Abstract
Purpose
Several meta-analyses of observational studies revealed a modest increase in the risk of gestational diabetes (GDM) among pregnant women with low levels of serum vitamin D. However, no study examined a dose-response meta-analysis as well as a high versus low analysis in this regard.
Methods
We systematically searched PubMed, Embase, ISI Web of Science, and Scopus up to August 2019 to find prospective observational studies investigating the association of serum 25(OH)D with the risk of developing GDM. Using a random-effects model, the reported risk estimates were pooled.
Results
Nine cohort studies and six nested case-control studies were included in the final analysis (40,788 participants and 1848 cases). Considering linear analysis, each 10 nmol/L increase in circulating 25(OH)D was associated with a 2% lower risk of GDM (effect size (ES): 0.98; 95% CI: 0.98, 0.99; I2 = 85.0%, P < 0.001). highest compared with the lowest category of circulating 25(OH)D was associated with a 29% lower risk of GDM, with low evidence of heterogeneity (I2 = 45.0%, P = 0.079).
Conclusions
In conclusion, lower levels of serum 25(OH)D were associated with a higher chance of GDM. Differential results existed between the overall and subgroup analysis, either based on vitamin D detection methods or based on maternal age, although these subgroups partially lowered the heterogeneity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gestational diabetes (GDM) is a common outcome of pregnancy, defined as any grade glucose intolerance diagnosed during pregnancy, mostly after 24 weeks of gestation [1]. GDM is associated with an increased risk of short and long-term consequences for the health of the mother and the fetus [2]. In 2017, 21.3 million births (16.2%) were reported to be affected by hyperglycemia in pregnancy worldwide, of which 84.4% was diagnosed with GDM [3]. Vitamin D deficiency is widely prevalent among pregnant women [4]; such that higher prevalence was reported in South Asian, Middle Eastern, and African women [5]. Since the prevalence of vitamin D deficiency and insufficiency is growing worldwide, much attention has been directed toward how vitamin D may influence health outcomes in pregnancy [6].
Vitamin D is among the major risk factors for the incidence of GDM. The need for vitamin D is increased during pregnancy [7]. Evidence on the effect of vitamin D supplementation on the prevention or treatment of diabetes is controversial [8,9,10,11]. Moreover, recent reviews regarding supplemental vitamin D during pregnancy could not find robust evidence [12, 13]. During the last decade, several observational studies revealed a significant association between serum concentrations of vitamin D and the risk of GDM [14,15,16]. Although some individual studies could not find any significant association [17], several meta-analyses and reviews have found a modest increase in odds of GDM among women with low levels of serum vitamin D [18,19,20,21]. In the current study, we performed a dose-response meta-analysis and a high versus low analysis of relevant cohort and nested case-control studies that investigated the association of circulating 25(OH)D with incident GDM. Such an analysis utilizes all the population data of each study rather than just the extreme categories by which the classical meta-analysis has been done.
Methods
Search strategy
We searched four electronic databases including PubMed, Embase, ISI Web of Science, and Scopus up to August 2019. Eligible studies that examined the association of serum vitamin D and the risk of GDM were searched using keywords and MeSH terms as follows: (“vitamin D” OR “cholecalciferol” OR “25-hydroxyvitamin D” OR “25(OH)D” OR “1,25-(OH)2D2” OR “1,25-dihydroxyvitamin D2”) AND (“Diabetes, Gestational” OR “gestational diabetes” OR “GDM”). The bibliography of relevant original and review articles was referred to in order not to miss any other articles.
Study selection
This meta-analysis is conducted based on the Preferred Reporting Items for Systematic Review and Meta-Analyses statement [22]. No restriction was performed in terms of the publication language. We included those reports with information on circulating vitamin D during pregnancy in relation to the risk of GDM. Nonhuman studies, review articles, editorials, and letters were not included in the current study. To be more conclusive, different screening criteria used for GDM diagnosis were accepted. Studies that reported a prepregnancy diagnosis of diabetes for included women were excluded. Studies were excluded if they did not report the odds ratio (OR), relative risk (RR), confidence interval (CI), or standard error (SE), the number of women in each category of serum 25(OH)D or insufficient data to compute such values. If several publications used the same population, only the latest publication was included.
Data extraction
Two researchers independently extracted following information: first author, publication year, country, study design, characteristics of participants (age, number of case/cohort), average serum 25(OH)D levels in each patient category, RRs or ORs and 95% CIs for GDM for each category, diagnostic criteria for GDM, method of vitamin D assessment, and variables adjusted for in the analysis. If the study reported several adjustment models, only the more complete one was considered.
Quality assessment of studies
The quality of included studies was determined using the Newcastle–Ottawa Scale specific methods for cohort studies [23]. A maximum of nine points was assigned to each cohort study according to this method: four for selection and assessment of exposure, two for comparability, and three for assessment of outcomes. We defined the quality scores of >6 as high-quality studies; otherwise, it was deemed to be low-quality. Disagreements were resolved by consensus. Findings from the risk of bias assessment are presented in Table 1.
