Abstract
REB2C2 (RE = Y and lanthanides) compounds have gained attention for their unique layered crystal structure. However, there have been few reports about Raman spectroscopy of REB2C2 compounds up to now. Here, the Raman spectrum of YbB2C2 is obtained by a micro-Raman spectroscope and the first-principles calculations. Raman active vibrational modes of YbB2C2 are confirmed as A1g (627 and 1311 cm−1), B1g (944 and 1172 cm−1), B2g (330 and 885 cm−1) and Eg (357 and 530 cm−1). Atomic displacements of these modes are different, they can be divided into two groups: A1g, B1g and B2g correspond to ring breathing (δin, in the plane) of B2C2 layer; Eg is due to ring deformation (δoop, out of the plane) of B2C2 layer. These results are helpful to understand the individual structure of REB2C2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
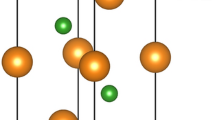
REB2C2 compounds have a feature of layered crystal structure with P4/mbm symmetry [1,2,3,4,5,6,7,8,9]. The crystalline structure can be described as a stacking of layers, –RE–B2C2–RE–B2C2–RE–, along the z-direction, where strong B–C covalent bonds in the B–C plane coexist with weak RE–B and RE–C bonds [1,2,3,4,5,6,7,8]. Within the B2C2 layer, each atom is bonded to three other atoms so as to form fused four- and eight-membered rings [3, 4]. Each four-membered ring consists of two boron atoms and two carbon atoms arranged alternately, whereas each eight-membered ring contains four boron and four carbon atoms with B–C contact [3, 4, 6]. In the unit cell, RE atoms locate at the 2a site (0, 0, 0), while B and C atoms occupy different 4 h sites (xB, xB + 1/2, 1/2) and (xC, xC + 1/2, 1/2), where xB and xC are the positional parameters of B and C atoms [7], as shown in Fig. 1 [5, 7]. The structure of REB2C2 resembles that of Mn+1AXn phases (“MAX phases,” where M is an early transition metal, A is an A-group element (mostly Groups 13 and 14) and X is C or N), which consist of alternately stacking of M–X octahedron layers and A layers, and strong covalent bond of M–X coexists with weak M–A bond in it [10,11,12,13].
As to the properties of REB2C2 compounds, previous work mainly focused on their electrical and magnetic properties. According to Sakai et al. [14], REB2C2 compounds indicate a metallic-type conductivity, which is confirmed by the negative sign of the Seebeck coefficients [14] and density functional calculations (DFT) [15]. Superconducting transitions are observed for YB2C2 and LuB2C2 at 3.6 and 2.4 K, respectively, whereas LaB2C2 remains in the normal metallic state down to 1.8 K [14]. And other RB2C2 (R = Nd, Sm, Gd, Tb, Dy, Ho, Er and Tm) show a rapid decrease in resistivity at low temperatures starting at 9, 53, 48, 23, 16, 7, 15 and 16.5 K, respectively [2, 14]. The results of electronic transport measurements are in good agreement with the magnetic ordering temperatures determined earlier by the same authors [14, 16], which indicates that most of the diboride dicarbides MB2C2 (M = Ce, Nd, Sm, Gd, Tb, Er and Tm) are antiferromagnetic. TbB2C2, DyB2C2 and HoB2C2 are ferromagnets with complex magnetic structures [2, 16]. And great experimental attention has been paid to the isostructural magnetic DyB2C2 due to its unusual antiferroquadrupolar ordering [17]. Experimentally, Zhao et al. [18, 19] have successfully synthesized high purity bulk YB2C2 and studied its physical and mechanical properties. It has excellent damage tolerance, easy machinability, high melting point (2500 °C < Tm < 2600 °C) and excellent high-temperature rigidity (the value of young’s modulus at 1500 °C can still maintain that at room temperature). These features might endow YB2C2 with great untapped potential for future ultrahigh temperature applications [18, 19].
