Abstract
The precipitation and evolution of secondary nitrides (S-nitrides) in the Alloy 690 electro-slag remelted (ESR) structure were investigated. Experimental results indicate that S-nitrides precipitated in the interdendritic region of the ESR structure at temperatures higher than 1100 °C. S-nitrides could spread throughout the entire interdendritic area after sufficient exposure, and they were more numerous and much finer than primary nitrides. Furthermore, after studying the evolution of S-nitride particles at 1100, 1200 and 1300 °C, it was determined that the precipitation of S-nitrides was controlled by the diffusion of nitrogen. In addition, by investigating the elemental segregation of ESR structure and calculating critical Ti concentrations, S-nitride precipitation was found to be thermodynamically inevitable in the Alloy 690 ESR structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Alloy 690, as one of the most important nickel-based alloys, is extensively used in the construction of nuclear reactors, gas turbines, spacecraft, liquid-fuel rocket engines and combustion systems because of its superior corrosion resistance, outstanding workability and good weldability [1, 2]. In these applications, Alloy 690 is typically required to be tolerant of aggressive environments including corrosive liquid, high-temperature and high-stress impact loading [3].
Electro-slag remelting (ESR) is a secondary melting process, and it has been widely employed for solidification control and chemical refining in the manufacture of nickel-based superalloys and special steels [4]. In order to improve the reliability of Alloy 690 components, currently ESR is also employed in the manufacture of Alloy 690 following the conventional melting, i.e., vacuum induction melting (VIM). During remelting, VIM ingot would be melted and purified in slag bath in drops. After descending from slag bath, metallic droplets would drop in a water-cooled copper crystallizer and form a molten pool in it. The ESR ingot would solidify from its bottom to its top in the crystallizer. Moreover, because of the presence of an external cooling system, the ESR ingot would also solidify in a fast rate. Thus, compared with conventionally solidified ingots, a denser structure with less elemental segregation could be expected in the ESR ingot [5].
Alloy 690 is quite simple in structure. It is almost only made up of a single-phase austenitic matrix and two kinds of precipitates, namely chromium carbide and titanium nitride. Generally, the existence of the precipitates is an important factor that influences the properties related to the second phases of metallic materials, such as the mechanical properties and the corrosion resistance. The carbides in Alloy 690 are mainly the M 23C6 with an FCC crystal structure [1]. M 23C6 are coarse and semi-continuous at high-angle grain boundaries, fine and faceted at low-angle grain boundaries, and needle-like at incoherent and twin-related boundaries [6]. Stiller et al. [7] assumed that dense intergranular carbides have a beneficial effect on retarding the propagation of stress corrosion cracking in high-temperature water environments.
Titanium-rich nitrides in Alloy 690 are basically cubic- or triangular-shaped blocks with several microns side length [8]. These nitrides are proved to be beneficial in refining grains [9]. However, on the other hand, they could also act as the preferential sites for pit initiation [10, 11]. Dutta et al. [12] attributed this to the large degree of misfit between the nitrides and the austenitic matrix. Presently, it is well acknowledged that the typical nitrides in Alloy 690 all form during solidification as a result of the titanium segregation [13]. However, due to the adoption of ESR, some submicron-sized secondary nitrides (S-nitrides) could also precipitate from solid. The precipitation behavior of primary nitride (P-nitride) has been extensively studied, but the literature about the S-nitride in Alloy 690 is still not available. Hence, in present study, the precipitation and evolution of submicron-sized S-nitrides were investigated.
2 Experimental
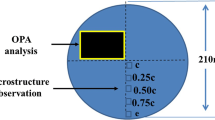
The manufacture and dissection of the large Alloy 690 ESR ingot have been presented elsewhere [14]. This ingot was produced by a double-route process involving VIM and ESR, and it was 1800 mm in height, 515 mm in diameter and 3 tons in weight. This ingot was longitudinally dissected along its central line to reveal the ESR macrostructure, and four rectangular plates were cut off from the longitudinal central section. These four plates were all of the size 515 mm × 450 mm × 25 mm and numbered I through IV according to their original locations in the ingot. On these four plates, three different parts, the hot top region, the steady-state region and the ramp-up region could be readily characterized by their macrostructural features. A majority of the ingot was in the steady-state region, and it exhibited the typical ESR structure. Among these four plates, Plate III contained the steady-state region exclusively, so it was selected as the experimental material. Specimens in the size of 10 mm × 10 mm × 5 mm were obtained at the center, the mid-radius and the edge of Plate III, respectively, as shown in Fig. 1. The chemical composition of the specimens was determined as follows (wt%): 0.02 C, 29.46 Cr, 0.19 Ti, 9.8 Fe, 0.0094 N, 0.18 Al, 0.24 Mn, 0.0021 O, <0.0006 S and balance Ni.