Statistical analysis
We used reported HRs or ORs and their 95% CIs for the risk of GDM to calculate log HR and log RR and its SEs. A linear dose-response meta-analysis per 10 nmol/L increment of serum levels of 25(OH)D was performed using generalized least squares trend estimation (GLST). These methods require the number of cases or person-year and the total number of subjects for at least three quantitative exposure categories. GLST also requires mean intake for each category of exposure levels. In cases that the range of serum vitamin D than mean levels was reported, the midpoint of the upper and lower limits in each category was chosen as the assigned dose. Then, we conducted a two-stage random-effects dose-response meta-analysis to determine the linear trend between serum 25(OH)D levels (dose) and GDM risk. First, we used a method suggested by Greenland and Longnecker [24] and Orsini et al. [25] to calculate the correlation within each study. Second, we combined study-specific estimates using a random-effects meta-analysis. Moreover, the effect sizes of the highest compared with the lowest categories were combined using the DerSimonian and Laird random-effects model [26]. To evaluate possible factors causing heterogeneity, we conducted subgroup analysis based on prespecified subgroups, including study design (cohort vs. nested case-control), study location (the US vs. non-US), vitamin D assessment method (immunoassay vs. non-immunoassay), the number of cases (<100 vs. >100), maternal age (>30 vs. <30 years), GDM diagnostic criteria, and adjustment for important risk factors including body mass index, gestational age, study site, family history of diabetes or previously diagnosed diabetes, smoking status, socioeconomic status, the season of sampling, and race/ethnicity. The heterogeneity was evaluated using I2 value and Cochran’s Q test (by metaan command in STATA) [27, 28]. P values for heterogeneity within studies in each subgroup (P-within) were obtained by comparing Q with a χ2 distribution with k-1 degree(s) of freedom, where k is the number of studies [29, 30]. To determine whether a statistically significant subgroup differences were detected, we considered the P values from the fixed-effect model (P-between) using the inverse variance-weighted estimation [31]. Sensitivity analysis was performed to explore the extent to which references might depend on a particular study or group of studies and the effect size was recalculated to estimate the statistical validity of the effect size [32]. Publication bias was assessed using visual inspection of funnel plots and the use of Egger’s regression asymmetry test. All the analyses were performed by the use of Stata, version 11.2 (Stata Crop). We considered P values of <0.05 as statistically significant. When serum 25(OH)D concentration was reported in different units, we converted them to the most frequently used unit.
Results
Findings from the systematic review
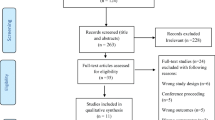
Out of 784 records identified through database searching, a total of 253 articles remained after removing duplicate publications. Following the first screening, 223 articles were removed based on their title and abstract and a total of 30 studies were reviewed through full-text assessment. We excluded ten more studies since they reported ORs or HRs for less than three categories of serum vitamin D [17, 33,34,35,36,37,38,39,40,41]. We also removed three review articles as well as two studies in which the number of cases or controls were not reported for each category of serum levels of vitamin D [42, 43]. Finally, nine cohort studies [14, 16, 44,45,46,47,48,49,50] and six nested case-control studies [51,52,53,54,55,56] were included in the final analysis (Fig. 1). Table 1 represents the characteristics of the included studies. A total of 40,788 pregnant women with an average age of 11 to 42 years participated in these studies, of which 1848 women developed GDM. Studies were published between 2008 and 2018, of them seven studies were from United States (US) [14, 16, 44, 47, 48, 51, 55], two studies were from Australia [49, 53], two studies were from Canada [52, 56], one study was from Korea [54], one study was from Norway [50], one study was performed in Singapore [46], and one study was conducted in Spain [45]. In 11 studies, serum 25(OH)D levels were measured using immunoassay methods including electrochemical luminescence immunoassay (ECLIA) [54, 56], immunodiagnostic systems (IDS-iSYS) [49], chemiluminescent immunoassay (CLIA) [14, 47, 52], enzyme-linked immunosorbent assay (ELISA) [44, 48], radioimmunoassay (RIA) [16, 50], and automated immunoassay system (AIAS) [53]. Whereas, other studies used chromatography methods including high-performance liquid chromatography (HPLC) [45] and liquid chromatography–mass spectrometry (LC–MS) [46, 51, 55]. Different criteria were used to diagnose GDM including American Diabetes Association (ADA) (n = 4), National Diabetes Data Group (NDDG) (n = 3), Carpenter–Coustan (C&C) (n = 2), World Health Organization (WHO) (n = 2), American College of Obstetricians and Gynecologists (ACOG) (n = 1), International Association of Diabetes and Pregnancy Study Groups (IADPSG) (n = 1), Australasian Diabetes in Pregnancy Society (ADPS) (n = 1), and Canadian Diabetes Association (CDA) (n = 1). One study evaluated only 25(OH)D3 [45] while other studies evaluated combined 25(OH)D3 and 25(OH)D2.