Raman spectroscopy is an ideal characterization tool depending on the fact that it is quick, sensitive and non-destructive, so it can offer high resolution structural and electronic information and can be applicable to both laboratory and mass-production scales [20,21,22,23,24]. Before Raman spectroscopy can be used for identifying and characterizing compounds, however, it is essential to determine the locations of the peaks and to correlate them with the various vibrational modes [22]. For layered materials, Raman spectroscopy has shown its great potential in the characterization of layer numbers, interlayer coupling and layer-stacking configurations [25]. Raman spectroscopy of graphitic materials has played an important role in their structural characterization since the 1970s, and this technique has also become a powerful tool for understanding the behavior of electrons and phonons in graphene [20, 26,27,28]. Barsoum et al. have reported on the Raman spectra of some representative compounds from the family of Mn+1AXn phases [11, 22, 29, 30]. In addition, Raman spectroscopy can also be employed to study ferromagnetic/antiferromagnetic materials [31, 32].
However, there have been few reports on the Raman spectroscopy of REB2C2, especially on the corresponding Raman active vibrational modes. YbB2C2 compound is an important member of REB2C2 compounds. V2AlC, Ti2AlC and Ti2AlN are common M2AX (211) phases, their first-order Raman spectra are similar [22]. Thus, the Raman spectroscopy of YbB2C2 should be helpful to study the Raman spectra of other REB2C2 compounds. Especially, Raman spectroscopy should also be helpful to study TbB2C2, DyB2C2 and HoB2C2 ferromagnets. The paper aims to confirm the Raman spectroscopy of YbB2C2 and to discuss its corresponding vibrational modes in detail.
2 Materials and Methods
2.1 Materials Preparation
YbB2C2 powder was synthesized by boro/carbothermic reduction method with a mixture of Yb2O3 (99%, 0.7 µm, Ganzhou Kemingrui, China), B4C (99%, 200 mesh, Jingangzhuan, China) and graphite (99%, 200 mesh, Tianyuan, China) powders. At first, raw powders with a 0.7:0.8:6.0 molar ratio were milled in a polypropylene jar for 6 h in alcohol to obtain a homogeneous mixture. The powder mixture was then dried in an oven at 60 °C for 24 h. After that, the powder mixture was placed in a graphite crucible heated at a rate of 10 °C/min to 1950 °C and held for 1.5 h under flowing Ar. After that, YbB2C2 powder was sieved through a − 500 mesh screen to obtain a homogeneous and fine powder. Then, the fine powder was shaped by pressing (in a steel mold with a pre-pressure of 100 MPa and a dwell time of 5 min) and cold isostatic pressure (with a pressure about 300 MPa) in turn to get a uniform and compact bulk sample. Finally, bulk YbB2C2 sample was obtained by sintering the green body at 2250 °C for 1 h under flowing Ar.
2.2 Characterization Methods
The phase composition was identified using an X-ray diffractometer with Cu Kα radiation (Rigaku D/max-2400, Tokyo, Japan). A micro-Raman spectroscope (Jobin Yvon HR800, excited by 532 and 633 nm He–Ne laser) was used to characterize the Raman spectrum of bulk YbB2C2 at room temperature. The grating of the micro-Raman spectroscope is 600 g/mm, and the spectral resolution of the spectrometer is better than 1.1 cm−1. The laser beam was focused on the surface of the sample before signal acquisition by adjusting the focal length, which helps to realize a confocal measurement. The spectrum was recorded with an exposure time of 240 s. The spectra were corrected for a baseline with the routine implemented in the software NGSLabSpec.
