Abstract
AlCoCrFeNi2.1-xNbC (x = 0, 2.5, 7.5, and 10 wt%, denoted as NbC0, NbC2.5, NbC7.5, and NbC10) high-entropy alloy (HEA) matrix composites were fabricated by mechanical alloying (MA) and spark plasma sintering (SPS) methods. The effect of NbC content on the microstructures, Vickers hardness, and wear properties of AlCoCrFeNi2.1 eutectic high entropy alloy (EHEA) was systematically investigated. The results indicate that the AlCoCrFeNi2.1 alloy consisted of FCC, B2, Al2O3, and Cr7C3 phases, while the AlCoCrFeNi2.1-xNbC (x > 0) alloys consisted of FCC, NbC, Al2O3, and Cr7C3 phases. The AlCoCrFeNi2.1-xNbC alloys exhibit excellent Vickers hardness with 679 HV, 664 HV, 677 HV, and 695 HV, respectively. In addition, with the addition of NbC, the average friction coefficient and wear rate of the AlCoCrFeNi2.1-xNbC alloys decrease from 0.59 to 0.42 and from 1.5 × 10–5 mm3 N−1 m−1 to 2.4 × 10–6 mm3 N−1 m−1, respectively. Wherein, NbC10 alloy shows the smallest average friction coefficient of 0.42 and lowest wear rate of 2.4 × 10–6 mm3 N−1 m−1, indicating that the NbC10 alloy displays the best wear resistance. And the wear mechanism of the NbC10 alloy was oxidation wear accompanied by slight adhesive wear.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, eutectic high entropy alloys (EHEAs) having the advantages of high entropy alloys (HEAs) and eutectic alloys have received extensive addition[1,2,3,4,5]. Wherein, the AlCoCrFeNi2.1 EHEA exhibited excellent mechanical properties emerging as a possible candidate material for engineering applications[1]. Recently, researchers used a series of methods to further improve the strength and plasticity of the AlCoCrFeNi2.1 EHEA [6,7,8,9,10,11,12,13]. For instance, Bhattacharjee et al. [6] prepared the AlCoCrFeNi2.1 EHEA with a nano-lamellar of L12 and B2 phases by cold-rolled and annealed, resulting in simultaneous strength and ductility improvements. Wang et al. [12] applied the friction stir processing to the AlCoCrFeNi2.1 EHEA, and the research results found the mechanical properties was improved significantly, such as the ultimate tensile strength increases from 1000 to 1360 MPa, the ductility changes from 6.5% to 10%. However, the wear resistance of AlCoCrFeNi2.1 EHEA was rarely researched [14, 15]. Therefore, the development of the AlCoCrFeNi2.1 EHEA in terms of wear resistance is important to further widen the advantages in industrial applications.
Many studies have shown that it is feasible to the addition of ceramic particles increase the wear resistance of alloys by refining the grains and improving hardness [16,17,18,19,20,21,22]. Ref. [22] synthesized CrFeCoNi HEAs with variety of weight percentages from 5 to 20 wt% WC particles by powder metallurgy process. The results exhibited that the alloys hardness increases up from 510.71 HV to 614.64 HV, and the wear rate is decreases from 1.70 × 10–4 mm3 N−1 m−1 to 3.81 × 10–5 mm3 N−1 m−1. Ye et al. [19] fabricated AlCoCrFeNi2.1 EHEA with different TiC contents by micro-plasma cladding technique, and the results showed that the addition of TiC greatly improved the hardness and wear resistance of the cladding layer. Although the ceramic particles can enhance the wear resistance of alloy, the selection of ceramic particles is very important. To obtain a particle reinforced HEAs with good interface bonding and excellent performance, it is not only necessary to consider the physical, chemical, and performance of particle reinforcement, but also the wettability and compatibility of particles and matrix. Wherein, the NbC particles having much better wettability can significantly refine the microstructure of alloy and effectively improve mechanical properties [23,24,25,26,27]. Li et al. [26] added NbC to Fe50Mn30Co10Cr10 HEAs coatings and found that nano-scale NbC particles can restrict the dendrite growth, making the microhardness and wear resistance of the coatings are significantly improved. In addition, powder metallurgy is conducive to the combination of particles and matrix, reducing the characteristics of uneven particle distribution and component segregation [28]. Besides, the mechanical alloying (MA) + spark plasma sintering (SPS) has particular advantages in synthesis and densification of HEAs and ceramic powders, such as rapidly sinter alloy powders, reduce the grain size, and maintain the fine microstructure.