Except one study [48], studies had controlled the analyses for maternal age. Out of 15 studies, 12 studies had controlled the analyses for body mass index [14, 16, 44,45,46,47,48,49, 51, 54,55,56]. Seven studies adjusted the analyses for gestational age [16, 45, 47, 51, 52, 54, 56]. Five studies controlled the analyses for study site [45, 49, 50, 53, 56] and five other studies performed adjustments for family history of diabetes or previously diagnosed diabetes [16, 44, 46, 53, 55]. Only six studies performed adjustment for parity [14, 16, 45, 46, 50, 53] and smoking status [14, 45, 46, 48, 49, 53]. Most studies adjusted the analyses for socioeconomic status [5, 14, 16, 45, 46, 51, 53], season of sampling [14, 47, 48, 50, 51, 53,54,55,56], and race/ethnicity [14, 16, 44, 46,47,48,49,50, 55]. Other studies further adjusted the analysis for child’s sex (n = 2), maternal weight (n = 2), alcohol consumption (n = 2), previous GDM (n = 1), previously diagnosed hypertension (n = 2), vitamin D intake (n = 1), dietary intakes of fish and calcium (n = 2), physical activity (n = 2), and skinfolds (n = 1). According to New-Castle Ottawa Scale, all of the included studies were of high quality (Table 1).
Findings from the dose-response meta-analysis
Nine studies including three cohort studies and six nested case-control studies reported sufficient information for dose-response meta-analysis on serum levels of 25(OH)D and the risk of GDM. We observed an inverse linear association between serum levels of 25(OH)D and the risk of developing GDM. Each 10 nmol/L increase in circulating 25(OH)D was associated with a 2% lower risk of GDM (effect size (ES): 0.98; 95% CI: 0.98, 0.99), with an evidence of high heterogeneity (I2 = 85.0%, P < 0.001) (Fig. 2). To address the main sources of heterogeneity, we implemented sensitivity analysis as well as subgroup analyses according to the prespecified characteristics. Findings from the subgroup analysis revealed that study design, vitamin D assessment methods, GDM criteria, and the number of cases could explain the source of heterogeneity. Findings from subgroup analysis showed a significant linear association in both cohort and nested case-control studies, studies conducted either in US or non-US countries, studies with <100 or >100 cases, and those that adjusted their analyses for <4 or ≥4 important variables. In addition, subgroup analysis showed no significant association in studies that used non-immunoassay methods to evaluate serum 25(OH)D (ES: 0.99; 95% CI: 0.99, 1.00; I2 = 0.00), those that diagnosed GDM using NDDG criteria (ES: 0.99; 95% CI: 0.99, 1.00; I2 = 0.00), and studies that recruited women with <30 (ES: 0.95; 95% CI: 0.88, 1.02; I2 = 0.00) (Table 2). In the sensitivity test that omitted one study each time to obtain the pooled effect size from the random-effects model, no study could significantly change the association between serum 25(OH)D and GDM risk (data not shown). There was not any publication bias based on Egger’s test (P = 0.88) and the symmetry of the funnel plot (Fig. 4a).
High vs. low meta-analysis
Seven cohort prospective studies [14, 16, 44, 46,47,48, 50] and one nested case-control study [52] reported sufficient information for high vs. low analysis. Highest compared with the lowest category of circulating 25(OH)D was associated with a 29 % lower risk of GDM (ES: 0.71; 95% CI: 0.51, 0.99), with low evidence of heterogeneity (I2 = 45.0 %, P = 0.079) (Fig. 3). In the subgroup analysis, the association remained significant if the mean age of the mother was more than 30 years (ES: 0.71, 95% CI: 0.54, 0.94), immunoassay methods were used to evaluate circulating 25(OH)D (ES: 0.69, 95% CI: 0.53, 0.90), and the study was from US countries (ES: 0.62, 95% CI: 0.42, 0.93). Furthermore, there was no significant association in studies with adjustment for ≥4 principal variables (ES: 0.91, 95% CI: 0.71, 1.16), studies with more than 100 cases (ES: 0.95, 95% CI: 0.70, 1.27), those that used WHO criteria to diagnose GDM (ES: 0.95, 95% CI: 0.71, 1.27), and cohort studies (ES: 0.82, 95% CI: 0.65, 1.04) (Table 3). Findings from sensitivity analysis showed that excluding 4 studies [14, 44, 48, 52] resulted in a nonsignificant linear association. There was no evidence of publication bias based on Egger’s test (P = 0.20) and visual inspection of the funnel plot (Fig. 4b).
Discussion
Giving great importance for vitamin D and the risk of GDM, an extensive body of evidence has been published to find the association between serum levels of vitamin D and the risk of GDM. During the past decade, seven meta-analyses of observational studies were published [18,19,20,21, 57,58,59]. They adopted nearly the same inclusion and exclusion criteria and updated each other. They reported a significant inverse relationship between serum levels of 25(OH)D and the risk of GDM. However, the optimal 25(OH)D levels for GDM prevention remain unknown. In the current study, we observed a 2% lower risk of GDM per 10 nmol/L increment of circulating 25(OH)D, although findings had high heterogeneity. The association was not significant anymore in the subgroups of studies with non-immunoassay methods, NDDG diagnostic criteria, and studies that included mothers younger than 30 years. Moreover, the highest compared with the lowest category of circulating 25(OH)D was associated with a 29% lower risk of GDM, with low evidence of heterogeneity. The result was not replicated in some subgroups.