To elucidate the Raman spectrum of YbB2C2, first-principle calculations based on density functional theory (DFT) were performed using the Cambridge Serial Total Energy Package (CASTEP) code [33], wherein the Vanderbilt-type ultrasoft pseudopotential [34] and the generalized gradient approximation (GGA) based on the Perdew–Burke–Ernzerhof (PBE) scheme [35] for the exchange–correlation functional were used. The k-points sampling and cut off energy were 5 × 5 × 7 and 500 eV, respectively. The Broyden–Fletcher–Goldfarb–Shanno (BFGS) minimization scheme [36] was used in geometry optimization. During the geometry optimization, the setting tolerances of total energy, maximum ionic Hellmann–Feynman force, maximum ionic displacement and maximum stress were 5 × 10−6 eV/atoms, 0.01 eV/Å, 5.0 × 10−4 Å and 0.02 GPa, respectively. Lattice parameters and atomic positions were modified to minimize the enthalpy and interatomic forces. Phonon dispersion curve and atomic vibrational character were obtained by lattice dynamics calculations employing a finite displacement method. The atomic Hellmann–Feynman forces were induced by small displacements of selected atoms in a 2 × 2 × 2 supercell. The phonon frequencies and the vibrational modes were obtained by diagonalization of the dynamical matrix. The computations have been made to determine the bond lengths, bond angles, and vibrational frequencies with its Raman activities and depolarization ratio of the Raman bands using the optimized molecular geometry of the most stable conformer.
3 Results and Discussion
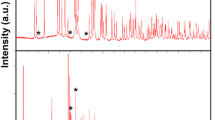
X-ray diffraction (XRD) pattern of as-prepared bulk YbB2C2 sample is shown in Fig. 2a, which confirms that high purity YbB2C2 compound has been successfully synthesized. Figure 2b shows the morphology of the bulk YbB2C2 sample. Obviously, the YbB2C2 sample consists of typical laminated plates with a well crystalline character. The laminated plates of YbB2C2 reflect the alternate arrangement of the B–C networks and Yb sheets, as shown in Fig. 1c.
Raman spectroscopy of YbB2C2 was studied both by a micro-Raman spectroscope and the first-principles calculations in this work. Raman spectrum of YbB2C2 powder detected by the micro-Raman spectroscope is shown in Fig. 3. It can be seen that Raman spectrum of YbB2C2 powder shows peaks at 329, 359, 528, 626, 887, 942, 1173 and 1309 cm−1.
The calculated phonon dispersion relation and the first Brillouin zone of YbB2C2 are shown in Fig. 4. Obviously, there is no imaginary phonon frequency throughout the examined high-symmetry directions in the Brillouin zone for the structure of YbB2C2 (Fig. 4a), which indicates that it is dynamically stable against mechanical perturbations. The phonon density of states (DOS) and the projected phonon DOS for YbB2C2 are shown in Fig. 5. Hereon, the site projected phonon density of states of Yb, B and C atoms was calculated to examine the contributions from vibrations of different atoms to the total density of states. The vibration of Yb atoms dominated the lowest frequency interval at around 50–150 cm−1 in structure of YbB2C2, which can be attributed either to the weak Yb–Yb bonding and low interatomic force constant of Yb or to the larger mass of Yb atom. Vibrations in low-frequency range of 200–1150 cm−1 are corresponding to B–C bonding within the fourfold cyclobutadiene-like ring, while the vibrations above 1150 cm−1 are corresponding to the B–C bonding connecting the fourfold cyclobutadiene-like rings in YbB2C2. The results are similar to those of YB2C2 [37].
The zone-center (Г-point) phonons can be classified by the irreducible representation of the point group D4h. Using the group theory, one can show that the irreducible representations of them are given by Eq. (1). Of these representations, three acoustic phonon modes are represented as Гacoustic = A2u + Eu, and the remaining optical modes are dissociated as Eq. (2).
Among those optical modes, the representations A1g, B1g, B2g and Eg are Raman active. Here, the zone-center (Г-point) phonons of YbB2C2 are similar to that of YB2C2 [37]. However, they are different from Mn+1AXn phases. For the M2AX (211) phases, there are a total number of 24 modes, out of which four are Raman active; three are only Raman active modes (A1g + 2E2g) and one is both Raman and infrared active (E1g) [22, 29]. And the M3AX2 (312) phases show a total of seven Raman active modes (2A1g + 2E1g + 3E2g) [22, 29]. The corresponding Raman tensors (dipole polarizability) of YbB2C2 are given as Eq. (3). The Raman tensor is defined as αij = ∂Pi/∂Ej, where Pi and Ej denote i and j components of dipole vector and external electric field, respectively [38].
