Abstract
Background
Metabolic syndrome (MetS) is one of the worldwide most chronic endocrine disorders. The disease can also lead to several other chronic disorders such as type 2 diabetes mellitus, cardiovascular diseases, renal diseases and cancers. This study aimed to evaluate the safety and efficacy of ginger powder on biochemical and anthropometric parameters in patients with MetS.
Methods
This randomized controlled clinical trial was conducted on 37 patients with MetS. We supplemented the individuals with MetS with either 2 g per day ginger powder or placebo for 12 weeks. Biochemical and anthropometric parameters before and after the intervention were compared between the two groups.
Results
After 12 weeks of treatment, the levels of triglyceride (TG), FBS, and insulin resistance were significantly improved in the patients receiving ginger in compare to the placebo group (P < 0·05). Moreover, significant changes were observed in terms of weight, waist circumference, LDL, HDL, total cholesterol, systolic and diastolic blood pressure and energy intake between the groups.
Conclusion
In general, our findings demonstrated that the ginger supplementation offers beneficial effects on patients with MetS, at least, in part due to the modulatory effects on TG, FBS, and insulin resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic syndrome (MetS) is a cluster of endocrine disturbances and is known as a main risk factor for several chronic disorders such as cardiovascular diseases, cancers and type 2 diabetes [1] which are among the major public health challenges. The salient features of MetS include insulin resistance, dyslipidemia, obesity and hypertension. Although many factors contribute to the pathogenesis of MetS, obesity and insulin resistance play a key role. MetS is a rapidly growing worldwide pandemic with an average prevalence of 31% [2] .
Common therapies such as treatment with synthetic drugs have shown to cause several undesirable side effects and limitations. In previous studies, researchers have shown the important role of lifestyle modification in the development of MetS; however, it is unknown whether a low intensive lifestyle program plus an herbal supplement might also be effective. Therefore, unraveling safe and traditional therapies, especially herbal medicine, has recently attracted more attention [3].
Herbs and spices have long been used for their both taste and health benefits in different nutritional patterns. There is unfortunately scarce scientific evidence for the effectiveness of these herbs; however, several empirical evidence have reported beneficial effects of using these herbs, and utilizing these plants is continuing even after the emergence of modern medicine [4].
Ginger (Zingiber officinale) is a nontoxic spice [5] used extensively in herbal medicine [6] and has recently attracted the attention of researchers due to its potential in reducing glucose and lipid levels, fat burning [6, 7] and prevention and treatment of chronic disorders [3, 8, 9].
The effect of oral ginger on MetS in humans has not been adequately studied. Therefore, in this study, we aimed to investigate the influence of ginger powder on the major components of MetS.
Methods
Subjects
From October 2016 to July 2017, 38 patients with MetS (who met MetS criteria according to the National Cholesterol Education Program Adult Treatment Panel III report [10]) aging between 18 and 70 years participated in a double-blinded, placebo-controlled parallel study. MetS was diagnosed as having three of the following five features: increased waist circumference (≥102 cm in men and ≥ 88 cm in women); elevated TAG levels (≥150 mg/dl (≥1.69 mmol)); reduced HDL-C levels (<40 mg/dl (<1.03 mmol/l) in men and < 50 mg/dl (<1.29 mmol/l) in women); elevated blood pressure (≥130.85 mmHg or on treatment for hypertension); elevated glucose levels (≥100 mg/dl (≥5.55 mmol/l)).
The eligibility criteria included age range from 18 to 70 years, body mass index of 18.5–30 kg/m2, confirmed diagnosis of MetS and no use of any dietary supplement or no adherence to special diets at least one month prior to baseline. The subjects were excluded from the study if they had renal, liver, heart, pituitary, thyroid or psychiatric disorders which could affect the ability of the participants to provide written informed consent; if they had cardiovascular diseases, cancers, and alimentary tract disorders affecting absorption; if they were currently pregnant and lactating and recently received insulin, anti-hypertensive drugs, multivitamin or nutrient supplements; and if they received weight loss drugs or supplements, received any changes in diet or daily exercise programs, had any allergic reaction to ginger, or received lipid-lowering medications, antibiotics, or sex hormone treatment.