Our meta-analysis suggests that each 10 nmol/L increase in circulating 25(OH)D was associated with a 2% lower risk of GDM, and the risk of developing GDM decreases by 29% for the highest compared with the lowest category of 25(OH)D levels. Our results are in line with those of previous meta-analyses on prospective studies that indicated a significantly lower risk of GDM in relation to higher levels of 25(OH)D [18, 19, 57]. Earlier evidence suggested that vitamin D deficiency was associated with insulin resistance and type 2 diabetes given its role in supporting insulin secretion and pancreatic β-cell function [60]. Moreover, serum 25(OH)D has been inversely associated with poorer glycemic control including higher levels of fasting glucose and fasting insulin during pregnancy [41, 61]. Several mechanisms have been suggested for the relationship between vitamin D and GDM including the effect of vitamin D on the performance of pancreatic β cells [62], intracellular calcium regulation [63], and the effect on systemic inflammation together with insulin resistance in patients with diabetes mellitus [64].
In the subgroup analysis, we observed a significant association in studies conducted on pregnant women older than 30 years. This might be influenced by the expected risk of GDM and vitamin D deficiency among older pregnant women [65, 66]. We also found that different vitamin D measurement tools may affect the final results; such that significant associations were only observed in studies that used immunoassay methods. The selected studies used different methods for the determination of 25(OH)D, among which immunoassays were the most widely used technique [67, 68]. Studies have shown that assay variations can affect the results of vitamin D measurement [68, 69]. However, the low number of studies in the non-immunoassay subgroup limited statistical power, and therefore more work is required to find out if the association reported here persists when using other methods. Notably, in the high vs low analysis, the significant association disappeared after the exclusion of one case-control study. Thus, the true association may result from potential biases of case-control studies such as selection bias, recall bias, and inverse causal bias [70]. In this regard, several meta-analyses confirmed the current results, showing that heterogeneity among studies included in a meta-analysis can be explained by study design [18, 57, 66]. Moreover, nonsignificant result among non-US countries is probably due to the paucity of studies in this subgroup since only one study came from Asia, one study was from Canada, and one study was from Europe. In addition, the significant association disappeared among studies with adjustment for ≥4 major confounders. Since the pathophysiology of GDM is multifactorial [71], some confounding bias may play a role in evaluating the association of serum vitamin D and the risk of GDM. Thus, the possible effect of vitamin D on GDM cannot be regarded as the sole causative factor.
Although we found a significant association of circulating vitamin D and GDM, findings from randomized controlled trials (RCTs) showed weak or even negative effects of vitamin D on diabetes prevention or treatment [8]. Most of these studies have suffered from more than one study design limitation such as recruiting participants without vitamin D deficiency [9,10,11] and insufficient dosage of vitamin D supplementation [72, 73]. Nevertheless, recent trials with the robust design did not find a significant effect of vitamin D supplementation on the glycemic profile of prediabetic and diabetic patients [74, 75]. Similarly, data from RCTs regarding vitamin D supplementation on GDM have also been inconsistent. Recent reviews on vitamin D supplementation during pregnancy could not find robust evidence related to GDM [12, 13].
Some limitations should be considered for our meta-analysis. First, since the included studies adjusted their analyses for separate confounders, high heterogeneity was apparent in the dose-response meta-analysis. However, based on a previous study, the results of the meta-analysis would not differ substantially when adopting fully adjusted models than using models that control only the most common confounders [76]. Moreover, other sources of heterogeneity among the original studies were partially explained by our subgroup analysis and sensitivity analysis. Second, due to a lack of relative data, we could not find the impact of potential confounding factors including skin color, weight gain during pregnancy, dietary intake of vitamin D, and sunlight exposure. Another limitation is the point that the included studies had used different methods and standards to diagnose GDM. Although we performed subgroup analysis in this regard, the interpretation of the results is complicated given the little number of studies in each subgroup. The strength of our study is that the present work was the first dose-response meta-analysis that examined the association between serum 25(OH)D and the risk of GDM using large-scale, high-quality, prospective cohort studies. The included studies were all population-based research, and therefore the results could be extended to the general population. Besides, to find the source of heterogeneity, we stratified our meta-analysis based on study design, study location, vitamin D assessment method, GDM criteria, maternal age, and adjustment for confounders, suggesting a possible independent association between serum 25(OH)D and GDM risk. The lack of publication bias indicates that we were unlikely to miss studies that could have changed the results of our meta-analysis.
Conclusion
Combining these findings, it seems that lower levels of serum 25(OH)D were associated with a higher chance of GDM. These results should be interpreted with caution due to high heterogeneity among the included studies. Although it is biologically plausible that low 25-OHD levels could be responsible for the GDM, owing to the observational nature of the data reviewed and inconsistency in RCTs we cannot infer causality from these findings. There is a need for well-conducted and adequately powered studies, especially in non-western regions, with different ethnic origins.
