Abstract
During the past decades, many researchers have tried to encapsulate medicines in biopolymer nanogels as injectable medicines. In the present study, dual-responsive bovine serum albumin (BSA)-loaded nanogels prepared from sodium alginate grafted poly (N-vinyl caprolactam) (PNVCL) have been reported. First, PNVCL-g-sodium alginate (PNVCL-g-Alg) was synthesized through free radical polymerization, and then nanogels were obtained from ionic crosslinking of sodium alginate in the presence of BSA. FTIR spectra showed that PNVCL-g-Alg nanogels were successfully prepared. Turbidimetry and rheometry analyses demonstrated the cloud point temperature near the human body. Particle size was evaluated using FE-SEM and dynamic light scattering and it was found that the size of particles in dry and swollen state are about 30 and 280 nm, respectively. The effect of temperature and pH on BSA release was evaluated. By comparing the drug release behavior, we found that the release of the protein at the temperature above the cloud point is faster than that at the temperature below the cloud point. The pH sensitivity of BSA-loaded PNVCL-g-Alg was evaluated at pH 5.5 and 7.4 and showed that the drug release was faster at acidic pH than at neutral pH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bio-polymers or bio-molecules, which constitute covalent bonding of biological units, have been widely studied in the past 2 decades. The prefix “bio” means that the digestible materials are produced by living organisms (Ottenbrite and Kim 2019; Ariga et al. 2011). Loading drugs and proteins in nanogels has many advantages, for example improved bioavailability, pharmacokinetic, enhanced cellular uptake, controlled drug release and decreased side effect. Encapsulated medicines contain small molecules or a complex of organic macromolecules (Hosseinkhani et al. 2013; Mottaghitalab et al. 2015). Recent advances in drug therapy research are focused on the identification of new molecular targets and active agents. The clinical use of new forms of controlled doses and local release of drugs can improve the treatment of the disease. Unfortunately, drug failure occurs in most cases due to the undesirable pharmacological and pharmacological dynamics of drug molecules. Nanotechnology has attracted much attention since the 1980s in many engineering fields such as electronics, mechanics, biomedical engineering and space engineering. In recent years, researchers have been interested in using nanoparticles as biological agents for the delivery of therapeutic molecules such as drugs and genes, as well as for tissue engineering (Deshmukh et al. 2013). In the sophisticated and evolving world of medicine, issues related to drug development, including delivery of specific drugs to the site and acceptable dosage of treatment, have always been of great importance. With the advancement of these new drug delivery systems (DDS), new methods have been used to facilitate medicine use in the body and improve pharmacokinetic profiles and bioavailability, as well as the efficacy and safety of treatment. The concept of overuse of targeted drugs, first developed by Paul Harlich in the early twentieth century, has been developed over the past decades to achieve this goal. Targeted drug delivery involves improving the properties of the system relative to drug targets associated with the body. Targeted drug delivery systems (TDDS) include injecting the DDS into the patient, directing the DDS to the target site (pathologic), releasing the active substances into or around the target, and avoiding non-specific toxicity in normal cells (Devarajan and Jain 2015). Smart polymers or stimuli-responsive polymers are types of materials that are widely applied for drug delivery applications. Smart materials can respond to a variety of stimuli such as temperature, pH, electrical field or magnetic field, light intensity, and biological stimuli such as enzymes, which create macroscopic responses to change the environment. The conjugation of different smart polymers provides many ways to optimize or improve different properties of stimuli-responsive materials (Das et al. 2020; Le et al. 2019). Among different types of environmentally sensitive polymers, thermoresponsive polymers have been studied more than other smart polymers for biomedicine applications. Increasing attention to temperature-sensitive polymers is due to their behavior in aqueous environments that can exhibit phase transition