The study was approved by the ethics committee of the Tehran University of Medical Sciences (the approval number: 27265). The objectives, procedures, risks and benefits of the study were explained to the participants, and then a written consent was received from each participant. This trial was registered at the clinical trials. Gov as NCT02535195.
Study design
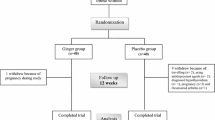
At the beginning of the study, 63 individuals were assessed for the inclusion criteria and finally 38 subjects were included in the study (the consort diagram, Fig. 1). The allocation was carried out by a random permuted block procedure and the subjects were allocated to the intervention (n = 19) and control (n = 19) groups (the allocation ratio was 1:1). Both the investigators and patients were blinded to treatment allocation. The test group received 2000 mg of ginger as four capsules daily, each containing 500 mg of ginger root (other ingredients: plant-derived capsule (modified cellulose), cellulose, silica) for 12 weeks. Concentrations of ginger constituents in a 100 mg product included: (10.51 ± 0.41 6-gingerol, 1.55 ± 0.20 8-gingerol, 2.55 ± 0.09 10-gingerol, 19.32 ± 1.25 6-shogaol, 2.53 ± 0.20 8-shogaol, 6.12 ± 0.15 10-shogaol, 6-paradol: not detected, 0.56 ± 0.13 1-dehydrogingerdione). The placebo group received four placebo capsules containing starch. Ginger capsules and placebos were produced by the Green Plants of Life Pharmaceutics Co., Iran. Supplements and placebo were given randomly by a person other than the researchers, so that both the subjects and investigators were blind to randomization categories. During each visit, each participant was given the ginger supplement or placebo for three weeks and throughout these weeks, consumption of the supplements or placebo by the subjects was ensured through phone calls or text messages.
In case of forgetting to consume one or two supplements, the subjects were recommended to consume the forgotten supplements in the following day according to the previous studies suggesting that up to a dose of 3 g/day was free of complications [11].
To ensure the acceptable use of the supplements, the participants were asked to return the unconsumed supplements. The participants who did not consume more than 10% of their capsules were excluded from the study.
Dietary intake, physical activity, anthropometric assessment, and blood pressure
At baseline, a three-day food record, physical activity questionnaires and general questionnaires were completed. All the participants were consulting for 20 min on the modification of lifestyle and followed a modified diet and physical activity plan, according to the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults from the National Institute of Health (NIH) and the North American Association for the study of obesity [12]. To increase the accuracy, all the food records were reviewed by a dietitian. Data from dietary intakes were analyzed by the N4 software, and national food composition tables were used to analyze food intake as a reference.
A classified physical activity questionnaire based on Metabolic Equivalent of Task (MET) [13] was used to measure physical activity. Height, weight, waist circumference and blood pressure were measured according to the standard protocols. Height and weight (Seca, Germany) were measured while the participants wore light clothing and no shoes with 0.1-kg accuracy for weight and 0.5-cm accuracy for height. Waist circumference was measured to the nearest 0.1 cm using a flexible tape at the mid-point between the lowest rib and iliac crest. Body mass index (BMI) was calculated by dividing the weight (kg) by height squared (m2).
The patients’ blood pressures were measured by a trained nurse before and after sitting for a 15 min period using a manual blood pressure cuff and the mean blood pressure included in analysis [14].
Blood collection and analyses
At both baseline and the end of study, 10 ml of blood sample was taken from each participant following 12 to 14 h fasting. The samples were then stored in a freezer (−80 °C) for further biochemical analysis.
