo occurring in the context of immunotherapy for non-melanoma malignancies, need to be better understood.
Last, further studies will be required to clarify the optimal approach to patients who desire treatment of MAV. As the pathogeneses of these disorders are further elucidated, it will provide a greater understanding of immunologic regulation in vitiligo and melanoma, which can guide future therapeutic approaches to both conditions.
References
Teulings HE, Willemsen KJ, Glykofridis I, Krebbers G, Komen L, Kroon MW, et al. The antibody response against MART-1 differs in patients with melanoma-associated leucoderma and vitiligo. Pigment Cell Melanoma Res. 2014;27(6):1086–96.
Boasberg PD, Hoon DS, Piro LD, Martin MA, Fujimoto A, Kristedja TS, et al. Enhanced survival associated with vitiligo expression during maintenance biotherapy for metastatic melanoma. J Invest Dermatol. 2006;126(12):2658–63.
Merimsky O, Shoenfeld Y, Baharav E, Altomonte M, Chaitchik S, Maio M, et al. Melanoma-associated hypopigmentation: where are the antibodies? Am J Clin Oncol. 1996;19(6):613–8.
Failla CM, Carbone ML, Fortes C, Pagnanelli G, D'Atri S. Melanoma and vitiligo: in good company. Int J Mol Sci. 2019;20(22):5731.
Fishman P, Azizi E, Shoenfeld Y, Sredni B, Yecheskel G, Ferrone S, et al. Vitiligo autoantibodies are effective against melanoma. Cancer. 1993;72(8):2365–9.
Daneshpazhooh M, Shokoohi A, Dadban A, Raafat J. The course of melanoma-associated vitiligo: report of a case. Melanoma Res. 2006;16(4):371–3.
Quaglino P, Marenco F, Osella-Abate S, Cappello N, Ortoncelli M, Salomone B, et al. Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: results from a single-institution hospital-based observational cohort study. Ann Oncol. 2010;21(2):409–14.
Yang M, Chang D. Vitiligo after immune checkpoint inhibitor therapy in a woman with metastatic melanoma. J Cancer Res Pract. 2018;5:161–4.
Lommerts JE, Teulings HE, Ezzedine K, van Geel N, Hartmann A, Speeckaert R, et al. Melanoma-associated leukoderma and vitiligo cannot be differentiated based on blinded assessment by experts in the field. J Am Acad Dermatol. 2016;75(6):1198–204.
Teulings HE, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33(7):773–81.
Rosenberg SA, White DE. Vitiligo in patients with melanoma: normal tissue antigens can be targets for cancer immunotherapy. J Immunother Emphasis Tumor Immunol. 1996;19(1):81–4.
Gathings R, Lewallen R, Yosipovitch G. Immunotherapy-induced leukoderma from treatment of melanoma with IL-2: a case report and a review of the literature. Acta Derm Venereol. 2015;95(2):197–200.
Khan R, Gupta S, Sharma A. Circulatory levels of T-cell cytokines (interleukin [IL]-2, IL-4, IL-17, and transforming growth factor-beta) in patients with vitiligo. J Am Acad Dermatol. 2012;66(3):510–1.
Shi YL, Li K, Hamzavi I, Lim HW, Zhou L, Mi QS. Elevated circulating soluble interleukin-2 receptor in patients with non-segmental vitiligo in North American. J Dermatol Sci. 2013;71(3):212–4.
Senzer NN, Kaufman HL, Amatruda T, Nemunaitis M, Reid T, Daniels G, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27(34):5763–71.
Iglesias P, Ribero S, Barreiro A, Podlipnik S, Carrera C, Malvehy J, et al. Induced vitiligo due to talimogene laherparepvec injection for metastatic melanoma associated with long-term complete response. Acta Derm Venereol. 2019;99(2):232–3.
Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22(4):886–94.
de Golian E, Kwong BY, Swetter SM, Pugliese SB. Cutaneous complications of targeted melanoma therapy. Curr Treat Options Oncol. 2016;17(11):57.
Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13(22 Pt 1):6681–8.
Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100(14):8372–7.
Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–32.
Belum VR, Benhuri B, Postow MA, Hellmann MD, Lesokhin AM, Segal NH, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12–25.
Hwang SJ, Carlos G, Wakade D, Byth K, Kong BY, Chou S, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74(3):455–61.e1.
Wolner ZJ, Marghoob AA, Pulitzer MP, Postow MA, Marchetti MA. A case report of disappearing pigmented skin lesions associated with pembrolizumab treatment for metastatic melanoma. Br J Dermatol. 2018;178(1):265–9.
Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152(1):45–51.
Larsabal M, Marti A, Jacquemin C, Rambert J, Thiolat D, Dousset L, et al. Vitiligo-like lesions occurring in patients receiving anti-programmed cell death-1 therapies are clinically and biologically distinct from vitiligo. J Am Acad Dermatol. 2017;76(5):863–70.
Nakamura Y, Tanaka R, Asami Y, Teramoto Y, Imamura T, Sato S, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2017;44(2):117–22.
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30.
Indini A, Di Guardo L, Cimminiello C, Prisciandaro M, Randon G, De Braud F, et al. Immune-related adverse events correlate with improved survival in patients undergoing anti-PD1 immunotherapy for metastatic melanoma. J Cancer Res Clin Oncol. 2019;145(2):511–21.
Nardin C, Jeand'heur A, Bouiller K, Valnet-Rabier MB, Dresco F, Castagna J, et al. Vitiligo under anti-programmed cell death-1 therapy is associated with increased survival in melanoma patients. J Am Acad Dermatol. 2020;82(3):770–2.
Hwang SJE, Park JJW, Wakade D, Chou S, Byth K, Fernandez-Penas P. Cutaneous adverse events of anti-programmed death 1 antibodies combined with anti-cytotoxic T-lymphocyte-associated protein 4 therapy use in patients with metastatic melanoma. Melanoma Res. 2019;29(2):172–7.
Quach HT, Dewan AK, Davis EJ, Ancell KK, Fan R, Ye F, et al. Association of anti-programmed cell death 1 cutaneous toxic effects with outcomes in patients with advanced melanoma. JAMA Oncol. 2019;5(6):906–8.
Babai S, Voisin AL, Bertin C, Gouverneur A, Le-Louet H. Occurrences and outcomes of immune checkpoint inhibitors-induced vitiligo in cancer patients: a retrospective cohort study. Drug Saf. 2020;43(2):111–7.
Nardin C, Pelletier F, Puzenat E, Aubin F. Vitiligo repigmentation with melanoma progression during pembrolizumab treatment. Acta Derm Venereol. 2019;99(10):913–4.
Plaquevent M, Greliak A, Pinard C, Duval-Modeste AB, Joly P. Simultaneous long-lasting regression of multiple nevi and melanoma metastases after ipilimumab therapy. Melanoma Res. 2019;29(3):311–2.
Schwager Z, Laird ME, Latkowski JA. Regression of pigmented lesions in a patient with metastatic melanoma treated with immunotherapy. JAAD Case Rep. 2018;4(5):421–3.
Mihailovic N, Dyballa J, Herz S, Fluck M, Alnawaiseh M, Merte RL, et al. Vogt-Koyanagi-Harada-like uveitis under immune checkpoint inhibitor treatment for metastasized malignant melanoma [in German]. Ophthalmologe. 2020;117(5):467–71.
Obata S, Saishin Y, Teramura K, Ohji M. Vogt-Koyanagi-Harada disease-like uveitis during nivolumab (anti-PD-1 antibody) treatment for metastatic cutaneous malignant melanoma. Case Rep Ophthalmol. 2019;10(1):67–74.
Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19(3):345–61.
Sanlorenzo M, Vujic I, Daud A, Algazi A, Gubens M, Luna SA, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151(11):1206–12.
Billon E, Walz J, Brunelle S, Thomassin J, Salem N, Guerin M, et al. Vitiligo adverse event observed in a patient with durable complete response after nivolumab for metastatic renal cell carcinoma. Front Oncol. 2019;9:1033.
Liu RC, Consuegra G, Chou S, Fernandez PP. Vitiligo-like depigmentation in oncology patients treated with immunotherapies for nonmelanoma metastatic cancers. Clin Exp Dermatol. 2019;44(6):643–6.
Yin ES, Totonchy MB, Leventhal JS. Nivolumab-associated vitiligo-like depigmentation in a patient with acute myeloid leukemia: a novel finding. JAAD Case Rep. 2017;3(2):90–2.
Kosche C, Mohindra N, Choi JN. Vitiligo in a patient undergoing nivolumab treatment for non-small cell lung cancer. JAAD Case Rep. 2018;4(10):1042–4.
Byrne EH, Fisher DE. Immune and molecular correlates in melanoma treated with immune checkpoint blockade. Cancer. 2017;123(S11):2143–53.
Lo JA, Fisher DE, Flaherty KT. Prognostic significance of cutaneous adverse events associated with pembrolizumab therapy. JAMA Oncol. 2015;1(9):1340–1.