Specimens were subjected to thermal exposures that were conducted at 1100, 1200 and 1300 °C for 30, 60, 120 and 240 min, respectively. All the specimens were water-quenched after exposure. Specimens for microstructure examination were mechanically ground, polished and electrolytically etched using a solution of 10 vol% phosphoric acid and 90 vol% H2O. Optical microscopy (OM) and scanning electron microscopy (SEM) were employed for observing the precipitation and growth of S-nitrides. Electro-probe microanalysis (EPMA) was used to evaluate the elemental segregation in the ESR structure. Transmission electron microscopy (TEM) was employed for characterizing the precipitates. TEM samples were perforated between −22 and −17 °C at 15 V by a twin-jet electro-polisher, with 10 vol% perchloric acid and 90 vol% ethanol solution. 3000 × SEM images were used to determine the size and quantity of S-nitrides by using the software Image-Pro Plus.
3 Results
3.1 Characterization of S-nitrides
The phenomenon of S-nitride precipitation was confirmed and studied at different locations in the ingot. Figure 2 illustrates the existence of S-nitrides in specimens that were thermally exposed at 1200 °C for 60 min. Figure 2a shows that there was no S-nitride in the original ESR structure, but S-nitrides were observed in all the exposed specimens. Moreover, the quantities of S-nitrides were different at different places in the ingot. The quantity of S-nitrides increased only slightly from the center to the mid-radius of this ingot. However, from the mid-radius to the edge, the amount of S-nitrides increased significantly.
The precipitation location of S-nitrides in the ESR structure is specified in Fig. 3. The images in Fig. 3 are all dark-field OM metallographs that were captured from the specimens thermally exposed at 1100 °C for 30 and 240 min. In these dark-field images, nitrides can be easily distinguished from the matrix because of their unique golden color [15]. It is clear that S-nitrides preferentially precipitated in the interdendritic area. However, when the exposure time was relatively short, S-nitrides were mostly concentrated along the grain boundary, as shown in Fig. 3b. The quantity of S-nitrides increased significantly when the exposure time was extended from 30 to 240 min, and Fig. 3c shows that S-nitrides could eventually spread across the entire interdendritic area. Figure 4 shows the TEM characterizations of S-nitrides; they were identified as TiN with an FCC structure and a lattice constant of 0.424 nm. Even though these S-nitrides were no greater than 1 μm in side length, they still had a typical faceted blocky appearance.
3.2 Evolution of S-nitrides
The evolution of S-nitrides was studied in specimens that were exposed at 1100, 1200 and 1300 °C for 30, 60, 120 and 240 min, as shown in Fig. 5. Statistical analysis was conducted on the average particle radius and average particle quantity of S-nitrides, and the results are presented in Fig. 6. The average particle quantities in Fig. 6b were determined by using 3000× SEM images covering an area of 1350 μm2.
Figures 5 and 6 show that the dimensions of the S-nitride particles simply increased with exposure time. However, the variations of particle quantity are different at the three temperatures. At 1100 °C, the number of S-nitride particles together with the particle radius monotonically increased throughout the entire exposure. At 1200 °C, the quantity of S-nitride particles peaked after being exposed for 60 min and then began to decline with further exposure. In the case of 1300 °C, S-nitride particles were basically independent of time.
The stability of S-nitride particles against dissolution is illustrated in Fig. 7. Two specimens were thermally exposed for 60 min at 1200 and 1350 °C, respectively. Clearly, there is no evidence of S-nitride dissolution, even when the temperature was as high as 1350 °C, which is only about 10 °C lower than the solidus of Alloy 690.
3.3 Elemental Segregation of the ESR Ingot
The elements Ti, N, Cr and Ni were qualitatively analyzed at the center, the mid-radius and the edge of the ingot’s steady-state region, as shown in Fig. 8. According to these mapping images, Ti segregated substantially to the spaces between dendrites, whereas N was homogeneously distributed in the matrix. Cr and Ni exhibited positive and negative segregation behaviors, respectively, in the interdendritic area. The bright spots in the mapping images of Ti and N indicate the presence of P-nitrides.
The dendritic arms marked on the SEM images in Fig. 8 were quantitatively analyzed. In Fig. 9, the concentration profiles of Cr, Ti and Ni are plotted as a function of the distance from the interdendritic center. Because the quantitative analyzing path crossed the dendritic arms perpendicularly, the concentrations of elements fluctuated. Taking the element Cr as an example, Cr was enriched in the interdendritic area and depleted in the dendrites. Thus, the highest point on the concentration profile of Cr can be considered as being obtained at the center of the interdendritic area, and this spot is designated as “0 μm” on the X-axis. The lowest point on the profile would then be obtained at the center of the intradendritic area. The X value of the lowest point represents the distance between the inter- and intradendritic centers.