For the first-principles calculations, it should be noted that B–C bonds of YbB2C2 can be separated as B–CL (with a larger length about 1.603 Å) and B–CS (with a smaller length about 1.517 Å) according to their relationship with the four-membered rings. These B–C bonds and atom numbering of YbB2C2 are shown in Fig. 6.
According to the first-principles calculations, Raman active vibrational modes of YbB2C2 are observed at different Raman shifts: A1g at 627 and 1311 cm−1, B1g at 944 and 1172 cm−1, B2g at 330 and 885 cm−1, Eg at 357 cm−1 (which are doubly degenerate and denoted as 357 cm−1 (1 and 2)) and 530 cm−1 (which are also doubly degenerate and denoted as 530 cm−1 (1 and 2)), respectively. Raman active vibrational modes and the corresponding Raman shifts of YbB2C2 are listed in Table 1 according to the first-principles calculation and experiment. It can be seen that Raman active vibrational modes of YbB2C2 studied by the first-principles calculations agrees well with that detected by micro-Raman spectroscope.
The atomic displacements of vibrational modes of YbB2C2 are shown in Fig. 7. The modes can be generally summarized as ring breathing (δin, in plane) and ring deformation (δoop, out of plane) of B2C2 layer, which are similar to those of 2,3,5,6-tetrachloroaniline, CH3TAF3B and graphene [20, 39, 40]. The former includes A1g, B1g and B2g modes, while the latter consists of Eg mode. All these modes are different, which can be described as follows. B2g (330 cm−1) mode (Fig. 7a): B1 and C4, B5 and C8 atoms vibrate toward each other in opposite directions; and C2 and B7, B3 and C6 atoms vibrate away from each other in opposite directions. As discussed above, Eg at 357 cm−1 is doubly degenerate including (1) and (2) vibrational modes, the atomic displacements of which are similar. For the former: B1, C2, B3 and C8 atoms vibrate in the negative direction of z-axis; and other B and C atoms vibrate in the positive direction of z-axis, as shown in Fig. 7b. The latter (Fig. 7c) is equivalent to the former rotating 90° along the axis perpendicular to B–C plane. Similarly, Eg at 530 cm−1 is also doubly degenerate including (1) and (2) vibrational modes. Figure 7d shows the atomic displacements of the former: B1, B3, C4 and C6 atoms vibrate in the negative direction of z-axis; and other B and C atoms vibrate in the positive direction of z-axis. The latter (Fig. 7e) corresponds to the former rotating 90° along the axis perpendicular to B-C plane. In A1g (627 cm−1, shown in Fig. 7f) mode, all B and C atoms vibrate along B–C bonds in anti-clockwise direction in x–y plane. B2g (885 cm−1) mode (Fig. 7g): B1 and C4 atoms vibrate from B1 to C4 along the line between them; C2 and B7 atoms vibrate from C2 to B7 along the line between them; B3 and C6 atoms vibrate from C6 to B3 along the line between them; and B5 and C8 atoms vibrate from B5 to C8 along the line between them. Figure 7h shows the atomic displacements of B1g (944 cm−1) mode: C2 and B3 atoms vibrate from C2 to B3 along B3–C2 bond; C4 and B5 atoms vibrate from B5 to C4 along B5-C4 bond; C6 and B7 atoms vibrate from C6 to B7 along B7-C6 bond; and C8 and B1 atoms vibrate from B1 to C8 along B1–C8 bond. B1g (1172 cm−1) mode (Fig. 7i): C2 and B3, C6 and B7 atoms vibrate away from each other along B–C bonds between them; C4 and B5, C8 and B1 atoms vibrate toward each other along B–C bonds between them. A1g (1311 cm−1) mode: C2 and B3, C4 and B5, C6 and B7, C8 and B1 atoms vibrate toward each other along B–C bonds between them, as shown in Fig. 7j. Obviously, the atomic displacements of A1g, B1g, B2g and Eg modes in YbB2C2 are corresponding to B–C bonding, which agrees well with the calculated phonon dispersion, phonon density of states and the projected phonon DOS.