behavior with increasing temperature (Mahinroosta et al. 2018; Kim and Matsunaga 2017). According to the phase transition behavior, temperature-responsive polymers are classified into two main types: LCST and UCST behaviors. Many polymers dissolve with increasing temperature, but these polymers produce two phases with increasing temperature and unique properties. The LCST behavior depends on the hydrogen bond between the water molecules and the structure of the monomer units in the polymer chains. USCT-type polymers show a single phase with increasing temperature (Vancoillie et al. 2014; Bordat et al. 2019). Many thermoresponsive polymers exhibit LCST behavior in aqueous solutions, and accordingly, many smart materials based on LCST polymers are designed and developed. LCST and UCST polymers are based on a hydrophilic/hydrophobic balance. Poly (N-vinylcaprolactam) is a thermoresponsive polymer that shows LCST behavior around 32 °C (Gandhi et al. 2015). Copolymerization of thermoresponsive polymers with hydrophobic or hydrophilic polymers causes changes in the LCST balance of thermoresponsive polymers (Safari et al. 2021). Polysaccharides are hydrophilic polymers that are mostly used for drug delivery systems. In the present work, we balanced the phase transition temperature of thermoresponsive nanogels by grafting PNVCL on sodium alginate, and the LCST of the nanogels was optimized around human body temperature. These nanogels are designed to deliver the target protein to tissues. For this purpose, serum bovine albumin was loaded as protein into nanogels. To analyze the thermoresponsive behavior, two different temperatures above and below the LCST were chosen to evaluate the protein release behavior. The pH release analysis was performed under two different pH values at 7.4 and 5.5. This study aims to improve the protein release behavior for tissues and tumors successfully. Dual-responsive nanogels provide many opportunities for loading a variety of drugs and therapeutic agents.
Materials and methods
Materials
N-Vinylcaprolactam (PNVCL 98%), sodium alginate high molecular weight, calcium chloride, (2,2,6,6-tetramethylpiperidin-1-yl) oxyl or (2,2,6,6-tetramethylpiperidin-1-yl) oxidanyl (TEMPO), and serum bovine albumin were supplied from Sigma Aldrich. NaCl, KCl, and n-hexane were purchased from Merck Chemical Company.
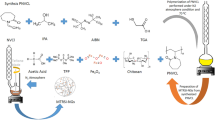
Synthesis PNVCL-g-Alg
PNVCL-G-Alg was prepared by free radical polymerization method in aqueous medium. First, a mixture of NVCL, sodium alginate and deionized water was incorporated to a three-neck sealed flask. To avoid oxidized reaction, oxygen was removed by vacuum pump, and then the initiator was incorporated to the reaction. The reaction was carried out under nitrogen gas purge at 75 °C for 5 h. At the final steps of polymerization, the synthesized process was inhibited by adding TEMPO. The obtained solution was purified by incorporating n-hexane as anti-solvent and PTFE filter paper under vacuum condition.
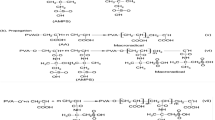
Preparation of poly (N-vinyl caprolactam) grafted sodium alginate polymer nanogels
Particle size is a crucial parameter in nanogels’ formation, which depends on the mixing speed during the crosslinking process, which is able to control the particle size and distribution. PNVCL-g-Alg was mixed for 1 h and then 0.002 g of bovine albumin was added to the solution. Then, the calcium chloride solution in deionized water (10% w/w) was added as a crosslinking agent to the reaction mixture and was stirred at 1000 rpm. The particles formed from the reaction mixture were washed for several times with deionized water until the residual poly (n-vinylcaprolactam) was not reacted and the calcium chloride was separated. The separated particles were completely dried under vacuum at 35 °C.
Characterization
FTIR spectral studies
Fourier transform infrared (FTIR) analysis was performed to analyze the graft copolymer and the formation of PNVCL-g-Alg according to the potassium bromide (KBr) disk technique, which was conducted on a Bruker-IFS-48 FTIR spectrometer (Ettlingen, Germany) in the range of 400–5000 cm−1.
Rheological analysis
Rheological analysis of 10% w/w dual-responsive nanogels suspension/buffer phosphate solutions (pH 7.4) was performed by an Anton Paar rheometer with a 50 mm diameter and angle 1° in cone and plate geometry. The suspension was analyzed under temperature sweep mode over the range of 5–65 °C and a heating rate of 1 °C/min.
