Abstract
This study highlights the range of non-melanoma cancers where ICI-induced vitiligo can be present and challenges the exclusivity of this phenomenon to melanoma. We believe our manuscript will encourage awareness in our colleagues and stimulate interest in further studies to elucidate the mechanisms of ICI-induced vitiligo in both melanoma and non-melanoma cancers, and to understand whether this phenomenon holds the same positive prognostic value in both cancer groups. This is a retrospective cohort study from a single-institution’s electronic medical record for cancer patients treated with ICIs who subsequently developed vitiligo. We identified 151 patients with ICI-induced vitiligo, 19 (12.6%) non-melanoma and 132 (77.4%) melanoma patients. Time to onset of vitiligo was nearly doubled in the non-melanoma cohort, however, this is confounded by possible delayed diagnosis or under reporting of this asymptomatic condition in patients who do not regularly receive skin exams. The majority of patients had a stable course of vitiligo with 91.4% receiving no treatment in this largely Caucasian cohort. Two patients with non-melanoma cancers and Fitzpatrick type IV or above skin received treatment with narrowband ultraviolet B light therapy and topical steroids with near-complete response. This study highlights the occurrence of ICI-induced vitiligo in a variety of non-melanoma cancers, where skin of color patients will be more prevalent and the need for treatment will potentially be more urgent. Further study is needed to elucidate the mechanism of ICI-induced vitiligo and determine if non-melanoma cancers have the same association between vitiligo and increased tumor response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immune checkpoint inhibitors (ICIs) are targeted inhibitors of T-lymphocyte surface receptors resulting in T-cell stimulation and subsequent upregulation of host anti-tumor response. The immunomodulatory effects of ICIs also lead to various immune-mediated adverse events. Cutaneous adverse events (CAEs) have a wide range of presentations and are among the most common with incidence as high as 44% with use of anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) monotherapy and > 50% on combination anti-CTLA-4 and anti-programmed cell death 1/anti-programmed cell death-ligand 1 (PD-1/PD-L1) therapy [1]. Vitiligo is a well-documented phenomenon that has long been associated with melanoma and viewed as a positive prognostic factor indicating the body’s heightened immune response towards melanocytes [2]. This has also been observed in the ICI-induced setting for melanoma patients, and although the mechanism remains unclear, it is commonly thought to occur because of melanocyte antigen sensitization to the melanoma resulting in subsequent off-target autoimmune response in benign melanocytes [2]. This mechanism comes into question as there are scattered case reports in the literature describing ICI-induced vitiligo in a variety of non-melanoma cancers [3,4,5,6,7,8] but not nearly at the same incidence as is seen in melanoma patients.
Although ICI-associated vitiligo is a known CAE, little has been reported about the course, prognosis, and management of this disease. In the predominantly-Caucasian melanoma patient population, vitiligo is low-acuity adverse event with rare symptoms and low quality of life impact. With the growing diversity of the patient population being treated with ICIs, the cosmetic and quality of life impact of ICI-associated vitiligo as well as patient desire for vitiligo treatment are increasing. This study seeks to outline what we know about the course of ICI-associated vitiligo and to highlight successful management strategies.
Materials and methods
We identified patients between January 23, 2014 and September 24, 2020 through our institutional electronic medical records system using natural language processing (NLP). We searched for patients with multiple mentions of checkpoint inhibitor names or abbreviations as well as the words “depigmented”, “depigmentation”, or “vitiligo” under an internal institutional review board-approved protocol. Charts were manually reviewed to examine timing and determine causation. Data collected include patient characteristics, cancer type, staging and response, ICI type and treatment information, and vitiligo characteristics, treatment, and outcome. Determining the incidence of vitiligo per cancer type would have required manual review of the 13,697 patients who were on checkpoint inhibitors over this time period to determine their primary cancer diagnosis and was beyond the scope of this project. CAE severity was determined through the Common Terminology Criteria for Adverse Events (CTCAE). The Kruskal–Wallis method was used to test statistical significance for time to onset.