Therefore, in this study, the NbC was selected added into AlCoCrFeNi2.1 EHEA to improve the wear resistance. And the AlCoCrFeNi2.1-xNbC (x = 0, 2.5, 7.5, and 10 wt%) HEA composites were fabricated by a combination of MA and SPS. In addition, the microstructures and mechanical properties including hardness and wear performance of AlCoCrFeNi2.1-xNbC HEA composites were system investigated. Meanwhile, the wear mechanism of AlCoCrFeNi2.1-xNbC HEA composites was thoroughly discussed.
2 Experimental
The high-purity Al, Co, Cr, Fe, and Ni powders (more than 99.5 wt%, and an average particle size of 45 µm) were employed as the AlCoCrFeNi2.1 HEA powder. The NbC particles with the content of 0, 2.5, 7.5, and 10 wt% (purity with 99.5 wt%, average particle size with 1–3 µm) were added to the AlCoCrFeNi2.1 HEA powder. The powers were milled at 300 r/min for 50 h in two 30 min intervals, and the ball to powder ratio was maintained at 10:1. The milled powders were subjected to spark plasma sintering furnace at 900 °C for 15 min with an applied pressure of 50 MPa to obtain cylindrical blocks of 15 mm diameter and 11 mm height. Figure 1 shows schematic diagram of the AlCoCrFeNi2.1-xNbC alloys preparation process. The density of the sintered alloy was measured using the Archimedes drainage method. The X-ray diffractometer (Rigaku Ultima IV, Japan) with Cu target, voltage of 40 kV, scanning range from 20° to 100°, and scanning speed of 4°/min was used to analyze the phase compositions. Scanning electron microscopy (SEM, Carl Zeiss) with an energy dispersive spectrometer (EDS) was used to analyze the microstructure of the samples and wear surfaces morphology. The Vickers hardness was measured with a Vickers hardness tester (MH-50) at a loading of 500 g and a dwell time of 15 s. The dry wear tests were carried out on multifunctional friction and wear testing machine (Rtec MFT-5000, USA) with ball-on-disk dry sliding reciprocating friction at room temperature. The friction counterpart used Al2O3 ceramic ball with a size of 9.8 mm. The round trip distance, sliding speed, loads, and sliding time of tests were 5 mm, 1 Hz, 10 N, and 30 min, respectively. The composition on the worn tracks was analyzed by a Raman spectrometer (DXR2, USA) using 532 nm wavelength laser light. A three-dimensional morphometer (Contour GT-K1, Germany) was used to measure the worn volume and morphology of the tracks. The corresponding wear rates (mm3 N−1 m−1) were calculated by the equation: W = V/FL, where V is the worn volume (mm3), F is the normal load (N) and L is the sliding distance (m).
3 Results and Discussion
3.1 Microstructures of HEA Matrix Composites
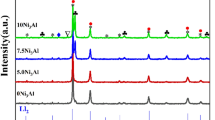
In order to investigate the effect of the NbC content on the phase structure in the AlCoCrFeNi2.1-xNbC alloys, XRD patterns are analyzed and given in Fig. 2. As can be seen in Fig. 2, NbC0 alloy is composed of FCC, ordered BCC (B2) solid phase and small amounts of Al2O3 and Cr7C3 phases. For NbC2.5 alloy, the B2 phase disappears with the formation of NbC phase, and the diffraction intensity of FCC phase increases compared to NbC0 alloy. With further increase in NbC content, the diffraction intensity of FCC phase decreases slightly, while the diffraction intensity of NbC phase increases. This phenomenon is consistent with the study of Li et al. [23].