Serum glucose, total cholesterol, triglyceride and HDL cholesterol concentrations were measured by the enzymatic colorimetric method (Pars Azmoon, Tehran, Iran). Moreover, LDL cholesterol concentration was measured using the Friedewald formula. Fasting insulin level was measured using the enzyme-linked immune-sorbent assay (Monobind Inc). Insulin resistance was quantified in the two groups by the HOMA-IR index (HOMA-IR = [fasting insulin (mU/L) × 3 fasting blood glucose (mg/dl)]/ 405 [15]. Moreover, insulin sensitivity was assessed by the quantitative insulin sensitivity check index (QUICKI) equation: QUICKI = 1/ (log insulin (mU/ml) + log glucose (mg/dl)) [16].
Statistical analyses
According to Shidfar et al.’s study [5], the sample size with a 95% confidence interval and 80% power of study was calculated according to the following formula. The sample size was 17 subjects in each group. However, considering a 10% probable withdrawing, 19 subjects were involved in each group.
- \( {Z}_{1-\frac{\alpha }{2}}=1.96 \) :
-
(α = 0.05), Z1 − β = 1.28 (β = 0.1)
- S 1 :
-
18 (standard deviation of decreased FBS level in the case group)
- S 2 :
-
16 (standard deviation of decreased FBS level in the control group)
- d:
-
19 (minimum difference between decreased FBS levels in the two groups that was considered important by the researcher)
To analyze the data, we used the SPSS 20.0 software. The continuous and categorical data were reported as means/standard deviations and frequency (%), respectively. In order to analyze the demographic variables, we used × 2 or t test. The normality was assessed by the Kolmogorov–Smirnov and Shapiro–Wilk tests. For within group comparisons, before and after the intervention, the student’s t test or Wilcoxon was used. The means and 95% CI for changes in outcome variables from baseline to the end of study were compared using ANCOVA models. To eliminate the effects of confounding factors, all the ANCOVA models were adjusted for BMI, WHR, MET and energy. Statistical significance was set at p < 0.05.
Results
Participants’ characteristics
From the 38 subjects included in this study, one participant in the placebo group did not complete the study (due to unwillingness to continue) and finally, 19 subjects in the intervention group and 18 subjects in the control group were included in the final analysis (Fig. 1). The baseline clinical and demographic characteristics of the participants are shown in Table 1. At baseline, height from anthropometric characteristics, and triglyceride and total cholesterol levels from clinical parameters, significantly differed between the two groups (p < 0.05). Other factors did not reveal significant differences between the two groups.
Findings recorded in the two groups of participants before and after treatment
At the end of the study, energy intake decreased and physical activity was shown to be elevated in the both groups; however, this difference was not statistically significant between two groups (P = 0.214 for energy intake and P = 0.132 for MET).
Table 2 shows the effects of ginger supplementation on MetS characteristics. Serum levels of FBS was improved in the intervention and placebo groups, but its reduction was also significantly greater in the ginger treated group compared to the placebo group (P = 0.001). Furthermore, this difference remained significant after adjusting the results for weight, MET, and energy intake (P < 0.001).
To evaluate the insulin resistance and insulin sensitivity, we used HOMA-IR and QUICKI formulas. Insulin resistance and insulin sensitivity were demonstrated to improve significantly in the intervention group compared to the placebo group before and after adjusting for confounding factors (P < 0.001).
Compared to the baseline, body weight, WC and body mass did not change significantly in the ginger group, as compared to the control group (P = 0.132, 0.456 and 0.481 respectively).
The mean reduction of the serum TG concentration indicated a significant difference showing 56.35 in the ginger group compared to 8.63 in the placebo treated individuals (P < 0.001). No significant difference was observed between the two groups regarding HDL (P = 0.31), LDL (P = 0.503), and total cholesterol (P = 0.39), following the adjustment for the confounding variables. Changes in systolic and diastolic blood pressure did not significantly alter between the groups in the end of study (P = 0.165 and P = 0.078, respectively). It should be mentioned that no severe side effects were reported due to the ginger or placebo supplementation by the patients.
