Abstract
Purpose
Gestational diabetes mellitus (GDM) is one of the most common medical complications in pregnancy. This systematic review aimed to evaluate the association between vitamin E and GDM.
Methods
Relevant articles from the Cochrane Library, PubMed, Scopus, Science Direct, Web of Science, and EMBASE databases up to December 2019 were searched. The inclusion criteria were observational full-text articles. The fixed and random effect models were used to analyze the pooled data using Review Manager 5.3.
Results
Thirteen studies, including 596 participants, of whom 285 were diagnosed with GDM were included in the meta-analysis. The vitamin E level was significantly lower in women with GDM (MD: - 0.10; 95% CI: [−0.15, − 0.05]). The level of vitamin E was not different between overweight women with GDM and healthy pregnant women (MD: 0.03; 95% CI: [−0.08, 0.013]). The level of vitamin E was significantly lower in the third trimester of pregnancy in GDM women in comparison to the healthy pregnant women(MD: -0.09; 95% CI: [−0.12, −0.06]).
Conclusion
This study showed that the level of vitamin E is significantly lower in GDM women compared to healthy pregnant women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gestational diabetes mellitus (GDM) is one of the most common medical complications in pregnancy [1]. Its prevalence is increasing globally and has been estimated at nearly 15–20% [1]. GDM is defined by hyperglycemia or glucose intolerance with the onset or first recognition at any stage of pregnancy [2]. The risk factors for GDM are high maternal age, maternal overweight or obesity, higher weight gain in pregnancy, history of macrosomia in previous pregnancies, family history of diabetes, and the impaired pancreatic B cell function during pregnancy [3].
The secretion of insulin increases during pregnancy physiologically, although the exact cause of this process is unknown, it can be due to the increased levels of diabetogenic hormones in the pregnancy such as cortisol, Human Placental Lactogen (HPL), and progesterone [4].
Gestational diabetes is associated with an increased risk of fetal and maternal complications. Fetal complications consist of macrosomia, shoulder dystocia, newborn asphyxia, neonatal respiratory distress syndrome, neonatal hypoglycemia, and macrosomia. Maternal complications include preeclampsia, a higher rate of cesarean section, and increased risk of developing type 2 diabetes in postpartum [5].
GDM is associated with the induction of oxidative stress and the reduction of antioxidant defense systems [6]. Vitamin E is one of the essential fat-soluble antioxidants that act as a part of an antioxidant defense system to trap free radicals and neutralize Reactive Oxygen Species (ROS), and preventing lipid peroxidation of the membrane [7]. This neutralization is essential, especially in situations of increased oxidative stress, such as GDM [8].It seems that glycosylation and oxidative stress are two critical factors in the occurrence of diabetic complications. Given the relatively high levels of glycine proteins in people with diabetes, it has been suggested that oxidative stress is involved in the formation of glycine proteins through increasing the production of ROS or reducing the levels of antioxidants in the body [9]. Therefore, oxidative stress has an important role in the development and progression of GDM complications [10].
A study by Resende et al. (2014) showed that GDM is not associated with changes in α-tocopherol concentration in colostrums [11]. Paolissoet al. (1995) showed that constant intake of pharmacological doses of vitamin E might be useful in insulin reduction [12]. The results of a study by Sivan et al. showed that supplementation with vitamin E confers a significant protective effect against diabetic embryopathy, and the researchers postulate that this protective effect is mediated by a reduction in the oxidative load induced by hyperglycemia [13].
There are controversial results about the levels of vitamin E in human and animal samples of diabetes and GDM. Plasma and tissue levels of vitamin E in gestational diabetes have been reported differently in various studies. Some studies found that the level of vitamin E remained unchanged in patients with GDM [14], while others reported its increase [15, 16] or decreased [17, 18]. To the best of our knowledge, no systematic review has been conducted so far to investigate the level of vitamin E in women with GDM. Given the fact that the results of various studies in this regard have been contradictory, the purpose of the present systematic review was to examine the relationship of gestational diabetes with vitamin E level.