Figure 3 shows the SEM images of the AlCoCrFeNi2.1-xNbC alloys. Obviously, it can be seen from the low-magnification SEM images, NbC0, and NbC10 alloys are almost no pores, while NbC2.5 and NbC7.5 alloys have a few holes (as shown in Fig. 3a, c, e, and g). The relative densities of all the alloys are more than 90%, which confirms a dense microstructure for the AlCoCrFeNi2.1-xNbC alloys. Figure 3b, d, f, and h presents the high-magnification SEM images of the AlCoCrFeNi2.1-xNbC alloys. All samples were composed of four phases as marked by red arrow. The measured compositions of the different regions are list in Table 1. It can be found that the region A is enriched in Al and Ni elements, the region B is enriched in Cr and C elements, and the region C is enriched in Co, Cr, Ni, and Fe elements, which is the same as the results reported in the literature [7]. The region D is enriched in Al and O elements, and the region E is enriched in Nb and C elements.
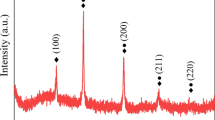
In order to further describe the elements distribution in AlCoCrFeNi2.1-xNbC alloys, the EDS mappings are shown in Fig. 4. Combined with the XRD and SEM results, the Al, Ni-rich region A is a B2 phase, and the C, Cr-rich region B is a Cr7C3. Obviously, Cr7C3 phase random distributed in the region A (B2 phase) and region C. The Co, Cr, Ni, and Fe-rich region C is an FCC phase, and the O, Al-rich region D is Al2O3 for NbC0 alloy. Similar Al2O3 and Cr7C3 formation had been observed in numerous MA–SPS alloy systems, from carbon/oxygen contamination during ball milling and the entrapped oxygen between the graphite punches and die during sintering [27]. And the C, Nb-rich region E is a NbC phase for NbC2.5, NbC7.5, and NbC10 alloys. Moreover, the distribution of NbC can only be judged by the content of Nb, because there is a large error in the content of C obtained by EDS.
The average particle size of NbC particle was measured by using the ipwin60 software, and the results are shown in Fig. 5. It can be seen that the NbC particles present nano-/micro-biscale distribution. With an increase in NbC content, the number of nano-NbC grains with 0.02–0.08 μm increases. In addition, the agglomeration of NbC particles causes certain particle size increase, wherein the micron grain (0.08–1.2 μm) changes greatly in alloys. However, when x = 10 wt%, the size distribution of NbC particles is relatively uniform, and the agglomeration phenomenon is significantly reduced. According to the grain refinement effect [29, 30] nano-scale NbC particles in the AlCoCrFeNi2.1 matrix can strengthen the alloy, while the microscale particles can directly bear the load when alloy exposed to external pressures. So, the nano-/micro-biscale distributed NbC can be played a significant role in the wear resistance of the AlCoCrFeNi2.1 alloy composite.
3.2 Effects of NbC Addition on Hardness and Wear Properties
Figure 6 shows the average hardness of AlCoCrFeNi2.1-xNbC alloys. It can be seen that the hardness of the AlCoCrFeNi2.1-xNbC alloys first decreases slightly from 679 HV (x = 0) to 664 HV (x = 2.5) and then, increases to 695 HV (x = 10) with the increase in NbC content. The decreased hardness in NbC2.5 alloy may due to the sudden disappearance of hard B2 phase. As x > 2.5, the increased hardness may be resulted from the strengthening induced by the increased volume fraction of the hard NbC phase. Moreover, it can be seen that the hardness value of all these alloys is maintained in a very high range with 660–700 HV, which will favor the wear resistance of AlCoCrFeNi2.1-xNbC alloys.