Results
We identified 151 consecutive, standard of care and clinical trials patients with ICI-induced vitiligo, of which 19 (12.6%) patients were being treated for non-melanoma cancers and 132 (87.4%) for melanoma. Demographics information, primary cancer type, and ICI data are summarized in Table 1.
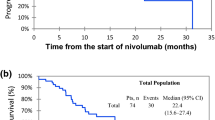
Time to onset, vitiligo outcome and treatments, time to next treatment (TTNT), and overall survival for the three most common melanoma ICI treatment groups are summarized in Table 2. Patients were stratified by primary cancer type into melanoma versus non-melanoma groups for relevant calculations. One patient was excluded from the non-melanoma group for overall survival calculation due to unknown date of passing. Two patients were excluded from the melanoma group, one for overall survival alone due to unknown date of passing and one for TTNT plus OS as patient was lost to follow-up. For melanoma patients, TTNT was 797 days. Overall survival was assessed for the three most common treatment arms within our melanoma group. Median OS in melanoma patients was 28.8 months, 34.9 months, and 48.8 months in patients treated with ipilimumab-plus-nivolumab, nivolumab monotherapy, and pembrolizumab monotherapy respectively. Overall, 5-year survival within the melanoma group was highest for patients treated with pembrolizumab (87.0%), followed by nivolumab (79.7%) and ipilimumab-plus-nivolumab therapy (56.8%). OS at 2 years showed a smaller variation among the same three treatment arms at 90.0%, 97.5% and 96.3% respectively. The comparison of each treatment arms’ 1, 2, and 5-year OS to previously reported clinical trial data can be found in Table 3. For non-melanoma patients, TTNT and OS data are stratified by cancer type in Table 2. Comparative statistics is not relevant with the small sample numbers for the non-melanoma cohorts.
Discussion
In this single institution series of ICI-induced vitiligo, we reviewed 151 consecutive patients with melanoma and non-melanoma cancers. In our cohort, all Food and Drug Administration (FDA)-approved ICIs have the capacity to induce vitiligo. ICIs are most frequently used in melanoma therapy and ICI-induced vitiligo is most strongly associated with treatment of melanoma [2]. As expected, our cohort had mostly melanoma patients (132, 87.4%), with the most prevalent race overall being Caucasians (108, 71.5%). Our cohort of 19 (12.6%) non-melanoma patients, however, definitively challenges the previously held notion that ICI-induced vitiligo is limited to melanoma patients. Specifically, ICI-induced vitiligo in patients with angiosarcoma, Hodgkin’s lymphoma, and mycosis fungoides were not previously reported in the literature. Thus, it appears that ICI-induced vitiligo can be associated with a much wider range of cancers than previously expected, and the mechanism of ICI-induced vitiligo deserves further exploration.
We noted that non-melanoma patients had a statistically significant difference (p = 0.005) in median time to onset of vitiligo compared to melanoma patients with 382 days versus 209 days, respectively. As ICI-induced CAEs generally occur on average within 4 weeks after ICI initiation [1], it is interesting to note the later onset. It is, however, unclear if ICI-induced vitiligo truly has a delayed onset like ICI-induced bullous pemphigoid, or if vitiligo has a delayed diagnosis. The low acuity and asymptomatic nature of vitiligo increase the risk of delayed diagnosis and under reporting. It is especially prone to delays in diagnosis in lighter skin types where vitiligo is more difficult to detect and there is lower cosmetic impact. Of note, the majority of patients in our cohort were Caucasian (71.5%). In combination, these factors may result in an erroneously low number of reported patients with vitiligo secondary to ICI. In studies looking at the correlation between vitiligo and tumor outcome in melanoma patients, prospective trials showed a higher incidence (25%) [9] than retrospective trials (3.4–13%) [10, 11]. Our patient cohort even has a low incidence of vitiligo (1%, 151/13697) to what was reported in retrospective melanoma clinical trials data (2–10%) [12]. Further, patients with non-melanoma cancers’ longer time to onset may be explained by these patients not receiving regular full-body skin exams from a dermatologist as their melanoma counterparts do. Definitive incidence and time to onset data would require more standardized skin exams and ideally, prospective reporting. Our aggregate incidence of vitiligo over the time period described above is 1%, which is likely due to a combination of factors including lower incidence of vitiligo in non-melanoma cancer patients and the factors described above.