Method
This systematic review and meta-analysis of observational studies were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement issued in 2009 [19] (Supplementary material 1). The protocol of this systematic review was registered in PROSPERO (Ref No: CRD 42019118826).
Search strategy
We searched studies describing the association between vitamin E and GDM in PubMed, Scopus, Science Direct, Cochrane Library, Web of Science, and EMBASE databases up to December 2019 (Supplementary Material 2).
Inclusion and exclusion criteria
Relevant studies were selected based on the following inclusion criteria: observational studies (case-control, cross-sectional or cohort), studies were published in any languages. Studies on type 1 or 2 diabetes or pre-gestational diabetes, animal studies, case report studies, and studies involving women who used the supplement of vitamin E were excluded from this review. In addition, studies with no clear statement about the diagnosis of GDM, or with no data or full texts on exposure and outcome were excluded.
Study participants
The study population including pregnant women, and case group consisting of women with GDM while the control group is pregnant women with normal glucose tolerance when the diagnosis of gestational diabetes is confirmed by an oral glucose tolerance test (OGTT).
Data extraction
Two authors performed data extraction and quality assessment independently (FS and SF). The Covidence software was used for screening and data extraction. Any conflict between authors was resolved by discussion. If the problem was persistent, the conflict was resolved by a third party (P.A.).
Extracted data included the author’s identifications, publication year, study location, study design, a sample size of the control and GDM groups, participant characteristics, gestational diabetes criteria, measurements of vitamin E and outcomes, and potential confounders.
Quality assessment
The quality of the included studies was assessed using the checklist of Downs and Black (1998) by two review authors (FS and SF). The following areas were covered using twenty-seven questions: ten questions for assessing reporting bias, three for assessing external validity, seven for evaluating internal validity, six for assessing selection bias, and one question for assessing the power of the study [20]. The total quality score was classified as follows:<14 = poor, 15–19 = fair and > 20 = good [21].
Statistical analysis
To obtain the differences in serum vitamin E between groups, we used the mean differences (M.D.) and 95% confidence intervals (95%CI). Forest plots were used to visually assess pooled estimates and corresponding 95% C.I.s. We performed a fixed-effect meta-analysis to combine the mean differences of each study. Statistical heterogeneity among studies was tested using the χ2 test, I2 statistics, and p values [22]. The I2 is the proportion of total variation contributed by between-study variation. In general, I2 values greater than 50% to 90%: may represent substantial heterogeneity [23]. In the presence of significant heterogeneity, a random-effects model was used to calculate the pooled effect size [24]. Sensitivity analyses were performed by removing individual studies to evaluate the role of moderating paper. We also conducted a subgroup analysis to compare groups regarding secondary outcomes. A funnel plot was added in an analysis where more than ten studies were included in the meta-analysis. All data were analyzed using Review Manager (RevMan 5.3). The significance level was set at p < 0.05.
Results
Selected articles
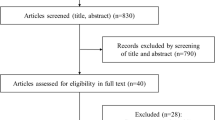
The flow chart of the selected studies is presented in the PRISMA chart (Fig. 1). We identified 387 articles relevant to our initial search, of which 124 were excluded after removing the duplicates, and 228 were excluded after screening titles or abstracts. A total of 35 eligible studies were selected, of which six studies were excluded due to wrong study design, five studies due to lack of information (e.g., conference paper), seven studies due to unclear exposure and outcome, two studies did not mention any guidelines for GDM diagnosis [11, 25] and four due to inaccessible data or full texts [26,27,28,29]. For these four articles, we sent several e-mails to the authors in order to obtain primary data but did not receive any replies, so finally, 11 studies were included in the meta-analysis.
Description of the studies
A summary of the included studies is presented in Table 1. These studies were published from 2000 to 2018, of which nine were conducted in Asia [6, 16, 18, 30,31,32,33,34,35] and two in Europe [36, 37]. The design of studies was as follows: seven studies were the case- control [16, 30,31,32,33,34,35], and the rest of the studies were cross-sectional [6, 18, 36, 37]. As far as timing of measurement was concerned, blood samples for measuring vitamin E were collected in the third trimester in eight studies [16, 30,31,32,33,34, 36, 37], and two studies at a delivery time [6, 35], and one study did not mention the timing of measurement [18].