References
H. Kleinwechter, U. Schafer-Graf, C. Buhrer, I. Hoesli, F. Kainer, A. Kautzky-Willer et al. Gestational diabetes mellitus (GDM) diagnosis, therapy and follow-up care: practice guideline of the german diabetes association(DDG) and the german association for gynaecologyand obstetrics (DGGG). Exp. Clin. Endocrinol. 122(7), 395–405 (2014)
J.S. Joergensen, R.F. Lamont, M.R. Torloni, Vitamin D and gestational diabetes: an update. Curr. Opin. Clin. Nutr. Metab. Care 17(4), 360–367 (2014)
International Diabetes Federation [Internet]. IDF Diabetes Atlas, 8th edition. Brussels, Belgium: International Diabetes Federation; http://www.diabetesatlas.org. Accessed 1 Aug 2019
C.L. Wagner, S.N. Taylor, A. Dawodu, D.D. Johnson, B.W. Hollis, Vitamin D and its role during pregnancy in attaining optimal health of mother and fetus. Nutrients. 4(3), 208–230 (2012)
A.R. Eggemoen, R.S. Falk, K.V. Knutsen, P. Lagerlov, L. Sletner, K.I. Birkeland et al. Vitamin D deficiency and supplementation in pregnancy in a multiethnic population-based cohort. BMC Pregnancy Childbirth 16, 7 (2016)
C. Palacios, L. Gonzalez, Is vitamin D deficiency a major global public health problem? J. Steroid Biochem. Mol. Biol. 144(Pt A), 138–145 (2014)
S. Shahgheibi, F. Farhadifar, B. Pouya, The effect of vitamin D supplementation on gestational diabetes in high-risk women: Results from a randomized placebo-controlled trial. J. Res. Med. 21, 2 (2016)
E. Maddaloni, I. Cavallari, N. Napoli, C. Conte, Vitamin D and diabetes mellitus. Front. Hormone Res. 50, 161–176 (2018)
A.G. Pittas, B. Dawson-Hughes, P. Sheehan, J.H. Ware, W.C. Knowler, V.R. Aroda et al. Vitamin D supplementation and prevention of Type 2 diabetes. N. Eng. J. Med. 381(6), 520–530 (2019)
G. El-Hajj Fuleihan, R. Baddoura, R.H. Habib, G. Halaby, A. Arabi, M. Rahme et al. Effect of vitamin D replacement on indexes of insulin resistance in overweight elderly individuals: a randomized controlled trial. Am. J. Clin. Nutr. 104(2), 315–323 (2016)
T.S. Moreira-Lucas, A.M. Duncan, R. Rabasa-Lhoret, R. Vieth, A.L. Gibbs, A. Badawi et al. Effect of vitamin D supplementation on oral glucose tolerance in individuals with low vitamin D status and increased risk for developing type 2 diabetes (EVIDENCE): A double-blind, randomized, placebo-controlled clinical trial. Diabetes Obes. Metab. 19(1), 133–141 (2017)
D.E. Roth, M. Leung, E. Mesfin, H. Qamar, J. Watterworth, E. Papp, Vitamin D supplementation during pregnancy: state of the evidence from a systematic review of randomised trials. BMJ (Clinical research ed). 359, j5237 (2017)
M.R.K. Rodrigues, S.A.M. Lima, G.M.Fd.S. Mazeto, I.M.P. Calderon, C.G. Magalhães, G.A.R. Ferraz et al. Efficacy of vitamin D supplementation in gestational diabetes mellitus: systematic review and meta-analysis of randomized trials. PLoS ONE. 14(3), e0213006-e (2019)
H.H. Burris, S.L. Rifas-Shiman, K. Kleinman, A.A. Litonjua, S.Y. Huh, J.W. Rich-Edwards et al. Vitamin D deficiency in pregnancy and gestational diabetes mellitus. Am. J. Obstet. Gynecol. 207(3), 182.e1–8 (2012)
V.T. Boyle, E.B. Thorstensen, D. Mourath, M.B. Jones, L.M.E. McCowan, L.C. Kenny et al. The relationship between vitamin D status and pregnancy outcomes. Reprod. Sci. 23(1), 277A (2016)
L.E. Tomedi, H.N. Simhan, L.M. Bodnar, Early-pregnancy maternal vitamin D status and maternal hyperglycaemia. Diabet. Med. 30(9), 1033–1039 (2013)
D.C. Whitelaw, A.J. Scally, D.J. Tuffnell, T.J. Davies, W.D. Fraser, R.S. Bhopal et al. Associations of circulating calcium and 25-hydroxyvitamin D with glucose metabolism in pregnancy: a cross-sectional study in European and South Asian women. J. Clin. Endocrinol Metab. 99(3), 938–946 (2014)
M.X. Zhang, G.T. Pan, J.F. Guo, B.Y. Li, L.Q. Qin, Z.L. Zhang, Vitamin D deficiency increases the risk of gestational diabetes mellitus: a meta-analysis of observational studies. Nutrients. 7(10), 8366–8375 (2015)
M. Lu, Y. Xu, L. Lv, M. Zhang, Association between vitamin D status and the risk of gestational diabetes mellitus: a meta-analysis. Arch. Gynecol. Obstet. 293(5), 959–966 (2016)