Vitiligo outcomes in our cohort showed 117 patients (78%) had stable disease, while 26 (17.3%) worsened and 7 (4.7%) improved. This supports vitiligo as a predominantly non-progressive disease. The majority (91.4%) of our patients were not treated for their vitiligo and were offered reassurance with sun protection alone. As many patients also opted not to treat the vitiligo, it is difficult to discern precisely whether no treatment was desired or no treatment was offered. This is not unexpected as vitiligo is predominantly an asymptomatic disease in fair-skinned patients, and thus low priority in the setting of metastatic cancer. With the expanding use of checkpoint inhibitors for non-melanoma cancers, however, the diversity of patients’ skin color is also expected to increase, leading to a greater impact of vitiligo on quality of life and greater importance of vitiligo outcomes. Of note, two African American patients were treated with narrowband ultraviolet B (NB-UVB). Both experienced vitiligo improvement, with one patient experiencing dramatic re-pigmentation. In a previous report, a Hispanic male with renal cell carcinoma experienced improvement of his ICI-induced vitiligo after being treated with NB-UVB in combination with topical steroids, after lack of improvement on topical steroids alone [14]. This points to the effectiveness of the current gold standard treatment of topical steroids and UV light therapy for vitiligo in the ICI-induced variant. In addition, one patient in our cohort with fair skin was depigmented with topical hydroquinone in cosmetically-sensitive areas to reduce the contrast and noticeability. Although not a standard treatment, depigmentation is an effective management strategy when it aligns with the patient’s goals.
Overall, it is reasonable to only treat ICI-induced vitiligo at the patient’s request, however, it is important for both patients and treating physicians to understand that there are potential management strategies available and to not minimize the quality of life concerns of this skin disease. As studies in skin-of-color (SOC) patients have shown that vitiligo can have a variety of negative impacts on mental health [15], treating vitiligo with NB-UVB alone or in combination with topical steroids can be impactful in this setting. [As ICIs are increasingly utilized in non-melanoma cancers where there is greater diversity of patients, physicians should be aware of potential quality of life impacts of ICI-induced vitiligo and potential effective treatment options.]
With the available data, we did a brief exploration into the hypothesis that vitiligo secondary to ICI therapy in melanoma patients is associated with increased tumor response, as it indicates the therapy is effectively targeting melanocytes. This is reflected through the association of ICI-induced vitiligo with increased progression-free survival, overall survival, and complete or partial response to treatment in melanoma patients [9, 10, 13]. Though the loss of melanocytes leading to vitiligo can be due to many factors, including genetics and the environment, is the autoimmunity etiology is the most highly studied. Harris and Boniface et al. discuss the likelihood that melanocytes carrying a specific somatic mutation are targeted by the immune system. This innate immunity leads to the activation of T cell immune responses that causes the loss of melanocytes [16]. Though similar in mechanism, but because of the ICI therapy rather than innate immunity, the ICIs are thought to induce a tumor response against antigens that are present on both benign and malignant melanocytes resulting in vitiligo [10, 13]. This theory, however, bears further exploration in light of the variety of non-melanoma cancers similarly associated with ICI-induced vitiligo. The heterogeneity and small size of our cohort does not allow for this analysis in the non-melanoma population of this study unfortunately.
Time to next treatment (TTNT) is defined as the time between start date of vitiligo-inducing ICI and the start date of next systemic treatment. This metric represents the clinical benefit of therapy by accounting for both tumor response as well as toxicity profile in disease or treatments which are highly symptomatic. In our cohort, TTNT was similar in melanoma and non-melanoma patients, with a median TTNT of 797 and 983 days respectively. Although the non-melanoma group is a heterogeneous cohort, this raises the question of whether ICI-induced vitiligo could be associated with increased clinical benefit in non-melanoma patients and would be worth further prospective analysis.