The friction coefficient of the AlCoCrFeNi2.1-xNbC alloys is obtained under the condition of dry friction, as given in Fig. 7a. It can be seen that the overall friction coefficient fluctuation is relatively considerable due to the uneven distribution of NbC particles in the AlCoCrFeNi2.1 matrix, which is consistent with the findings reported in the literature [26]. The average friction coefficient values of AlCoCrFeNi2.1-xNbC alloys are about 0.59, 0.55, 0.50, and 0.42, respectively, as shown in Fig. 7b. It can be found that the friction coefficient of the alloys containing NbC particles is lower than that of the alloy without NbC particles. The NbC particles gradually emerge from the surface of the AlCoCrFeNi2.1 EHEA during the wear process, reducing the contact area between the alloy surface and the friction pair to prevent further wear of the surface. And the NbC10 alloy has the smallest friction coefficient of 0.42, indicating that the alloy has the best friction behavior.
Figure 7 c, d shows wear sectional profiles and wear rate of the AlCoCrFeNi2.1-xNbC alloys, respectively. It can be found that with the increase in NbC content, the depth/width of wear track and wear rate first increased and then decreased. The depth/width of wear track and wear rate of NbC2.5 alloy are greater than those of NbC0 alloy, while the NbC10 and NbC7.5 alloys have observably narrower/shallower wear tracks and the lower wear rate than the NbC0 alloys, which is consistent with the friction coefficient (see Fig. 7a). The NbC10 alloy with the lowest wear scar volume and wear rate (2.4 × 10–6 mm3 N−1 m−1) exhibits excellent wear resistance. In conclusion, the proper NbC additions into AlCoCrFeNi2.1 EHEA could be improved the wear resistance significantly. The 3D morphology of the worn surface of the AlCoCrFeNi2.1-xNbC alloys is presented in Fig. 8. It can be found that the 3D worn morphology of the AlCoCrFeNi2.1-xNbC alloys is compatible with the cross-sectional contour of the wear scar. And it is clear that the NbC10 alloy has the best wear resistance and the lightest surface wear.
In order to analyze the wear mechanism of the wear track, the wear morphology and composition of the AlCoCrFeNi2.1-xNbC alloys after the dry sliding wear were systematically analyzed by SEM and the results are shown in Fig. 9 and Table 2, respectively. From Fig. 9a–d, it can be found that the NbC0 and NCb2.5 alloys exhibited the similar wear morphology with long and deep grooves on the surface and no wear debris around. However, the surface grooves of the NbC2.5 alloy are slightly deeper than those of the NbC0 alloy, which is consistent with the results of wear sectional profiles (see Fig. 7c). There are many pits on the wear surface of NbC7.5 alloy, which is notably different from NbC0 and NbC2.5 alloy wear surfaces, as illustrated in Fig. 9e, f. This may be due to the hard NbC particles fall off easily to flake and form small pits under the impact of the friction. As illustrated in Fig. 9g–h, the wear surface of the NbC10 alloy exhibits a smooth and dense friction layer, as well as a significant amount of wear debris. In addition, some smooth and dense areas such as (A, B, C, and F) are found on the wear surface of all the alloys. Combined with the EDS results, it can be seen that the smooth and dense areas are riched O element. This indicates that the oxidation reaction occurred on the wear surface, which is formed by the frictional heat generated by the reciprocal squeezing of the alloy surface by the counterpart ball.