The lowest points of these three Cr profiles in Fig. 9a were all placed on a base line with a value of 0 wt% on the Y-axis. The data displayed on the Y-axis are an elemental segregation quantity, which is equal to the concentration difference between the individual spot being analyzed and the intradendritic center. Obviously, spots at X = 0 μm have the maximum segregation quantities, and these values could be used to evaluate the segregation tendency of the elements. According to the maximum segregation quantity of these profiles in Fig. 9, the different elements exhibited different segregation tendencies at the center, mid-radius and edge of the ingot. The three elements all showed the highest segregation tendency at the ingot center, whereas the elemental segregation at the mid-radius was slightly milder. At the ingot surface, the segregation of elements was quite weak. In addition, the interdendritic concentrations of the elements, intradendritic concentrations and the segregation indexes (\(\xi_{i} = C^{i}_{{\text{intra}}} /C^{i}_{{\text{inter}}}\)) at the center, the mid-radius and the edge of the ingot are listed in Table 1.
4 Discussion
4.1 Precipitation Mechanism of S-nitrides
Generally, the type of function that describes the evolution of precipitates can be derived from the relationship between average particle radius (\(\bar{r}\)) and exposure time (t). Ferguson et al. [16] showed that the precipitation of TiN in nitrided austenitic steel during annealing followed a linear relationship between \(\bar{r}^{3}\) and t:
where \(\bar{r}_{\text{0}}\) is the initial average particle radius and k is the growth rate coefficient. Slozov [17] and Wagner [18] reported that the growth process that obeys this cube rate law is controlled by the diffusion of the solute.
In order to determine the true S-nitride precipitation function type in the Alloy 690 ESR ingot, linear relationships of \(\bar{r}^{2} \propto t\),\(\bar{r}^{3} \propto t\) and \(\bar{r}^{4} \propto t\) were all studied. Specifically, a coefficient of determination (COD) was employed to evaluate the quality of the linear fitting. The COD is a parameter with a value between 0 and 1, whose value is proportional to the fitness of a linear analysis. According to the experimental results obtained at 1100 °C, the CODs for \(\bar{r}^{2} \propto t\),\(\bar{r}^{3} \propto t\) and \(\bar{r}^{4} \propto t\) are 0.99717, 0.99949 and 0.99648, respectively. Apparently, \(\bar{r}^{3} \propto t\) shows the highest fitting quality, and the same results can also be acquired in the cases of 1200 and 1300 °C. In other words, the growth of S-nitride particles in the Alloy 690 ESR ingot obeyed a cube rate law and is therefore presumed to be controlled by solute diffusion [19].
The relationship between the cube of the average particle radius (\(\bar{r}^{3}\)) and the exposure time (t) is presented in Fig. 10a. Since the growth of S-nitride particle has been confirmed as diffusion-controlled, it can be assumed that k (the slope of \(\bar{r}^{3}\) against t) in Eq. (1) can be simply described by a Boltzmann law [20]:
where Q is the apparent activation energy for growth, T k is the annealing temperature in units of K, and R is the universal gas constant (R = 8.314 J K−1 mol−1). As Eq. (3) shows, Q can be calculated from the linear relationship between ln k and \(1\text{/}T_{\text{k}}\). The temperature dependency of ln k is demonstrated in Fig. 10b, and Q was determined to be 162.1 kJ mol−1.
The apparent activation energies for titanium diffusion and nitrogen diffusion in austenite are 251 kJ mol−1 [21] and 168 kJ mol−1 [22], respectively. Ferguson et al. [16] claimed that the growth of TiN particles in Fe-35 wt% Ni was controlled by the diffusion of titanium atom. Indeed, the diffusion rate of titanium is much lower than that of nitrogen, and generally, the slower process tends to be the controlling factor. In this study, however, the concentration difference between titanium and nitrogen should be considered as well.
In this ESR ingot, the content of nitrogen in the matrix was much lower than that of titanium; moreover, titanium substantially segregated to interdendritic areas, whereas nitrogen was uniformly distributed as shown in Fig. 8. Table 1 suggests that the titanium content in the interdendritic region of the mid-radius specimen is 0.24 wt%. If all the titanium atoms in the interdendritic region are consumed in the evolution of S-nitrides, the volume fraction of S-nitride between dendrites should be 0.47 vol%. However, in this study, the highest volume fraction of S-nitride in the interdendritic region is 0.42 vol%, which was achieved in the sample exposed at 1300 °C for 240 min. Therefore, the quantity of titanium in the interdendritic area is more than what is needed to sustain S-nitride particle evolution.