Discussion
In the present study, 12 weeks of supplementation with ginger was shown to significantly reduce FBS, serum triglyceride and insulin resistance, even after adjusting for confounding factors. Serum total cholesterol was significantly reduced in the treatment group; however, this effect was not significant after adjusting for confounding variables. According to our findings, there was no correlation between supplementation with ginger and weight loss, WC, BP, HDL and LDL.
Insulin resistance is one of the key components of MetS and type 2 diabetes. In people with MetS, lower glucose consumption by peripheral tissues causes an increase in blood insulin to adjust blood glucose levels.
In previous animal and human studies, researchers evaluated the effects of ginger on blood glucose and insulin resistance. Shanmugam et al. have shown that treatment of diabetic rat with 200 mg/kg ginger extract caused a reduction in blood glucose [17]. A similar positive effect was shown in some human-based studies. Arablou et al. found in a clinical trial that consumption of 1600 mg ginger powder for 12 weeks in patients with diabetes remarkably reduces the serum glucose and insulin resistance [18]. Consistently, Mahluji et al. have shown ameliorating effects of ginger supplementation on hyperglycemia [19].
It appears that beneficial effects of ginger on insulin resistance are due to its effect on increasing glucose uptake by some adipocytes and muscle tissue cells. Moreover, it is assumed that ginger decreases blood glucose by antagonistic effects against serotonin receptors, inhibiting the activity of intestinal glucosidase and amylase, but increases energy metabolism in mitochondria [20, 21]. Several lines of studies have also shown that ginger components such as gingerols, shogaols and paradols can enhance the protein content of GLUT-4 and accelerate B cell functions that improve the insulin sensitivity [22].
In our study, supplementation with ginger reduced serum TG. However, total levels of cholesterol, LDL and HDL did not differ significantly between the two groups. Our data was in agreement with Arabloo et al.’s study reporting that taking 1.6 g of ginger for 12 weeks resulted in a significant reduction in serum triglyceride levels in patients with type 2 diabetes [18]. In another study, supplementation with 3 g of ginger for 45 days in patients with hyperlipidemia caused a reduction in TG and total cholesterol [11].
Further, in an animal study, supplementation with the ethanolic extract of Zingiber officinale led to a significant reduction in total cholesterol, LDL and TG in rats fed with a high-fat diet for 6 weeks; however, HDL cholesterol was not significantly altered [9]. Mahmoud et al. compared ginger versus orlistat effects on lipid profile in Albino rats and showed that both ginger and orlistat had the ability to reduce lipid profile; nevertheless, the ginger supplementation showed a higher efficacy [23]. The controversial results may be due to different dosages of ginger consumption, length of treatment and dosage of effective compounds in the ginger supplement.
Despite the differences in the results of various studies, they portray levels of agreements in terms of the modulatory effects of ginger supplement on serum triglyceride. One of the proposed reducing mechanisms of ginger on TG levels is attributed to the influential activity of ginger components on lipoprotein lipase and as a result on hydrolysis of TG in circulation. It has also been proposed that ginger controls the activity of the carbohydrate-responsive element-binding protein (ChREBP) in the liver. ChREBP is a regulator of lipid and glucose metabolism, which causes the conversion of excess glucose to triglyceride. Reducing the expression of ChREBP, Acetyl-CoA carboxylase 1 (ACC1), and Acetyl acid synthase and Stearoyl-CoA desaturase 1 (SCD1) are other suggestive effects of ginger, by which it reduces the levels of triglyceride [24, 25].
Our data did not reveal any significant association between ginger consumption and systolic and diastolic blood pressure. Few studies evaluated ginger effect on blood pressure. In this regard, a clinical trial conducted by Azimi et al. examined the effect of 3 g ginger powder on blood pressure in patients with diabetes. Their observations showed that ginger consumption significantly reduced systolic blood pressure, but not diastolic blood pressure [26].