UV–Visible spectroscopy
A UV–Visible spectrophotometer was utilized to determine the phase transition temperature of nanogels as parameters of LCST. Measurements were performed by a Shimadzu UV–Visible spectrophotometer equipped with a temperature controller. The average of three evaluated temperatures was considered as the LCST of thermoresponsive nanogels.
Dynamic light scattering
Dynamic light scattering is a physical method used to assess the distribution of particles in emulsion and suspensions and submicron particles. It is a non-destructive and rapid method for determining particle sizes in the nanometer to micrometer range. The evaluation was carried out with SZ-100 Horbia Join Joyovin at 25 °C and 655 nm.
BSA loading efficacy
Before evaluating the drug release behavior from the PNVCL-g-Alg nanogel reservoir, the encapsulation efficiency was investigated. Encapsulated drug nanogels containing a certain amount of BSA and PNVCL-g-Alg nanogels (0.2 mg/mL) are able to form PNVCL-g-Alg nanogels. Protein loading efficiency (LE) and loading capacity (LC) of PNVCL-g-Alg nanogels were evaluated by determining free bovine serum albumin after encapsulation. To evaluate the residual amount of free BSA, PNVCL-g-Alg nanogels were centrifuged at 4000 rpm for 5 min and the floating surface fluid was analyzed by UV-spectrophotometry analytical tools. The following equation was used to calculate LE and LC (Cheng et al. 2015):
BSA release study
Thermoresponsive release study was carried out in a pH 7.4 phosphate buffer at 25 ± 1 and 40 ± 1 °C. Encapsulated PNVCL-g-Alg nanogels (0.2 mg/mL) were incorporated inside the dialysis bags, and then the dialysis bags were placed in 250 mL phosphate buffer solution, while the magnet stirring speed was 60 rpm and the temperature was 25 and 40 °C.
To analyze the pH release behavior of PNVCL-g-Alg nanogels, the samples were placed in PBS at different pHs (7.4 and 5.5) and a constant temperature of 37 °C. In both methods of analysis, 3 mL of the solution was drawn at each determined time every 24 h, and the fresh medium equivalent to the drawn solution was replaced. Protein release was investigated using UV–Visible spectroscopy and was shown as a function of time in the graph.
Results and discussion
FTIR spectral analysis
The FTIR spectra of PNVCL-g-Alg, crosslinked nanogels and sodium alginate nanogels are shown in Fig. 1, which confirms the grafting reaction and the formation of nanogels. By comparing the FTIR spectra of crosslinked alginate and PNVCL-g-Alg, new peaks appearing at 2925, 1196 and 925 cm−1 can be observed, which are, respectively, related to the vibration of C-H, C-O and = CH2, which were previously observed in the spectra of PNVCL samples. In preparation of PNVCL-g-Alg, new peaks are observed in the FTIR spectrum, which are related to the vibration of the OH and CH2 groups and the asymmetric bond of the C = O group to the nitrogen atom, which appeared at 1372 and 1721 cm−1, respectively, due to the reaction of PNVCL amine groups and hydroxyl groups. Due to mentioned reason, hydroxyl peak of sodium alginate shifted from 3525 cm−1 to 3441 cm−1 (Kozanoǧlu et al. 2011; Mohammad Gholiha et al. 2021b). The hydrogel formation was confirmed by the peaks appearing at 1670 and 1430 cm−1, which correspond to -COO- bonded to Ca2+, indicating the formation of three-dimensional nanogels.
Particle size analysis
To analyze the particle size in dry and swollen state, FESEM and dynamic light scattering were used. Figure 2 displays the FESEM images of pure sodium alginate nanogels and PNVCL-g-Alg nanogels. FESEM images were analyzed after drying the nanogels. The images display the particle size of pure sodium alginate nanogels that is in the range of 15–20 nm, while the particle size of PNVCL-g-Alg is slightly higher than that of pure nanogels, which can be attributed to the presence of PNVCL chains in grafted sodium alginate nanogels that caused a decrease in the degree of ionization compared to non-grafted nanogels (Zhang et al. 2018). On the other hand, the FE-SEM images exhibit the spherical shape of the nanogels, which demonstrates that the nanogels did not undergo significant deformation even after dehydration. Maintaining the spherical shape of nanoparticles is very important in drug delivery systems, because it can be very important in the process of excretion from the internal organs of the body after drug release and simplify the excretion process. FE-SEM analyses of both samples shows that the particle size distribution in the dry state was almost narrow (Mohammad Gholiha et al. 2021a, b).