Atomic displacements of vibrational modes of YbB2C2: a B2g (330 cm−1); b Eg (357 cm−1 (1)); c Eg (357 cm−1 (2)); d Eg (530 cm−1 (1)); e, Eg (530 cm−1 (2)); f A1g (627 cm−1); g B2g (885 cm−1); h B1g (944 cm−1); i B1g (1172 cm−1); j A1g (1311 cm−1). *Atomic displacements are represented by arrows and the lengths of them refer to the magnitudes
4 Conclusion
In this work, Raman spectrum of YbB2C2 was detected by micro-Raman spectroscope, which showed peaks at 329, 359, 528, 626, 887, 942, 1173 and 1309 cm−1. And according to the first-principles calculations, Raman active vibrational modes of YbB2C2 are observed as: A1g at 627 and 1311 cm−1, B1g at 944 and 1172 cm−1, B2g at 330 and 885 cm−1, Eg at 357 cm−1 and 530 cm−1, which agrees well with that detected by micro-Raman spectroscope. Then, the atomic displacements of vibrational modes of YbB2C2 were discussed in detail with the help of the first-principles calculations. Although these atomic displacements are different from each other, they can be generally summarized as ring breathing (δin) and ring deformation (δoop) of B2C2 layer. The former group consists of A1g, B1g and B2g modes, and the latter group refers to Eg mode. Eg at both 357 and 530 cm−1 are doubly degenerate including (1) and (2) vibrational modes, the atomic displacements of which are similar, respectively. The study of YbB2C2 compound’s Raman spectroscopy and its corresponding vibrational modes is helpful to study the Raman spectra of other REB2C2 compounds and is also helpful to identify and characterize these compounds.
References
V. Babizhetskyy, Visnyk Lviv Univ. Ser. Chem. 57, 77 (2016)
V. Babizhetskyy, J. Bauer, R. Gautier, K. Hiebl, A. Simon, J.F. Halet, Including Actinides (Elsevier, Amsterdam, 2018), p. 145
J. Bauer, O. Bars, Acta Crystallogr. Sect. B: Struct. Sci. 36, 1540 (1980)
J. Bauer, J.F. Halet, J.Y. Saillard, Coord. Chem. Rev. 178, 723 (1998)
K. Kaneko, K. Ohoyama, H. Onodera, Y. Yamaguchi, J. Phys. Soc. Jpn. 69, 3762 (2000)
S. Khmelevskyi, P. Mohn, J. Redinger, H. Michor, Supercond. Sci. Technol. 18, 422 (2005)
K. Ohoyama, K. Kaneko, K. Indoh, H. Yamauchi, A. Tobo, H. Onodera, Y. Yamaguchi, J. Phys. Soc. Jpn. 70, 3291 (2001)
T. Onimaru, H. Onodera, K. Ohoyama, H. Yamauchi, Y. Yamaguchi, J. Phys. Soc. Jpn. 68, 2287 (1999)
Y. Yang, T. Hong, Phys. B 525, 154 (2017)
M.W. Barsoum, Prog. Solid State Chem. 28, 201 (2000)
G.W. Bentzel, M. Naguib, N.J. Lane, S.C. Vogel, V. Presser, S. Dubois, J. Lu, L. Hultman, M.W. Barsoum, E.N. Caspi, J. Am. Ceram. Soc. 99, 2233 (2016)
P. Eklund, M. Beckers, U. Jansson, H. Högberg, L. Hultman, Thin Solid Films 518, 1851 (2010)
M. Sokol, V. Natu, S. Kota, M.W. Barsoum, Trends Chem. 1, 210 (2019)
T. Sakai, G.-Y. Adachi, J. Shiokawa, J. Less Common Met. 84, 107 (1982)