Contradictory to some previous results, this study suggested that ginger supplementation fails to offer significant alteration in weight loss and waist circumference. Nonetheless, in consistent with our data, several other studies reported no significant association between ginger supplementation with weight and WC change [5, 18, 27]. Conversely, Ebrahimzadeh et al. reported supplementation with 2 g/day ginger to significantly decrease BMI in obese women [28]. Overall, our study presented several strength points including its design as a controlled clinical trial and precise control of the participants’ diet and physical activity. Our study had a limitation. The initial data of TG and TC levels were higher in the ginger group than in the control group (Table 1). This may be due to that ginger is more effective in reduction of TG and TC levels than those in the control group. However, we could not find any evidence proving different effect of ginger on various levels of TG and TC.
Conclusions
This study demonstrated that daily consumption of 2 g per day ginger supplement for 12 weeks by patients with MetS can improve insulin sensitivity, triglyceride and fasting blood glucose levels, suggesting its beneficial impact to be used as a herbal agent in patients with MetS.
References
Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004;89:2595–600.
Engin A. The definition and prevalence of obesity and metabolic syndrome. Adv Exp Med Biol. 2017;960:1–17.
Karimi N, Dabidi Roshan V, Fathi BZ. Individually and combined water-based exercise with ginger supplement, on systemic inflammation and metabolic syndrome indices, among the obese women with breast neoplasms. Iran J Cancer Prev. 2015;8:e3856.
Panickar KS. Beneficial effects of herbs, spices and medicinal plants on the metabolic syndrome, brain and cognitive function. Cent Nerv Syst Agents Med Chem. 2013;13:13–29.
Shidfar F, Rajab A, Rahideh T, Khandouzi N, Hosseini S, Shidfar S. The effect of ginger (Zingiber officinale) on glycemic markers in patients with type 2 diabetes. J Complement Integr Med. 2015;12:165–70.
Wei CK, Tsai YH, Korinek M, Hung PH, El-Shazly M, Cheng YB, et al. 6-Paradol and 6-Shogaol, the pungent compounds of ginger, promote glucose utilization in adipocytes and Myotubes, and 6-Paradol reduces blood glucose in high-fat diet-fed mice. Int J Mol Sci. 2017;18.
Suk S, Seo SG, Yu JG, Yang H, Jeong E, Jang YJ, et al. A bioactive constituent of ginger, 6-Shogaol, prevents Adipogenesis and stimulates lipolysis in 3T3-L1 adipocytes. J Food Biochem. 2016;40:84–90.
Li Y, Tran VH, Kota BP, Nammi S, Duke CC, Roufogalis BD. Preventative effect of Zingiber officinale on insulin resistance in a high-fat high-carbohydrate diet-fed rat model and its mechanism of action. Basic Clin. Pharmacol. Toxicol. 2014;115:209–15.
Nammi S, Sreemantula S, Roufogalis BD. Protective effects of ethanolic extract of Zingiber officinale rhizome on the development of metabolic syndrome in high-fat diet-fed rats. Basic Clin Pharmacol Toxicol. 2009;104:366–73.
Expert Panel on Detection E. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285:2486.
Alizadeh-Navaei R, Roozbeh F, Saravi M, Pouramir M, Jalali F, Moghadamnia AA. Investigation of the effect of ginger on the lipid levels. A double blind controlled clinical trial. Saudi Medical Journal. 2008;29:1280–4.
Initiative NOE, Heart N, Lung, Institute B, Obesity NAAftSo, Identification EPot, et al. The practical guide: identification, evaluation, and treatment of overweight and obesity in adults: The Institute; 2000.
Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504.
Hardy R, Kuh D, Langenberg C, Wadsworth ME. Birthweight, childhood social class, and change in adult blood pressure in the 1946 British birth cohort. Lancet (London, England). 2003;362:1178–83.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Hrebicek J, Janout V, Malincikova J, Horakova D, Cizek L. Detection of insulin resistance by simple quantitative insulin sensitivity check index QUICKI for epidemiological assessment and prevention. Journal of Clinical Endocrinology & Metabolism. 2002;87:144–7.