Dynamic light scattering (DLS) is usually utilized to evaluate particle size in swollen states. By comparing the particle size in the FESEM and DLS images, it is seen that there are significant changes in particle size that indicates the ability of PNVCL-g-Alg nanoparticles to load drug in high amounts. On the other hand, due to the increase in the diameter of nanoparticles some defects may occur, such as reducing the ability of nanoparticles to pass through the arteries and affecting pharmacokinetics, leading to defects in drug delivery of drug to the internal organs (Danaei et al. 2018). As an approximation in drug delivery systems, nanogels about 250 nm in size are suitable for drug delivery systems, but larger nanoparticles can be easily identified by macrophages. The removal of particles with a size higher than 200 nm is done with the help of the mononuclear phagocytic system as mediator cells of the liver, spleen and bone marrow (Zein et al. 2020). In general, nanoparticles that have an average diameter of 50–250 nm are appropriate for drug delivery to various tissues of the body and tumors. Therefore, the obtained nanogels can be used as intelligent carriers in drug delivery systems (Sahu et al. 2011; Rizvi and Saleh 2018). The challenge between particle size and efficacy is one of the crucial issues in drug delivery systems.
Investigation of phase transition behavior
Rheological behavior of temperature-responsive nanogels
The temperature-responsive behavior was evaluated by UV–Visible spectroscopy equipped with a heating system. UV–Visible spectroscopy determined cloud points, which indicate phase transition behavior. Poly (N-vinyl caprolactam) exhibits phase transition behavior at around 32 °C, while PNVCL-g-Alg shows it at around 40 °C. Thermoresponsive polymers depend on hydrophilic/hydrophobic balance in the chain structure. Most of macromolecules show better solubility by increasing temperature, but LCST types of thermoresponsive polymers display low solubility at higher cloud point temperature that leads to phase separation and chain collapse. The cloud point temperature is a highly important matter for drug delivery, because it can release the drug at a designated point close to human body temperature. The mentioned polymers are completely soluble in water at temperatures below LCST, but at temperatures above LCST, particles settle due to the increased density of polymer particles (Ng et al. 2019; Gumfekar et al. 2019). This behavior can be explained by the breaking of hydrogen bonds between the particles and the aqueous medium, which leads to phase transition (Umapathi and Venkatesu 2018). Sodium alginate is a hydrophobic polymer that causes changes in the balance of hydrophilic and hydrophobic structure of PNVCL. By grafting PNVCL on sodium alginate chain, a new cloud point was achieved that showed about 40 °C, which is suitable for drug delivery system (Mohammad Gholiha et al. 2021a, b). The phase transition behavior in the temperature-responsive samples was investigated independently of the effect of pH on the phase transition behavior. The behavior of LCST in polymers is a reversible process. The reversibility of this process greatly helps to control drug release in vivo environment, because these nanoparticles can initiate or prevent drug release by heating or cooling (Khan et al., 2022) (Fig. 3).
Rheological analysis in temperature sweep mode was utilized to confirm the results of UV–Visible spectroscopy. During the analysis, the shear was constant while the temperature was continuously increased. Investigation of rheological behavior is one of the ways to determine temperature response behavior in these polymers, which carry out in a wide range of temperatures. Rheological behavior can help explain LCST processes in nanogels by their performance in response to the amount of applied shear at different temperatures (Feng et al. 2020). Figure 4 shows the results of rheological analysis. The elastic modulus shows the amount of energy stored and recovered in each cycle of behavior change. Therefore, the modulus of elasticity was low at temperatures below LCST, but increased upon heating up to the LCST temperature. The rheology analysis in the temperature sweep mode demonstrated the phase transition temperature at about 40 °C, which indicates a temperature close to human body temperature and appropriate for smart drug delivery system. Injectable nanogels can provide conditions to turn the drug release system on and off, resulting in a tunable drug release system that depends on human fever. In most diseases, as the disease worsens, the body temperature increases, and the drug can be released from the smart nanogel system according to the fever, and when the fever decreases, the drug release process slows down, because it is a reversible process.