In comparing our cohort of melanoma patients with vitiligo to that of previously studied consecutively treated standard of care melanoma patients from our institution, we noticed a trend toward higher median TTNT in the vitiligo patients (Table 3). Because of the disparity in sample sizes, meaningful time-dependent analysis was not possible with our data. Of note, in our sub-group of melanoma patients treated with combination ICI therapy, the median TTNT was almost three times longer than in previous studies with 623 and 210 days respectively (Table 4).
Survival data comparisons is tricky as our cohort is heterogeneous in terms of severity of disease at presentation and intent of therapy (adjuvant or definitive). Our cohort’s 1-, 2-, and 5-year overall survival rates were found to be higher than previously reported clinical trials among all treatment arms for melanoma patients (Table 3) [17,18,19,20,21]. Overall, 5-year survival has been previously reported at 34% with pembrolizumab, 52% with ipilimumab-plus-nivolumab, and 44% with nivolumab therapies in clinical trials. OS within our cohort was at 87%, 56.8% and 79.7% for the same respective categories of therapy [18, 21].
Although direct comparison between real-world and clinical trials patients cannot be definitively made, the increased OS rates and TTNT in our study may represent associated clinical benefit in the setting of ICI-induced vitiligo. Further study into the mechanism may aid in our understanding of the effect of ICI-associated vitiligo on TTNT and overall survival outcomes in melanoma patients. At this time, we are only discussing an association, which may have confounders such as patients having good enough response and low enough toxicity profile to remain on ICI therapy long enough to develop vitiligo. Mechanistic causation has yet to be established.
One limitation we noted in our chart review is that reporting of vitiligo was often inconsistent with skipped notes in documentation and non-specific descriptions of the disease. In combination with the single cohort nature of this study, this inconsistency complicates the calculation of incidence numbers as well as time to onset due to potential delays in reporting. With time to onset being dependent on patient or physician observation of vitiligo, a lower number of cases may also have been reported. The distribution of the patients’ disease may also affect the likelihood of diagnosis or documentation. This is supported in our cohort by vitiligo distribution, 95 (62.9%) patients had face/neck involvement and 104 (68.9%) had upper extremities involvement, areas that are easily noticed by the patient and clinicians. In contrast, only 53 (35.1%) had trunk and 51 (33.8%) had lower extremities involvement. Incidence was not calculated in this study due to the above recognized limitations.
Another limitation is that of the 151 patients reviewed, 39 had ICI treatments prior to the ICI they were on when vitiligo was noted in their electronic medical record. This represents a confounding variable in the treatment that definitively caused the vitiligo. Since time to onset can be delayed, it is possible that the prior ICI is the treatment that caused the vitiligo rather than the ICI the patient was currently being treated with when the vitiligo started. Though the exact vitiligo-inducing ICI may be unknown in these patients, it remains reasonable to assume that they had ICI induced vitiligo and should remain as part of the cohort.
In conclusion, ICI-induced vitiligo can be seen with many different cancer types and is not just limited to melanoma patients. Although vitiligo likely has a low incidence, there are multiple reasons for delayed and under reporting. As the cancer types treated with ICIs expands, it is important for patient care teams to recognize the quality-of-life impact on an increasingly diverse patient population and to treat their skin disease accordingly.
Data availability
De-identified data is available upon request.