Y.H. Poel, P. Hummel, P. Lips, F. Stam, T. van der Ploeg, S. Simsek, Vitamin D and gestational diabetes: a systematic review and meta-analysis. Eur. J. Inter. Med. 23(5), 465–469 (2012)
S.Q. Wei, H.P. Qi, Z.C. Luo, W.D. Fraser, Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta-analysis. J. Maternal Fetal Neonatal Med. 26(9), 889–899 (2013)
D. Moher, A. Liberati, J. Tetzlaff, D.G. Altman, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6(7), e1000097 (2009)
G.A.S.B. Wells, D. O’Connell, J. Peterson, V. Welch, M. Losos, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute. http://www.ohri.ca/programs/clinical
S. Greenland, M.P. Longnecker, Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am. J. Epidemiol. 135(11), 1301–1309 (1992)
N. Orsini, R. Bellocco, S. Greenland, Generalized least squares for trend estimation of summarized dose–response. Data. 6(1), 40–57 (2006)
R. DerSimonian, N. Laird, Meta-analysis in clinical trials. Controlled Clin. Trials 7(3), 177–188 (1986)
E. Kontopantelis, D. Reeves. Metaan: random-effects meta-analysis. Stata J. 10(3), 395–407 (2010)
J.P. Ioannidis, N.A. Patsopoulos, E. Evangelou, Uncertainty in heterogeneity estimates in meta-analyses. BMJ 335(7626), 914–916 (2007)
W.G.J.B. Cochran. The combination of estimates from different experiments. Biometrics 10(1), 101–129 (1954)
J.P.T. Higgins, S.G. Thompson, J.J. Deeks, D.G. Altman, Measuring inconsistency in meta-analyses. BMJ 327(7414), 557–560 (2003)
N. Mikolajewicz, S.V. Komarova, Meta-analytic methodology for basic research: a practical guide. Front. Physiol. 10, 203 (2019)
B.H. Willis, R.D.J. Sim Riley, Measuring the statistical validity of summary meta-analysis and meta-regression results for use in clinical practice. Stat Med. 36(21), 3283–3301 (2017)
J. Zhou, L. Su, M. Liu, Y. Liu, X. Cao, Z. Wang et al. Associations between 25-hydroxyvitamin D levels and pregnancy outcomes: a prospective observational study in southern China. Eur. J. Clin. Nutr. 68(8), 925–930 (2014)
A. Bener, A.O. Al-Hamaq, N.M. Saleh, Association between vitamin D insufficiency and adverse pregnancy outcome: global comparisons. Int. J. Women’s Health 5, 523–531 (2013)
H.C. Cheng, C. de Costa, A. McLean, C. Woods, Vitamin D concentrations in pregnant women with diabetes attending for antenatal care in Far North Queensland. Australian N. Z. J. Obstet. Gynaecol. 54(3), 275–278 (2014)
H.J.W. Farrant, G.V. Krishnaveni, J.C. Hill, B.J. Boucher, D.J. Fisher, K. Noonan et al. Vitamin D insufficiency is common in Indian mothers but is not associated with gestational diabetes or variation in newborn size. Eur. J Clin. Nutr. 63(5), 646–652 (2009)
J.L. Josefson, A. Reisetter, D.M. Scholtens, H.E. Price, B.E. Metzger, C.B. Langman. Maternal BMI associations with maternal and cord blood Vitamin D levels in a north American subset of hyperglycemia and adverse pregnancy outcome (HAPO) study participants. PLoS ONE. 11(3), e0150221 (2016)
C.K. Kramer, B. Swaminathan, A.J. Hanley, P.W. Connelly, M. Sermer, B. Zinman et al. Vitamin D and parathyroid hormone status in pregnancy: effect on insulin sensitivity, beta-cell function, and gestational diabetes mellitus. J. Clin. Endocrinol. Metab. 99(12), 4506–4513 (2014)
M. Lacroix, M.C. Battista, M. Doyon, G. Houde, J. Menard, J.L. Ardilouze et al. Lower vitamin D levels at first trimester are associated with higher risk of developing gestational diabetes mellitus. Acta Diabetol. 51(4), 609–616 (2014)
N. Perez-Ferre, M.J. Torrejon, M. Fuentes, M.D. Fernandez, A. Ramos, E. Bordiu et al. Association of low serum 25-hydroxyvitamin D levels in pregnancy with glucose homeostasis and obstetric and newborn outcomes. Endocr. Pract. 18(5), 676–684 (2012)
R.J. Clifton-Bligh, P. McElduff, A. McElduff, Maternal vitamin D deficiency, ethnicity and gestational diabetes. Diabet. Med. 25(6), 678–684 (2008)
M. Davies-Tuck, C. Yim, M. Knight, R. Hodges, J.C. Doery, E. Wallace, Vitamin D testing in pregnancy: Does one size fit all? Australian N. Z. J. Obstet. Gynaecol. 55(2), 149–155 (2015)