The increase in temperature leads to the change of chain conformation from random coil to globular, which is due to the disappearance of hydrogen interactions between PNVCL chains and water molecules (Wu et al. 2013). Thermoresponsive suspension shows its phase transition temperature by the collapse of nanogels in aqueous media. The vinyl group in the PNVCL chain plays an important role in the temperature response, because increasing the temperature above the LCST causes the hydrogen bond to break, which leads to the precipitation of nanoparticles. This change in conformity or particle precipitation, which is accompanied by an increase in the volume of these nanoparticles, leads to an increase in the modulus, which can be considered equivalent to the phase transition temperature or cloud point. Responsive nanogels, on the other hand, behave like solids at temperatures below LCST, where the dissipation modulus is larger than the storage modulus (G′ ˃ G´´). This behavior changes with increasing temperature of the solution or suspension, and the storage modulus approaches the loss modulus and becomes approximately equal. Rheological analysis illustrates that PNVCL-g-Alg displays a lower modulus compared to pure sodium alginate. The molecular mass of sodium alginate chains is much higher than the molecular mass of PNVCL chains, and therefore, poly (vinyl caprolactam) chains act as emollients and reduce the modulus compared to pure sodium alginate nanogels.
Drug release
To investigate the effect of cloud point on the protein release behavior of thermoresponsive nanogels, the drug release test was performed at 25 and 40 °C and pH 7.4. In the drug release process, various physical and chemical factors, such as release conditions (temperature and pH), nanoparticle preparation conditions, polymer chemical structure, type and molecular mass of drug, concentration, and drug release environment can be effective.
As shown in Fig. 5, the drug release behavior at 40 ºC was much faster than that at 25 °C, which demonstrates the importance of phase transition temperature. The drug release behavior at 40 °C indicates that more than 50% of the PNVCL-g-Alg sample is released from the nanoparticles in the first 3 days, while the drug release rate at 25 °C is much slower than the drug release at 40 °C, and pure alginate nanogels display almost the same behavior in drug release behavior at 25 and 40 °C. Total amount of BSA loaded to nanogels was 200 PPM, at 40 °C, it was about 85% equal to 170 PPM, while at 25 °C, it was about 41.2% equal to 82 PPM.
At the beginning of the process, the drug is released more rapidly, which is due to the release of drugs that are close to the surface of the nanoparticles, and then the release rate slows down. Drug release occurs faster at temperatures above the LCST than at temperatures below the LCST, because polymers above the LCST are hydrophobic in nature, which results in the loss of interactions with hydrophilic drugs. Removal of these interactions leads to the release of hydrophilic drugs, while at temperatures below the cloud point, PNVCL is a hydrophilic polymer and can retain the drug inside the nanogels (Mohammed et al. 2018).
Figure 6 displays the protein release behavior at pH 7.4 and 5.5. The results illustrate that pH strongly affects the release rate of bovine serum albumin. According to the obtained data, the amount of BSA released was more than 55% of the BSA released in 3 days at pH 5.5, whereas the amount noted for pH 7.4 was dramatically lower than the acidic pH. The obtained results indicate the importance of the effect of pH on the PNVCL-g-Alg dual-responsive nanogels, which shrink at pH 7.4, which causes protein retention inside the nanogels. The higher protein release ratio at pH 5.5 is related to the swelling properties of the nanogels. For the release behavior at pH 5.5, the release was about 62% equal to 124 PPM and at pH 7.4 about 40%, which was equal to 80 PPM. In addition, it was observed that the nanogels first swell and then lose their spherical shape (Sarıyer et al. 2020). The protein release behavior of dual-responsive nanogels represents that the release behavior depends on pH and temperature. The effect of the mentioned chain extension directly affected the protein release. When acidic pH or temperature is higher than LCST, the protein chains in the nanogels are released faster, while the release rate was slower at neutral pH or lower temperature than LCST. Consequently, this made the smart drug delivery system tunable for BSA release and usable as injectable nanogels for internal organs drug delivery system (Schemes 1, 2).