This review included a total of 596 participants, including 285 of women diagnosed with GDM, and 311 healthy pregnant women. Seven different criteria were used for diagnosis of gestational diabetes: American Diabetes Association (ADA) [6, 30], American College of Obstetrics and Gynecology (ACOG) [16, 34],World Health Organization (WHO) [31, 36], National Diabetes Data Group (NDDG) [35], O’Sullivan and Mahan [18, 28], Carpenter and Coustan [37], and International Association of Diabetes and Pregnancy Study Groups/ American Diabetes Association (IADPSC/ ADA) [33].
Main analysis
The initial results indicated that the level of vitamin E was significantly lower in women with GDM (MD: −0.06; 95% CI: [−0.06, −0.05]) (Fig. 2). Because of the high level of heterogeneity (P < 0.00001, I2 = 94%), we considered sensitivity analysis to sequentially exclude each study with odd results from the pooled analysis to reduce the rate of heterogeneity. Using random effect model and omitting five studies [6, 16, 32,33,34], the differences between GDM patients and control group regarding the level of vitamin E remained significant (MD: - 0.10; 95% CI: [−0.15, − 0.05]) and the heterogeneity was reduced to 38%. These results indicate that the level of vitamin E in GDM women was significantly lower than that in healthy pregnant women. Funnel plot of studies is shown in Fig. 3. As evident from this figure, all included studies crossed the line of no effect, meaning that there is no significant difference between two study arms. Studies also have a very narrow confidence interval that passes the line of no effect. This means a lower standard error. Hence, the graph will not look like a funnel but rather a line suggesting that there is no publication bias.
Subgroup analyses
Subgroup analyses were conducted to explore the relationship between GDM and vitamin E based on BMI lower or higher than 25, different times of pregnancy and serum level of vitamin E.
Overweight subgroup analysis
The relationship between vitamin E and overweight in women with GDM and healthy pregnant women is depicted in Fig. 4 and is a result of a pooled analysis of three studies [16, 25, 32]. The level of vitamin E with the fixed-effect model in overweight women having GDM was significantly lower than that of overweight women without GDM (MD: -0.03; 95% CI: [−0.05, −0.01]) (Fig. 4). Because of the high level of heterogeneity (I2 = 94%, P < 0.00001), the random effect model was used, and the results showed that the level of vitamin E in overweight women with GDM was not significantly different from that of overweight women without GDM (MD: 0.03; 95% CI: [−0.08, 0.013]).
Subgroup analysis based on the timing of vitamin E measurement
The subgroup analysis based on the timing of vitamin E measurement is shown in Fig. 5 [6, 16, 30,31,32,33,34,35,36,37]. The random-effect model was chosen because of the high level of heterogeneity in the two subgroups (third trimester: MD: 0.03; 95% CI: [−0.03, 0.1], I2 = 96%); and at delivery time: MD: 0.28; 95% CI: [−0.65, 1.22], I2 = 100%). There was no significant difference in vitamin E levels between the GDM and non-GDM women in the third trimester and at delivery time. Sensitivity analysis was used to reduce the heterogeneity. By omitting five studies in the third trimester, the heterogeneity was reduced (MD: -0.09; 95% CI: [−0.12, −0.06], I2 = 32%). Therefore, women with GDM had significantly lower vitamin E in the third trimester in comparison to healthy pregnant women. However, there was no difference at delivery time between women with and without GDM (MD = 0.07; 95% CI: [−0.19, 0.33], I2 = 99%).
The quality assessment of the included studies is shown in Table 2. The median total quality score was 17 which has a fair quality according to the Kennelly et al. [21].
Discussion
The aim of this systematic review was to evaluate the relationship between maternal vitamin E and gestational diabetes mellitus. This meta-analysis of 11 observational studies on 285 women with GDM and 311 healthy pregnant women demonstrated that serum vitamin E level was significantly lower in women with GDM.