Le Gal FA, Avril MF, Bosq J, Lefebvre P, Deschemin JC, Andrieu M, et al. Direct evidence to support the role of antigen-specific CD8(+) T cells in melanoma-associated vitiligo. J Invest Dermatol. 2001;117(6):1464–70.
Yee C, Thompson JA, Roche P, Byrd DR, Lee PP, Piepkorn M, et al. Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of T cell-mediated vitiligo. J Exp Med. 2000;192(11):1637–44.
Byrne KT, Turk MJ. New perspectives on the role of vitiligo in immune responses to melanoma. Oncotarget. 2011;2(9):684–94.
Okamoto T, Irie RF, Fujii S, Huang SK, Nizze AJ, Morton DL, et al. Anti-tyrosinase-related protein-2 immune response in vitiligo patients and melanoma patients receiving active-specific immunotherapy. J Invest Dermatol. 1998;111(6):1034–9.
Edmondson LA, Smith LV, Mallik A. Nivolumab-induced vitiligo in a metastatic melanoma patient: a case report. J Oncol Pharm Pract. 2017;23(8):629–34.
Ramondetta A, Ribero S, Conti L, Fava P, Marra E, Broganelli P, et al. Clinical and pathological relevance of drug-induced vitiligo in patients treated for metastatic melanoma with anti-PD1 or BRAF/MEK inhibitors. Acta Derm Venereol. 2020;100(1):adv00001.
Pala L, Conforti F, Cocorocchio E, Ferrucci PF. Extensive vitiligo associated to response to c-kit inhibitor in metastatic mucosal melanoma. Anticancer Drugs. 2020. https://doi.org/10.1097/CAD.0000000000000906.
Arowojolu OA, Orlow SJ, Elbuluk N, Manga P. The nuclear factor (erythroid-derived 2)-like 2 (NRF2) antioxidant response promotes melanocyte viability and reduces toxicity of the vitiligo-inducing phenol monobenzone. Exp Dermatol. 2017;26(7):637–44.
Jian Z, Li K, Song P, Zhu G, Zhu L, Cui T, et al. Impaired activation of the Nrf2-ARE signaling pathway undermines H2O2-induced oxidative stress response: a possible mechanism for melanocyte degeneration in vitiligo. J Invest Dermatol. 2014;134(8):2221–30.
Toosi S, Orlow SJ, Manga P. Vitiligo-inducing phenols activate the unfolded protein response in melanocytes resulting in upregulation of IL6 and IL8. J Invest Dermatol. 2012;132(11):2601–9.
Kapoor R, Dhatwalia SK, Kumar R, Rani S, Parsad D. Emerging role of dermal compartment in skin pigmentation: comprehensive review. J Eur Acad Dermatol Venereol. 2020. https://doi.org/10.1111/jdv.16404.
Taieb A, Picardo M. Clinical practice: vitiligo. N Engl J Med. 2009;360(2):160–9.
Hartmann A, Bedenk C, Keikavoussi P, Becker JC, Hamm H, Brocker EB. Vitiligo and melanoma-associated hypopigmentation (MAH): shared and discriminative features. J Dtsch Dermatol Ges. 2008;6(12):1053–9.
Fukuda K, Harris JE. Vitiligo-like depigmentation in patients receiving programmed cell death-1 inhibitor reflects active vitiligo. J Am Acad Dermatol. 2018;78(1):e15–e1616.
Nakashima C, Ishida Y, Nakagawa K, Irie H, Hirata M, Kataoka T, et al. Identification of CD49a+ CD8+ resident memory T cells in vitiligo-like lesions associated with nivolumab treatment for melanoma. J Eur Acad Dermatol Venereol. 2020;34(2):e79–82.
Spritz RA. The genetics of generalized vitiligo: autoimmune pathways and an inverse relationship with malignant melanoma. Genome Med. 2010;2(10):78.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was in part funded by the Leo Foundation (Grant number LF16101).
Conflict of interest
Brandon E. Cohen, Prashiela Manga, Krysta Lin, and Nada Elbuluk have no conflicts of interest that might be directly relevant to the content of this article.
Rights and permissions
About this article
Cite this article
Cohen, B.E., Manga, P., Lin, K. et al. Vitiligo and Melanoma-Associated Vitiligo: Understanding Their Similarities and Differences. Am J Clin Dermatol 21, 669–680 (2020). https://doi.org/10.1007/s40257-020-00524-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-020-00524-0