Conclusion
The temperature-responsive drug delivery systems based on poly (vinyl caprolactam) have been studied. First, PNVCL was grafted on sodium alginate and then crosslinked by calcium chloride to obtain the nanogels. The grafting reaction was confirmed by FTIR spectroscopy, and the phase transition temperature was tuned near the human body. For this purpose, LCST behavior was evaluated by UV–Visible spectroscopy with cloud point determination as a function of phase transition temperature and data confirmed by rheometry. The particle sizes of PNVCL-g-Alg and Alg nanogels were estimated to be about 17 and 21 nm in the dry state and 250 nm in the swollen state, respectively, which indicate their suitability for drug delivery applications. The drug release behavior showed that more than 50% of the drug was released from the nanogels at 40 °C for 3 days. The present study suggests that high-potential drug delivery system based on temperature-responsive LCST-type nanogels are suitable for protein delivery. The modification of PNVCL cloud point and the formation of thermoresponsive nanogels are the main achievements of the present work. These results indicate that PNVCL-g-Alg nanogels are suitable for injectable protein delivery systems.
Data availability
No datasets have been used.
References
Ariga K, Lvov YM, Kawakami K, Ji Q, Hill JP (2011) Layer-by-layer self-assembled shells for drug delivery. Adv Drug Deli Rev 63:762–771
Bordat A, Boissenot T, Nicolas J, Tsapis N (2019) Thermoresponsive polymer nanocarriers for biomedical applications. Adv Drug Deliv Revi 138:167–192
Cheng L, Bulmer C, Margaritis A (2015) Characterization of novel composite alginate chitosan–carrageenan nanoparticles for encapsulation of BSA as a model drug delivery system. Curr Drug Deliv 12(3):351–357
Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, Mozafari MR (2018) Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharma 10:57
Das SS, Bharadwaj P, Bilal M, Barani M, Rahdar A, Taboada P, Kyzas GZ (2020) Stimuli-responsive polymeric nanocarriers for drug delivery, imaging, and theragnosis. Polym 12:1397
Deshmukh PK, Ramani KP, Singh SS, Tekade AR, Chatap VK, Patil GB, Bari SB (2013) Stimuli-sensitive layer-by-layer (LbL) self-assembly systems: targeting and biosensory applications. J Cont Rel 166:294–306
Devarajan PV, Jain S (2015) Targeted drug delivery: concepts and design. Springer, Cham, p 286
Feng J, Dou J, Zhang Y, Wu Z, Yin D, Wu W (2020) Thermosensitive hydrogel for encapsulation and controlled release of biocontrol agents to prevent peanut aflatoxin contamination. Polym 12:547
Gandhi A, Paul A, Sen SO, Sen KK (2015) Studies on thermoresponsive polymers: phase behaviour, drug delivery and biomedical applications. Asian J Pharm Sci 10:99–107
Gumfekar SP, Vajihinejad V, Soares JB (2019) Advanced polymer flocculants for solid-liquid separation in oil sands tailings. Macromol Rapid Commun 40:1800644
Hosseinkhani H, Hong PD, Yu DS (2013) Self-assembled proteins and peptides for regenerative medicine. Chem Rev 113:4837–4861
Khan F, Atif M, Haseen M, Kamal S, Khan MS, Shahid S, Nami SA (2022) Synthesis, classification and properties of hydrogels: their applications in drug delivery and agriculture. J Mat Chem B 10(2):170–203
Kim YJ, Matsunaga YT (2017) Thermo-responsive polymers and their application as smart biomaterials. J Mat Chem B 5(23):4307–4321
Kozanoǧlu S, Özdemir T, Usanmaz A (2011) Polymerization of N-vinylcaprolactam and characterization of poly (N-vinylcaprolactam). J Macro Sci Part A 48(6):467–477