There is now considerable evidence that oxidative stress plays an important role in the glycation of hemoglobin [38]and beta-cell dysfunctional [39] in type 2 diabetes mellitus (T2DM). Vitamin E is a common antioxidant that suppresses ROS generation in the pancreas and maintains the structural integrity of pancreatic islets in experimental diabetes [40]. Moreover, vitamin E may play a role in the preservation of pancreatic beta-cell function and even could reverse the beta-cell apoptosis caused by oxidative stress [40, 41].
Some studies have reported that the serum level of vitamin E was significantly reduced in both diabetes type I and GDM. This reduction may be due to oxidative stress associated with hyperglycemia and insulin resistance and increased antioxidant activity against oxidative stress damage [18, 25, 29, 30, 32, 36, 37]. Also, a study of Biriet al., suggest the presence of oxidant stress in gestational diabetes, the reason probably being impaired antioxidant defense mechanism and increased free radical production through xanthine oxidase (X.O.) activation [42].
On the other hand, since lipid peroxidation may increase in women with GDM, an abnormal rise in lipid peroxides could increase the consumption of antioxidants like vitamin E and cause a reduction in vitamin E levels [36], thus confirming the results obtained in this research.
Our subgroup analyses of three studies showed that there was no significant difference between the level of vitamin E in overweight women with and without GDM. In our study, none of the studies examined the level of vitamin E in obese women with and without GDM. In obese women, the body’s antioxidant activity decreases, which increases the level of oxidative stress due to the high secretion of inflammatory mediators. Oxidative stress is the result of disrupting the balance between cellular destruction by free radicals and the body’s antioxidant defense. This finding does not apply to overweight women, which may be due to the lack of free radical activity in these women [43,44,45,46]. Further, our subgroup analysis of eight studies showed a significant decrease in serum vitamin E levels in the third trimester of GDM in comparison to non-GDM. Evidence revealed lower maternal levels of vitamin E in abnormal pregnancies [47]. Rosa et al., in their study, found that women with lower intake of vitamin E in the third trimester of pregnancy had newborns with poorer Apgar and a lower level of vitamin E in their milk [48].
Limitations of the study
This systematic review had several limitations. First, the diagnostic criteria for GDM and the methods of vitamin E measurement were different among the studies. Second, potential confounding factors in several studies could not be adjusted. Third, some of the studies did not provide enough clinical information, so we could not include them in the meta-analysis. And the last, the nature of observational studies in this systematic review does not allow us to draw a causal relationship between vitamin E and GDM.
Conclusion
This systematic review showed that the level of vitamin E is significantly lower in GDM women. There was no significant difference between the level of vitamin E in overweight women with and without GDM. Women with GDM had significantly lower vitamin E in the third trimester, but not at partum.
References
Association A.D. Standards of medical care in diabetes. Diabetes Care. 2014;37(Suppl. 1):S14–80.
Cho NH. IDF diabetes atlas. 7th ed. Brussels: International Diabetes Federation; 2015.
Buchanan TA, Xiang A, Kjos SL, Lee WP, Trigo E, Nader I, et al. Gestational diabetes: antepartum characteristics that predict postpartum glucose intolerance and type 2 diabetes in Latino women. Diabetes. 1998;4:1302–10. https://doi.org/10.2337/diab.47.8.1302.
Lind L, Hanni A, Lithell H, Hvarfner A, Sörensen OH, Ljunghall S. Vitamin D is related to blood pressure and other cardiovascular risk factors in middle-aged men. Am J Hypertens. 1995;8:94–901.
Metzger BE, International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups on the diagnosis and classification of recommendations hyperglycemia in pregnancy. Diabetes Care. 2010; 33(3):676–682. https://doi.org/10.2337/dc09-1848.
Suhail M, Patil S, Khan S, Siddiqui S. Antioxidant vitamins and Lipoperoxidation in non-pregnant, pregnant, and gestational diabetic women: erythrocytes osmotic fragility profiles. J Clin Med Res. 2010;2:266–73.
Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38.