X. Rocquefelte, S.E. Boulfelfel, M. Benahia, J. Bauer, J.Y. Saillard, J.F. Halet, Angew. Chem.-Int. Edit. 44, 7542 (2005)
T. Sakai, G. Adachi, J. Shiokawa, Solid State Commun. 40, 445 (1981)
K. Hirota, N. Oumi, T. Matsumura, H. Nakao, Y. Wakabayashi, Y. Murakami, Y. Endoh, Phys. Rev. Lett. 84, 2706 (2000)
G. Zhao, dissertation, University of Chinese Academy of Sciences, 2017
G. Zhao, J. Chen, Y. Li, M. Li, Scr. Mater. 124, 86 (2016)
A.C. Ferrari, D.M. Basko, Nat. Nanotechnol. 8, 235 (2013)
Y.R. Hu, J.K. Liang, A.S. Myerson, L.S. Taylor, Ind. Eng. Chem. Res. 44, 1233 (2005)
V. Presser, M. Naguib, L. Chaput, A. Togo, G. Hug, M.W. Barsoum, JRSp 43, 168 (2012)
G. Schoukens, K. De Clerck, Poly 46, 845 (2005)
M. Zolfaghari, Phys. B 555, 1 (2019)
X. Zhang, Q.H. Tan, J.B. Wu, W. Shi, P.H. Tan, Nanoscale 8, 6435 (2016)
M.S. Dresselhaus, A. Jorio, R. Saito, in Annual Review of Condensed Matter Physics. ed. by J.S. Langer (Wiley, Hoboken, 2010), p. 89
M.S. Dresselhaus, A. Jorio, A.G. Souza, R. Saito, Philos. Trans. R. Soc. A-Math. Phys. Eng. Sci. 368, 5355 (2010)
F. Tuinstra, J.L. Koenig, J. Chem. Phys. 53, 1126 (1970)
O.D. Leaffer, S. Gupta, M.W. Barsoum, J.E. Spanier, J. Mater. Res. 22, 2651 (2007)
J.E. Spanier, S. Gupta, M. Amer, M.W. Barsoum, Phys. Rev. B 71, 012103 (2005)
W. Ben Nasr, K. Karoui, A. Bulou, A. Ben Rhaiem, Phys. E 93, 339 (2017)
Y.J. Sun, Q.H. Tan, X.L. Liu, Y.F. Gao, J. Zhang, J. Phys. Chem. Lett. 10, 3087 (2019)
M.D. Segall, P.J.D. Lindan, M.J. Probert, C.J. Pickard, P.J. Hasnip, S.J. Clark, M.C. Payne, J. Phys.: Condens. Matter 14, 2717 (2002)
D. Vanderbilt, Phys. Rev. B 41, 7892 (1990)
J.P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett. 77, 3865 (1996)
B.G. Pfrommer, M. Cote, S.G. Louie, M.L. Cohen, J. Comput. Phys. 131, 233 (1997)
Y. Zhou, H. Xiang, X. Wang, W. Sun, F.-Z. Dai, Z. Feng, J. Mater. Sci. Technol. 33, 1044 (2017)
Y.F. Li, B. Xiao, L. Sun, X.Z. Wang, Y.M. Gao, Y.R. Wang, Comput. Mater. Sci. 134, 67 (2017)
H.M. Badawi, W. Förner, A.A. Al-Saadi, J. Mol. Struct. 938, 41 (2009)
M.M. Oliva, G. Hennrich, J. Casado, J.T. López Navarrete, J. Mol. Struct. 369, 834–836 (2007)
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China under Contract Nos. 50802099 and 51072201. The authors gratefully thank Shaofan Ge from School of Materials Science and Engineering, University of Science and Technology of China for his linguistic assistance during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Available online at http://springerlink.bibliotecabuap.elogim.com/journal/40195
Rights and permissions
About this article
Cite this article
Li, Z., Yang, J., Wang, J. et al. Raman Spectroscopy of Layered Compound YbB2C2. Acta Metall. Sin. (Engl. Lett.) 34, 1021–1027 (2021). https://doi.org/10.1007/s40195-020-01181-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40195-020-01181-2