V.T. Boyle, E.B. Thorstensen, D. Mourath, M.B. Jones, L.M.E. McCowan, L.C. Kenny et al. The relationship between 25-hydroxyvitamin D concentration in early pregnancy and pregnancy outcomes in a large, prospective cohort. Br. J. Nutr. 116(8), 1409–1415 (2016)
C. Zhang, C. Qiu, F.B. Hu, R.M. David, R.M. van Dam, A. Bralley, et al. Maternal plasma 25-hydroxyvitamin D concentrations and the risk for gestational diabetes mellitus. PLoS ONE 3(11), e3753 (2008)
A. Rodriguez, R. García-Esteban, M. Basterretxea, A. Lertxundi, C. Rodríguez-Bernal, C. Iñiguez et al. Associations of maternal circulating 25-hydroxyvitamin D3 concentration with pregnancy and birth outcomes. BJOG: An Int. J. Obstet. Gynaecol. 122(12), 1695–1704 (2015)
S.L. Loy, N. Lek, F. Yap, S.E. Soh, N. Padmapriya, K.H. Tan, et al. Association of maternal Vitamin D status with glucose tolerance and caesarean section in a multi-ethnic asian cohort: The growing up in singapore towards healthy outcomes study. PLoS ONE 10(11), e0142239 (2015)
C.J. Nobles, G. Markenson, L. Chasan-Taber, Early pregnancy Vitamin D status and risk for adverse maternal and infant outcomes in a bi-ethnic cohort: the behaviors affecting baby and you (B.A.B.Y.) study. Br. J. Nutr. 114(12), 2116–2128 (2015)
S.K. Flood-Nichols, D. Tinnemore, R.R. Huang, P. G. Napolitano, D.L. Ippolito. Vitamin D deficiency in early pregnancy. PLoS ONE. 10(4) (2015)
R.L. Wilson, A.J. Leviton, S.Y. Leemaqz, P.H. Anderson, J.A. Grieger, L.E. Grzeskowiak, et al. Vitamin D levels in an Australian and New Zealand cohort and the association with pregnancy outcome. BMC Pregnancy Childb. 18(1), 251 (2018)
A.R. Eggemoen, C.W. Waage, L. Sletner, H.L. Gulseth, K.I. Birkeland, A.K. Jenum, Vitamin D, gestational diabetes, and measures of glucose metabolism in a population-based multiethnic cohort. J. Diabetes Res. 2018, 8939235 (2018)
A.M. Baker, S. Haeri, C.A. Camargo Jr, A.M. Stuebe, K.A. Boggess, First-trimester maternal vitamin D status and risk for gestational diabetes (GDM) a nested case-control study. Diabetes Metab. Res. Rev. 28(2), 164–168 (2012)
L. Parlea, I.L. Bromberg, D.S. Feig, R. Vieth, E. Merman, L.L. Lipscombe, Association between serum 25-hydroxyvitamin D in early pregnancy and risk of gestational diabetes mellitus. Diabet. Med. 29(7), e25–e32 (2012)
F.J. Schneuer, C.L. Roberts, C. Guilbert, J.M. Simpson, C.S. Algert, A.Z. Khambalia et al. Effects of maternal serum 25-hydroxyvitamin D concentrations in the first trimester on subsequent pregnancy outcomes in an Australian population. Am. J Clin. Nutr. 99(2), 287–295 (2014)
S. Park, H.K. Yoon, H.M. Ryu, Y.J. Han, S.W. Lee, B.K. Park et al. Maternal vitamin D deficiency in early pregnancy is not associated with gestational diabetes mellitus development or pregnancy outcomes in Korean pregnant women in a prospective study. J. Nutr. Sci. Vitaminol. 60(4), 269–275 (2014)
D.L. Arnold, D.A. Enquobahrie, C. Qiu, J. Huang, N. Grote, A. VanderStoep et al. Early pregnancy maternal vitamin D concentrations and risk of gestational diabetes mellitus. Paediatr. Perinat. Epidemiol. 29(3), 200–210 (2015)
L. Dodds, C.G. Woolcott, H. Weiler, A. Spencer, J.C. Forest, B.A. Armson et al. Vitamin D status and gestational diabetes: effect of smoking status during pregnancy. Paediatr. Perinat. Epidemiol. 30(3), 229–237 (2016)
F. Aghajafari, T. Nagulesapillai, P.E. Ronksley, S.C. Tough, M. O’Beirne, D.M. Rabi, Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ. 346, f1169 (2013)
M. Amraei, S. Mohamadpour, K. Sayehmiri, S.F. Mousavi, E. Shirzadpour, A. Moayeri, Effects of vitamin D deficiency on incidence risk of gestational diabetes mellitus: a systematic review and meta-analysis. Front. Endocrinol. 9, 7 (2018)
Y. Zhang, Y. Gong, H. Xue, J. Xiong, G. Cheng, Vitamin D and gestational diabetes mellitus: a systematic review based on data free of Hawthorne effect. BJOG. 125(7), 784–793 (2018)
X. Palomer, J.M. Gonzalez-Clemente, F. Blanco-Vaca, D. Mauricio, Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes. Metab. 10(3), 185–197 (2008)