Valdés-Ramos R, Ana Laura GL, Beatriz Elina MC, Alejandra Donají BA. Vitamins and Type 2 Diabetes Mellitus. Endocr Metab Immune Disord Drug Targets. 2015;15(1):54–63. https://doi.org/10.2174/1871530314666141111103217.
Thomas JE, Foody JM. The pathophisiology of cardiovascular disease in diabetes mellitus and the future of therapy. J Cardiometab Syndr. 2007;2:108–13.
Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–12.
Resende F, Clemente H, Bezerra D, Grilo E, Melo L, Bellot P, et al. Alpha-tocopherol concentration in serum and colostrum of mothers with gestational diabetes mellitus. Rev Paul Pediatr. 2014;32:178–86.
Paolisso G, Gambardella A, Giugliano D, Galzerano D, Amato L, Volpe C, et al. Chronic intake of pharmacological doses of vitamin E might be useful in the therapy of elderly patients with coronary heart disease. Am J Clin Nutr. 1995;61:848–52.
Sivan E, Reece E, Wu Y, Homko C, Polansky M, Borenstein M. Dietary vitamin E prophylaxis and diabetic embryopathy:Morphologic and biochemical analys. Am J Obstet Gynecol. 1996;175:793–9.
Martinoli L, Di Felic M, Seghieri G, Ciuti M, De Giorgio LA, Fazzini A, et al. Plasma retinol and alpha-tocopherol concentrations in insulin-dependent diabetes mellitus:their relationship to microvascular complications. Int J Vitam Nutr Res. 1993;63:87–92.
Asayama K, Nakane T, Ushida N, et al. Serum antioxidant status in streptocizin-induced diabetic rat. Horm Metab Res. 1994;26:313–5.
Santra D, Sawhney H, Aggarwal N, Majumdar S, Vasishta K. Lipid peroxidation and vitamin E status in gestational diabetes mellitus. J Obstet Gynaecol Res. 2003;29:300–4.
Cinar MG, Ulker S, Alper G, Evinc A. Effect of dietary vitamin E supplementation on vascular reactivity of thoracic aorta in streptozotocin diabetic rats. Pharmacology. 2001;62:56–64.
Kharb S. Lipid peroxidation in pregnancy with preeclampsia and diabetes. Gynecol Obstet Investig. 2001;50:113–6.
Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA group, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Downs SH, Black N. The feasibility of creating a checklist for the assessmentof the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–84.
Handler A, Kennelly J, Peacock N. Reducing Racial/Ethnic Disparities in Reproductive and Perinatal Outcomes: The Evidence from Population-Based Interventions, Springer Science+Business Media, LLC2011. Boston, MA: Springer. https://doi.org/10.1007/978-1-4419-1499-6.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;2:1539–58.
Higgins JP, Thompson SG, Eeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Vijayalaxmi KG, Urooj A. Biochemical profile and outcome in normal and high risk subjects. Indian J Clin Biochem. 2009;24:269–74.
Al Senaidy AM. Plasma α- and γ-tocopherol have different pattern during normal human pregnancy. Mol Cell Biochem. 1996;154:71–75.
Bayer R. Indications for vitamin E therapy in endangered pregnancies. Arch Gynakol. 1967;204:249–50.
Bodzek P, Wielkoszyński T. Evaluation of vitamin C and E concentration--some nonenzymatic indicators of antioxidant protective barrier in pregnant women. Wiad Lek. 2003;56:508–14.
Grissa O, Atègbo JM, Yessoufou A, Tabka Z, Miled A, Jerbi M. Antioxidant status andcirculating lipids are altered in human gestational diabetes and macrosomia. Transl Res. 2007;150:164–71.
Hekmat K, Bagheri HR, Abedi P, Tabesh H. The relationship of fat soluble antioxidants with gestational diabetes in Iran: a case–control study. J Matern Fetal Neonatal Med. 2014;27:1676–9.
Kharb S. Total radical-trapping antioxidant potential in gestational diabetes. Int J Gynaecol Obstet. 2008;103:257–8. https://doi.org/10.1016/j.ijgo.2008.06.017.