To further investigate the wear mechanism of the AlCoCrFeNi2.1-xNbC alloys, the wear surface is analyzed by the Raman spectrum, and the obtained results are shown in Fig. 10. It can be found that the Al2O3 and Cr2O3 peaks were detected on the surface of NbC0 alloy, while the Al2O3 [31], Cr2O3 [32], Fe3O4 [33], NiO [34] , Nb2O5 [35], and CoO [36] oxide peaks were detected on the surface of NbC2.5, NbC7.5, and NbC10 alloys. Therefore, it further indicates that oxidative wear occurs, and the oxidation degree of each alloy is different during the wear process. Combining Figs. 9 and 10, the conclusions can be drawn that the main wear mechanism of NbC0 and NbC2.5 alloys is abrasive wear accompanied by slight oxidation wear, the NbC7.5 alloy is fatigue wear together with slight abrasive and oxidation wear, and the NbC10 alloy is oxidation wear accompanied by slight adhesive wear.
3.3 Oxidation Mechanism
For the dry wear experiment, oxidation wear resulted from the oxygen to react with the metal elements in the alloy surface to produce metal oxides due to elevated temperature at the friction interface. The Ellingham diagram expresses the affinity of the metal elements for oxygen [37], in which the oxide has a larger negative Gibb's free energy indicating that the stronger binding capacity for metal elements with oxygen, and the oxide will easier form. Figure 11 shows the Gibb’s free energy for metal oxides. It can be seen that the absolute values of Gibb's free energy are ordered in the following order CoO > Al2O3 > Nb2O5 > Cr2O3 > NiO > Fe3O4. The relevant oxidation chemical reaction equations are as follows:
During the dry sliding process, the produced oxides will undergo a dynamic change process of formation–destruction–formation–destruction again. Repeated friction will produce a dense oxide protection interface to decrease wear due to the production rate of oxides is higher than the destruction rate. On the other hand, the new formed oxide film will protect wear during oxides build more slowly than they are destroyed. On the contrary, the created oxide film will increase the wear rate as the formation rate of oxides is less than the destruction rate. According to the Archard’s law [38], the wear resistance is positively related to the hardness of materials. Therefore, the metal oxides with a higher hardness will reduce the rate of being destroyed and will form a protective layer on metal surface. Table 3 presents the hardness of several metal oxides. Wherein, the A2O3 and Cr2O3 have the highest hardness, indicating that they will be retained in the formation of dense oxide layer. And this prediction is consistent with the retained metal oxides measured by Raman spectrum in Fig. 10.
Figure 12 presents the illustration of the oxide formation of the AlCoCrFeNi2.1-xNbC alloys. The oxide layer will create during cyclic friction, and the oxide layer with relatively low hardness will be destroyed soon. Wherein, part of the destroyed oxide debris participates in the formation of next oxide, and the other part will be used as abrasive particles to further wear the surface. Therefore, during the wear process of the AlCoCrFeNi2.1-xNbC alloys, the oxide layer produced will undergo different destruction to form a destruction cycle and finally leave different oxide layers. In addition to the oxide, NbC particles may play a certain role in the formation of the oxide layer.
4 Conclusions
AlCoCrFeNi2.1 EHEA matrix composites reinforced by 0, 2.5, 7.5, and 10 wt% NbC particles were successfully fabricated using MA + SPS method. The effect of NbC particles addition on the microstructure, Vickers hardness, and wear resistance of the AlCoCrFeNi2.1-xNbC HEAs was investigated. The main conclusions are summarized as follows:
-
1.
The crystal structure of AlCoCrFeNi2.1-xNbC alloys changed from the B2 + FCC main phases and Al2O3 and Cr7C3 minor phases to the NbC + FCC main phases and Al2O3 and Cr7C3 minor phases. In addition, with the increase in NbC content, the volume fraction of NbC phase increased, and the NbC particles in the NbC2.5, NbC7.5, and NbC10 alloys present the biscale distribution with micro-/nano-scale.
-
2.
All of the AlCoCrFeNi2.1-xNbC alloys exhibited excellent Vickers hardness with 679 HV, 664 HV, 677 HV, and 695 HV, respectively. With the addition of NbC particles, the average friction coefficient of the AlCoCrFeNi2.1-xNbC alloys gradually decreases from 0.59 to 0.42, and the wear rate increases first from 1.5 × 10–5 mm3 N−1 m−1 to 1.7 × 10–5 mm3 N−1 m−1 and then, decrease to 2.4 × 10–6 mm3 N−1 m−1.