S.L. Lau, J.E. Gunton, N.P. Athayde, K. Byth, N.W. Cheung, Serum 25-hydroxyvitamin D and glycated haemoglobin levels in women with gestational diabetes mellitus. Med. J. Australia 194(7), 334–337 (2011)
A.W. Norman, J.B. Frankel, A.M. Heldt, G.M. Grodsky, Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 209(4458), 823–825 (1980)
B. Draznin, K.E. Sussman, R.H. Eckel, M. Kao, T. Yost, N.A. Sherman, Possible role of cytosolic free calcium concentrations in mediating insulin resistance of obesity and hyperinsulinemia. J. Clin. Investig. 82(6), 1848–1852 (1988)
J.A. Alvarez, A. Ashraf, Role of vitamin d in insulin secretion and insulin sensitivity for glucose homeostasis. Int. J. Endocrinol. 2010, 351385 (2010)
A. Prentice, G.R. Goldberg, I. Schoenmakers, Vitamin D across the lifecycle: physiology and biomarkers. Am. J. Clin. Nutr. 88(2), 500s–506s (2008)
M. Amraei, S. Mohamadpour, K. Sayehmiri, S.F. Mousavi, E. Shirzadpour, A. Moayeri, Effects of vitamin D deficiency on incidence risk of gestational diabetes mellitus: a systematic review and meta-analysis. Front. Endocrinol. 9, 7 (2018)
G.D. Carter, J.C. Jones, J.L. Berry, The anomalous behaviour of exogenous 25-hydroxyvitamin D in competitive binding assays. J. Steroid Biochem. Mol. Biol. 103(3–5), 480–482 (2007)
G.D. Carter, R. Carter, J. Jones, J. Berry, How accurate are assays for 25-hydroxyvitamin D? Data from the international vitamin D external quality assessment scheme. Clin. Chem. 50(11), 2195–2197 (2004)
M. Rabenberg, C. Scheidt-Nave, M.A. Busch, M. Thamm, N. Rieckmann, R.A. Durazo-Arvizu et al. Implications of standardization of serum 25-hydroxyvitamin D data for the evaluation of vitamin D status in Germany, including a temporal analysis. BMC Public Health 18(1), 845 (2018)
J.A. Kopec, J.M. Esdaile, Bias in case-control studies. a review. J. Epidemiol. Community Health 44(3), 179–186 (1990)
K. Hodson, S. Robson, R. Taylor, Gestational diabetes: emerging concepts in pathophysiology. Obstet. Med. 3(4), 128–132 (2010)
A. Javed, A. Vella, P.B. Balagopal, P.R. Fischer, A.L. Weaver, F. Piccinini et al. Cholecalciferol supplementation does not influence β-cell function and insulin action in obese adolescents: a prospective double-blind randomized trial. J. Nutr. 145(2), 284–290 (2015)
M.M. Oosterwerff, E.M. Eekhoff, N.M. Van Schoor, A.J. Boeke, P. Nanayakkara, R. Meijnen et al. Effect of moderate-dose vitamin D supplementation on insulin sensitivity in vitamin D-deficient non-Western immigrants in the Netherlands: a randomized placebo-controlled trial. Am. J. Clin. Nutr. 100(1), 152–160 (2014)
H.J. Wallace, L. Holmes, C.N. Ennis, C.R. Cardwell, J.V. Woodside, I.S. Young et al. Effect of vitamin D3 supplementation on insulin resistance and β-cell function in prediabetes: a double-blind, randomized, placebo-controlled trial. Am. J Clin. Nutr. 110(5), 1138–1147 (2019)
A. Mousa, N. Naderpoor, M.P. de Courten, H. Teede, N. Kellow, K. Walker et al. Vitamin D supplementation has no effect on insulin sensitivity or secretion in vitamin D-deficient, overweight or obese adults: a randomized placebo-controlled trial. Am. J. Clin. Nutr. 105(6), 1372–1381 (2017)
R.J. de Souza, A. Mente, A. Maroleanu, A.I. Cozma, V. Ha, T. Kishibe et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ. 351, h3978 (2015).
Acknowledgements
The study was approved (No. IR.AJUMS.REC.1398.798) and financially supported (grant number 98S75) by the School of Paramedicine, Ahvaz Jundishapur University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
The authors’ responsibilities were as follows—MS, SR, and SAH: conducted the research; MS, OS, and MA: wrote the paper; AZ: had primary responsibility for the final content.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sadeghian, M., Asadi, M., Rahmani, S. et al. Circulating vitamin D and the risk of gestational diabetes: a systematic review and dose-response meta-analysis. Endocrine 70, 36–47 (2020). https://doi.org/10.1007/s12020-020-02360-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02360-y