Surapaneni KM, Vishnu PV. Antioxidant enzymes and vitamins in gestational diabetes. J Clin Diagn Res. 2008;2:1081–5.
Shang M, Zhao J, Yang L, Lin L. Oxidative stress and antioxidant status in women with gestational diabetes mellitus diagnosed by IADPSG criteria. Diabetes Res Clin Pract. 2015;109:404–10.
Dey P, Gupta P, Acharya NK, Rao SN, Ray S, Chakrabarty S. Antioxidants and lipid peroxidation in gestational diabetes - A preliminary study. Indian J Physiol Pharmacol. 2008;52:149–56.
Sobki SH, Al-Senaidy AM, Al-Shammari TA, Inam SS, Al-Gwiser AA, Bukhari SA. Impact of gestational diabetes on lipid profiling and indices of oxidative stress in maternal and cord plasma. Saudi Med J. 2004;25:876–880.
Harsem NK, Braekke K, Torjussen T, Hanssen K, Staff A.C. Advanced Glycation end products in pregnancies complicated with diabetes mellitus or preeclampsia. Hypertens Pregnancy. 2008;27:374–86. https://doi.org/10.1080/10641950802000968.
Peuchant E, Brun JL, Rigalleau V, Dubourg L, Thomas MJ, Daniel JY, et al. Oxidative and antioxidative status in pregnant women with either gestational or type 1 diabetes. Clin Biochem. 2004;37:293–8.
Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–7.
Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004;279:42351–4.
Pazdro R, Burgess JR. The role of vitamin E and oxidative stress in diabetes complications. Mech Ageing Dev. 2010;131:276–86.
Jin L, Xue HY, Jin LJ, Li SY, Xu YP. Antioxidant and pancreas-protective effect of aucubin on rats with streptozotocin-induced diabetes. Eur J Pharmacol. 2008;582:162–7.
Biri A, Onan A, Devrim E, Babacan F, Kavutcu M, Durak I. Oxidant status in maternal and cord plasma and placental tissue in gestational diabetes. Placenta. 2006;27(2–3):327–32.
Amirkhizi F, Siyasi F, Hamedi S, Jalali M. Evaluation of oxidative stress and total antioxidant capacity in plasma in women with general and abdominal obesity. Med J Mashhad Univ Med Sci. 2012;55:170–7.
Furukawa S, Fujita T, Shimbukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–61.
Keaney JF, Larson MG, Vasan RS, Wilson PWF, Lipinska I, Corey D, et al. Obesity and systemic oxidative stress. Clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–439.
Melissas J, Malliaraki N, Papadakis JA, Taflampas P, Kampa M, Castanas E. Plasma antioxidant capacity in morbidly obese patients before and after weight loss. Obes Surg. 2006;16:314–20.
Mandach U, Huch R, Huch A. Maternal and cord serum vitamin E levels in normal and abnormal pregnancy. Int J Vitam Nutr Res. 1994;64:26–32.
Ortega RM, López-Sobaler AM, Andrés P, Martínez RM, Quintas ME, Requejo AM. Maternal vitamin E status during the third trimester of pregnancy in Spanish women: influence on breast milk vitamin E concentration. Nutr Res. 1999;19:25–36.
Acknowledgments
We would like to thank Dr. Mahmoud Maniati for English language editing.
Availability of data and material
N.A.
Author information
Authors and Affiliations
Contributions
F.S., PA, and S.J. were involved in conception. F.S., S.F., Z.M., M.Z. Involved in search, data extraction, and data analysis. F.S., PA, and S.J. were responsible for data interpretation, writing, and finalizing manuscript in English.
Corresponding author
Ethics declarations
Conflict of interest
There is no competing interest for authors.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharifipour, F., Abedi, P., Ciahkal, S.F. et al. Serum vitamin E level and gestational diabetes mellitus: a systematic review and meta-analysis. J Diabetes Metab Disord 19, 1787–1795 (2020). https://doi.org/10.1007/s40200-020-00582-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-020-00582-5