-
3.
The AlCoCrFeNi2.1-10NbC alloy has the best wear performance, and the wear mechanism was oxidation wear accompanied by slight adhesive wear, while the main wear mechanism for NbC0 and NbC2.5 alloys is abrasive wear accompanied by slight oxidation wear, for NbC7.5 alloy, fatigue wear together with slight abrasive and oxidation wear.
References
Y.P. Lu, Y. Dong, S. Guo, L. Jiang, H.J. Kang, T.M. Wang, B. Wen, Z.J. Wang, J.C. Jie, Z.Q. Cao, H.H. Ruan, T.J. Li, Sci. Rep. 4, 6200 (2014)
I. Baker, M. Wu, Z. Wang, Mater. Charact. 147, 545 (2019)
M.L. Wan, Y.P. Lu, T.M. Wang, C. Zhang, Z.Q. Cao, T.J. Li, P.K. Liaw, Scr. Mater. 204, 114132 (2021)
H. Jiang, L. Li, R. Wang, K.M. Han, Q.W. Wang, Acta Metall. Sin. -Engl. Lett. 34, 1565 (2021)
W.N. Jiao, T.X. Li, X.X. Chang, Y.P. Lu, G.M. Yin, Z.Q. Cao, T.J. Li, J. Alloys Compd. 902, 163814 (2022)
T. Bhattacharjee, I.S. Wani, S. Sheikh, I.T. Clark, T. Okawa, S. Guo, N. Tsuji, Sci Rep. 8, 3276 (2018)
R. John, A. Karati, J. Joseph, D. Fabijanic, B.S. Murty, J. Alloys Compd. 835, 15524 (2020)
T. Xiong, S.J. Zheng, J.G. Pang, X.L. Ma, Scr. Mater. 186, 336 (2020)
X.H. Chen, W.Y. Xie, J. Zhu, Z.D. Wang, Y.L. Wang, Y.F. Ma, M. Yang, W.W. Jiang, H.W. Yu, Y.D. Wu, X.D. Hui, Intermetallics 128, 107024 (2021)
B.S. Dong, Z.Y. Wang, Z.G. Pan, O. Muránsky, C. Shen, M. Reid, B. Wu, X.Z. Chen, H.J. Li, Mater. Sci. Eng. A 802, 140639 (2021)
W. Chen, Y.T. Wang, L.L. Wang, J.Q. Zhou, Eng. Fract. Mech. 246, 107615 (2021)
T. Wang, M. Komarasamy, S. Shukla, R.S. Mishra, J. Alloys Compd. 766, 312 (2018)
P.J. Shi, W.L. Ren, T.X. Zheng, Z.M. Ren, X.L. Hou, J.C. Peng, P.F. Hu, Y.F. Gao, Y.B. Zhong, P.K. Liaw, Nat. Commun. 10, 1 (2019)
V. Hasannaeimi, A.V. Ayyagari, S. Muskeri, R. Salloom, S. Mukherjee, N.P.J. Mater, Degrad. 3, 16 (2019)
F. Kafexhiu, B. Podgornik, D. Feizpour, Metals 10, 208 (2020)
Y. Guo, C.G. Li, M. Zeng, J.Q. Wang, P. Deng, Y. Wang, Mater. Chem. Phys. 242, 122522 (2020)
H. Wu, S.R. Huang, C.Y. Zhu, J.F. Zhang, H.G. Zhu, Z.H. Xie, Acta Metall. Sin. -Engl. Lett. 33, 1091 (2020)
M. Ghanbariha, M. Farvizi, T. Ebadzadeh, A.A. Samiyan, Wear 484, 43 (2021)
F.X. Ye, Y. Yang, Z. Lou, L.Z. Feng, L. Guo, J.X. Yu, Mater. Lett. 284, 128859 (2021)
Y.B. Peng, W. Zhang, T.C. Li, M.Y. Zhang, B. Liu, Y. Liu, L. Wang, S.H. Hu, Surf. Coat. Technol. 385, 125326 (2020)