Shanmugam KR, Mallikarjuna K, Kesireddy N, Sathyavelu RK. Neuroprotective effect of ginger on anti-oxidant enzymes in streptozotocin-induced diabetic rats. Food Chem Toxicol. 2011;49:893–7.
Arablou T, Aryaeian N, Valizadeh M, Sharifi F, Hosseini A, Djalali M. The effect of ginger consumption on glycemic status, lipid profile and some inflammatory markers in patients with type 2 diabetes mellitus. Int J Food Sci Nutr. 2014;65:515–20.
Mahluji S, Attari VE, Mobasseri M, Payahoo L, Ostadrahimi A, Golzari SE. Effects of ginger (Zingiber officinale) on plasma glucose level, HbA1c and insulin sensitivity in type 2 diabetic patients. Int J Food Sci Nutr. 2013;64:682–6.
Al-Amin ZM, Thomson M, Al-Qattan KK, Peltonen-Shalaby R, Ali M. Anti-diabetic and hypolipidaemic properties of ginger (Zingiber officinale) in streptozotocin-induced diabetic rats. Br J Nutr. 2006;96:660–6.
Li Y, Tran VH, Duke CC, Roufogalis BD. Preventive and protective properties of Zingiber officinale (ginger) in diabetes mellitus, diabetic complications, and associated lipid and other metabolic disorders: a brief review. Evid Based Complement Alternat Med. 2012;2012:516870.
Henriksen EJ. Exercise training and the antioxidant alpha-lipoic acid in the treatment of insulin resistance and type 2 diabetes. Free Radic Biol Med. 2006;40:3–12.
Mahmoud RH, Elnour WA. Comparative evaluation of the efficacy of ginger and orlistat on obesity management, pancreatic lipase and liver peroxisomal catalase enzyme in male albino rats. Eur Rev Med Pharmacol Sci. 2013;17:75–83.
Brahma Naidu P, Uddandrao VV, Ravindar Naik R, Suresh P, Meriga B, Begum MS, et al. Ameliorative potential of gingerol: promising modulation of inflammatory factors and lipid marker enzymes expressions in HFD induced obesity in rats. Mol Cell Endocrinol. 2016;419:139–47.
Gao H, Guan T, Li C, Zuo G, Yamahara J, Wang J, et al. Treatment with ginger ameliorates fructose-induced fatty liver and hypertriglyceridemia in rats: modulation of the hepatic carbohydrate response element-binding protein-mediated pathway. Evid Based Complement Alternat Med. 2012;2012:570948.
Azimi P, Ghiasvand R, Feizi A, Hosseinzadeh J, Bahreynian M, Hariri M, et al. Effect of cinnamon, cardamom, saffron and ginger consumption on blood pressure and a marker of endothelial function in patients with type 2 diabetes mellitus: a randomized controlled clinical trial. Blood Press. 2016;25:133–40.
Mozaffari-Khosravi H, Talaei B, Jalali BA, Najarzadeh A, Mozayan MR. The effect of ginger powder supplementation on insulin resistance and glycemic indices in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Complement Ther Med. 2014;22:9–16.
Ebrahimzadeh Attari V, Ostadrahimi A, Asghari Jafarabadi M, Mehralizadeh S, Mahluji S. Changes of serum adipocytokines and body weight following Zingiber officinale supplementation in obese women: a RCT. Eur J Clin Nutr. 2016;55:2129–36.
Acknowledgements
This study was supported by a grant from the Tehran Medical University. We hereby appreciate the participation of the patients as well as the cooperation of the staff at the National Nutrition and Food Technology Research Institute of the Shahid Beheshti University. We also appreciate Mr. Mousa Vatanmakanian for the critical revision and language editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors disclose no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rahimlou, M., Yari, Z., Rayyani, E. et al. Effects of ginger supplementation on anthropometric, glycemic and metabolic parameters in subjects with metabolic syndrome: A randomized, double-blind, placebo-controlled study. J Diabetes Metab Disord 18, 119–125 (2019). https://doi.org/10.1007/s40200-019-00397-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-019-00397-z