Le NTT, Nguyen TNQ, Cao VD, Hoang DT, Ngo VC, Hoang Thi TT (2019) Recent progress and advances of multi-stimuli-responsive dendrimers in drug delivery for cancer treatment. Pharm 11(11):591
Mahinroosta M, Farsangi ZJ, Allahverdi A, Shakoori Z (2018) Hydrogels as intelligent materials: a brief review of synthesis, properties and applications. Mat Tod Chem 8:42–55
Mohammad Gholiha H, Ehsani M, Saeidi A, Ghadami A, Alizadeh N (2021a) Magnetic dual-responsive semi-IPN nanogels based on chitosan/PNVCL and study on BSA release behavior. Prog Biomat 10(3):173–183
Mohammad Gholiha H, Ghadami A, Monajjemi M, Ehsani M (2021b) Enhanced physical and mechanical properties of flake–shape/vinyl-ester nanocomposites through surface modification of graphene and glass flake: a comparison with simulated data. Biointerface Res Appl Chem 11(4):11316–11337
Mohammed MN, Yusoh KB, Shariffuddin JHBH (2018) Poly (N-vinyl caprolactam) thermoresponsive polymer in novel drug delivery systems: a review. Mater Express 8(1):21–34
Mottaghitalab F, Farokhi M, Shokrgozar MA, Atyabi F, Hosseinkhani H (2015) Silk fibroin nanoparticle as a novel drug delivery system. J Control Release 206:161–176
Ng J, Osborn I, Harbottle D, Liu Q, Masliyah JH, Xu Z (2019) Stimuli-responsive hybrid polymer for enhanced solid-liquid separation of industrial effluents. Environ Sci Tech 53(11):6436–6443
Ottenbrite RM, Kim SW (eds) (2019) Polym drug and drug deliv syst. CRC Press, Boca Raton
Rizvi SA, Saleh AM (2018) Applications of nanoparticle systems in drug delivery technology. Saudi Pharm J 26(1):64–70
Safari S, Ehsani M, Zandi M (2021) Stimuli-responsive electrospun nanofibers based on PNVCL-PVAc copolymer in biomedical applications. Prog Biomat 10(4):245–258
Sahu SK, Maiti S, Maiti TK, Ghosh SK, Pramanik P (2011) Hydrophobically modified carboxymethyl chitosan nanoparticles targeted delivery of paclitaxel. J Drug Targ 19(2):104–113
Sarıyer S, Duranoğlu D, Doğan Ö, Küçük İ (2020) pH-responsive double network alginate/kappacarrageenan hydrogel beads for controlled protein release: effect of pH and crosslinking agent. J Drug Deliv Sci Technol 56:101551
Umapathi R, Venkatesu P (2018) Assessing the efficiency of imidazolium-based ionic liquids on the phase behavior of a synthetic biomedical thermoresponsive polymer. J Coll Interfa Sci 511:174–183
Vancoillie G, Frank D, Hoogenboom R (2014) Thermoresponsive poly (oligo ethylene glycol acrylates). Prog Polym Sci 39:1074–1095
Wu L, Zhou H, Sun HJ, Zhao Y, Yang X, Cheng SZ, Yang G (2013) Thermoresponsive bacterial cellulose whisker/poly (NIPAM-co-BMA) nanogel complexes: synthesis, characterization, and biological evaluation. Biomacro 14(4):1078–1084
Zein R, Sharrouf W, Selting K (2020) Physical properties of nanoparticles that result in improved cancer targeting. J Oncol 2020:5194780
Zhang R, Guo J, Liu Y, Chen S, Zhang S, Yu Y (2018) Effects of sodium salt types on the intermolecular interaction of sodium alginate/antarctic krill protein composite fibers. Carbohydr Polym 189:72–78
Acknowledgements
This research was supported by the Science and Research branch of Islamic Azad University and also Iran polymer and petrochemical institute.
Funding
The authors of this manuscript received no funding for the work reported in their manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohammad Gholiha, H., Ehsani, M., Saeidi, A. et al. Albumin-loaded thermo/pH dual-responsive nanogels based on sodium alginate and poly (N-vinyl caprolactam). Prog Biomater 12, 41–49 (2023). https://doi.org/10.1007/s40204-022-00211-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40204-022-00211-9