Y.M. Zou, Z.G. Qiu, C.J. Huang, D.C. Zeng, R. Lupoi, N. Zhang, S. Yin, Surf. Coat. Technol. 434, 128205 (2022)
A. Hegazy, M. Bhlol, O. Dawood, O. Elkady, J. Mech. Eng. 7, 1127 (2022)
X.F. Li, Y.H. Feng, B. Liu, D.H. Yi, X.H. Yang, W.D. Zhang, G. Chen, Y. Liu, P.K. Bai, J. Alloys Compd. 788, 485 (2019)
H. Wu, S.R. Huang, C.M. Zhao, H.G. Zhu, Z.H. Xie, C.L. Tu, X.D. Li, Intermetallics 127, 106983 (2020)
Q.S. Gou, J. Xiong, Z.X. Guo, J.B. Liu, L. Yang, X.R. Li, Int. J. Refract. Met. Hard Mater. 94, 105375 (2021)
X.F. Li, X.H. Yang, D.H. Yi, B.S. Liu, J.C Zhu, J.X Li, C. Gao, L.Z. Wang, Intermetallics 138, 107309 (2021)
Y. Shao, Z.H. Guo, Y.B. Wang, H. Ma, Int. J. Refract. Met. Hard Mater. 94, 105388 (2021)
S. Praveen, J. Basu, S. Kashyap, R.S. Kottada, J. Alloys Compd. 662, 361-367 (2016)
G.A. Malygin, Phys. Usp. 181, 1129 (2011)
J.Y. Wang, H.L. Yang, Z.L. Liu, S.X. Ji, R.D. Li, J.M. Ruan, J. Alloys Compd. 772, 272 (2019)
P. Thomas, V. Ramakrishnan, V. Vaidyan, Thin Solid Films 170, 35 (1989)
O. Monnereau, L. Tortet, C. Grigorescu, D. Savastru, C.R. Iordanescu, F. Guinneton, R. Notonier, A. Tonetto, T. Zhang, I.N. Mihailescu, J. Optoelectron. Adv. Mater. 12, 1752 (2010)
N. Lee, P.J. Schuck, P.S. Nico, B. Gilbert, J. Phys. Chem. Lett. 6, 970 (2015)
U.N. Mironova, A. Kuzmin, I. Steins, J. Grabis, I. Sildos, M. Pärs, J. Phys. Conf. Ser. 93, 12039 (2007)
M. Yashima, J.H. Lee, M. Kakihana, M. Yoshimura, J. Phys. Chem. Solids. 58, 1593 (1997)
M. Rashad, M. Rüsing, G. Berth, K. Lischka, J. Nanomater. 2013, 1 (2013)
M. Hasegawa, Treatise Process Metall. 1, 507 (2014)
J. Archard, J. Appl. Phys. 24, 981 (1953)
Acknowledgements
This work was supported by the Natural Science Foundation of Shandong Province (No. ZR2020QE019), the National Natural Science Foundation of China (No. 51901116), and the China Postdoctoral Science Fund (No. 2021M692724).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors state that there are no conflicts of interest to disclose.
Additional information
Available online at http://springerlink.bibliotecabuap.elogim.com/journal/40195.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, H., Li, L., Wang, J. et al. Wear Properties of Spark Plasma-Sintered AlCoCrFeNi2.1 Eutectic High Entropy Alloy with NbC Additions. Acta Metall. Sin. (Engl. Lett.) 36, 987–998 (2023). https://doi.org/10.1007/s40195-023-01529-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40195-023-01529-4