References
Geisler AN, Phillips GS, Barrios DM et al (2020) Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol 83(5):1255–1268. https://doi.org/10.1016/j.jaad.2020.03.132
Failla CM, Carbone ML, Fortes C, Pagnanelli G, D’Atri S (2019) Melanoma and vitiligo in: good company. Int J Mol Sci 20(22):5731. https://doi.org/10.3390/ijms20225731
Lolli C, Medri M, Ricci M et al (2018) Vitiligo-like lesions in a patient treated with nivolumab for renal cell carcinoma. Medicine (Baltimore) 97(52):e13810. https://doi.org/10.1097/MD.0000000000013810
Liu RC, Consuegra G, Chou S, Fernandez Peñas P (2019) Vitiligo-like depigmentation in oncology patients treated with immunotherapies for nonmelanoma metastatic cancers. Clin Exp Dermatol 44(6):643–646. https://doi.org/10.1111/ced.13867
Uenami T, Hosono Y, Ishijima M et al (2017) Vitiligo in a patient with lung adenocarcinoma treated with nivolumab: a case report. Lung Cancer 109:42–44. https://doi.org/10.1016/j.lungcan.2017.04.019
Bulat V, Likic R, Bradic L, Speeckaert R, Azdajic MD (2021) Pembrolizumab-induced vitiligo in a patient with lung adenocarcinoma: a case report. Br J Clin Pharmacol 87(6):2614–2618. https://doi.org/10.1111/bcp.14663
Kosche C, Mohindra N, Choi JN (2018) Vitiligo in a patient undergoing nivolumab treatment for non-small cell lung cancer. JAAD Case Rep 4(10):1042–1044. https://doi.org/10.1016/j.jdcr.2018.08.009
Rodríguez-Lomba E, Molina-López I, Suárez-Fernández R, Baniandrés-Rodríguez O (2018) Vitiligo-like lesions and immune checkpoint inhibition therapy: is it truly an adverse event exclusive to patients with melanoma? Clin Exp Dermatol 43(5):598–599. https://doi.org/10.1111/ced.13382
Hua C, Boussemart L, Mateus C et al (2016) Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol 152(1):45. https://doi.org/10.1001/jamadermatol.2015.2707
Teulings H-E, Limpens J, Jansen SN et al (2015) Vitiligo-like depigmentation in patients with stage iii–iv melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. JCO 33(7):773–781. https://doi.org/10.1200/JCO.2014.57.4756
Freeman-keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS (2016) Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res 22(4):886–894
Villadolid J, Amin A (2015) Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res 4(5):560–575
Quaglino P, Marenco F, Osella-Abate S et al (2010) Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: results from a single-institution hospital-based observational cohort study. Ann Oncol 21(2):409–414. https://doi.org/10.1093/annonc/mdp325
Karri PV, Tahseen D, Patel AB (2020) Treatment of checkpoint inhibitor-induced vitiligo in a patient with metastatic renal cell cancer. Dermatitis. https://doi.org/10.1097/DER.0000000000000670
Grimes PE, Miller MM (2018) Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol 4(1):32–37. https://doi.org/10.1016/j.ijwd.2017.11.005
Seneschal J, Harris JE, Le Poole IC, Passeron T, Speeckaert R, Boniface K (2021) Editorial: immunology of vitiligo. Front Immunol 12:711080
Hodi FS, Chesney J, Pavlick AC et al (2016) Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 17:1558–1568. https://doi.org/10.1016/S1470-2045(16)30366-7
Larkin J, Chiarion-Sileni V, Gonzalez R et al (2019) Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 381(16):1535–1546. https://doi.org/10.1056/NEJMoa1910836
Topalian SL, Sznol M, McDermott DF et al (2014) Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 32(10):1020–1030. https://doi.org/10.1200/JCO.2013.53.0105
Robert C, Schachter J, Long GV et al (2015) Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372(26):2521–2532. https://doi.org/10.1056/NEJMoa1503093
Hamid O, Robert C, Daud A et al (2019) Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol 30(4):582–588. https://doi.org/10.1093/annonc/mdz011
Funding
The authors declare that no funds, grants, or other supports were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
AP conceived the project. JL conducted the chart review, wrote initial drafts, and continued to edit and revise the manuscript until submission. HH and MK assisted in the chart review, manuscript writing and editing process. AP directly supervised the first three authors and worked closely with them on this manuscript in multiple aspects, including verification of chart review data and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was conducted under protocol PA15-0959, approved by our institutional IRB.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lo, J., Hanania, H.L., Keiser, M.F. et al. Immune checkpoint inhibitor-induced vitiligo in cancer patients: characterization and management. Arch Dermatol Res 315, 1697–1703 (2023). https://doi.org/10.1007/s00403-023-02577-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-023-02577-7