The growth process of S-nitrides is illustrated in Fig. 11. Firstly, S-nitrides substantially precipitate in the interdendritic region, the place where titanium is enriched, after being exposed to elevated temperature. With the precipitation of S-nitrides, the local titanium and nitrogen are consumed. As discussed above, the content of titanium between dendrites is sufficient for S-nitride precipitation. However, the local nitrogen would be depleted gradually and a resultant gradient of nitrogen concentration would be formed. Driven by this composition gradient, nitrogen constantly diffuses to the interdendritic area to sustain the growth of S-nitrides. In addition, according to the above results, the activation energies for S-nitride growth and nitrogen diffusion are quite similar. Therefore, it is reasonable that the growth of S-nitride particles is controlled by the diffusion of nitrogen.
Schematic illustration of the growing mechanism of S-nitride particles. a S-nitride particles have formed between dendritic arms; b nitrogen diffuses to the interdendritic region for sustaining the growth of S-nitride particles. The brightness of red color and intensity of greenish color represent the concentration of titanium and nitrogen, respectively
The difference in the development of S-nitride particles with exposure time could be ascribed to the different diffusion rates of N at different temperatures. At 1100 °C, the size of S-nitride particles increased slightly, whereas the quantity of them increased significantly, as shown in Fig. 5. The diffusion of N was relatively low at this temperature, and consequently, the growth of S-nitride particles proceeded in a low rate. However, the level of supercooling was already very high at 1100 °C. As a result, the nucleation of S-nitride was significantly enhanced and the number of particles increased dramatically. As the growth rate of S-nitride particles was quite low, so the consumption of the N in the interdendritic area is low. Generally, the precipitation of S-nitride remained in its initial stage during the exposure at 1100 °C, and the amount of N between dendrites was fairly enough to sustain the development of S-nitride particles within the whole exposure process.
When the annealing temperature was elevated to 1200 °C, the diffusion rate of N was increased. The N in the dendritic area could promptly diffuse to the S-nitride particles to support the growth of them. However, for the nitrogen in the intradendritic region, it would take them a relatively long period of time to diffuse to the interdendritic region because of a much longer diffusion distance. At the beginning of exposure, both the number and size of S-nitride particles increased significantly. However, after this fast growth, the N in the interdendritic area was almost depleted, and the N in the intradendritic region had not yet arrived. In general, with a shortage of solute supply, a dispersion of particles with limited solubility in the matrix tends to coarsen by the growth of large particles at the expense of smaller ones [23]. Hence, when the local nitrogen was depleted, nitrogen diffused from small particles to larger ones and the S-nitride particles coarsened.
When the exposure temperature was increased to 1300 °C, the diffusion of nitrogen increased significantly. Nitrogen could diffuse from the intragranular area to the intergranular area within a short period of time. Besides, the nucleation rate of S-nitride particles significantly declined at this high temperature. Therefore, all the particles grew together with a steady number.
Figure 2 shows that the quantities of S-nitride particles were different at different locations in the ingot. Clearly, the number of S-nitride particles at the ingot surface is higher than that in the interior locations (the center and the mid-radius). It is easy to see that P-nitride precipitation at the ingot surface was seriously suppressed by the intense external cooling. As a result, compared with the interior locations, less nitrogen was consumed at the ingot surface during solidification, and hence, more nitrogen was left over and could be used in the precipitation of S-nitrides.
4.2 Thermodynamic Inevitability of the Secondary Precipitation
According to the segregation indexes listed in Table 1, although the ESR ingot was fabricated in a crucible with an external cooling system, elements like titanium and chromium still exhibited quite evident segregation tendencies. Because of such evident titanium enrichment, a study of the relationship between titanium segregation and S-nitrides precipitation is necessary.
The precipitation of nitrides is believed to be controlled by the solubility product [24]:
where [Ti] and [N] are the matrix concentrations (wt%) of Ti and N, A and B are constants, and T p is the temperature at which the precipitation of nitrides occurs. In brief, the precipitation of S-nitrides is controlled by the concentration of Ti and N as well as by the temperature. As shown in Fig. 8, the distribution of nitrogen in the matrix was uniform. If the temperature is fixed, a certain critical titanium concentration for the secondary nitride precipitation would exist. In other words, at this fixed temperature, if the local Ti content surpasses the critical Ti content somewhere, the formation of S-nitrides would be induced. Since the phenomenon of S-nitride precipitation has been confirmed in this ingot, we just need to calculate the highest critical Ti content and determine whether the precipitation of S-nitride is inevitable in this ingot.
In order to obtain the highest critical Ti content, the lowest nitrogen content should be used in this calculation. Because the center of the ingot experienced the mildest cooling condition, the largest amount of N was consumed there during P-nitride precipitation. As a consequence, the ingot center had the least amount of N in the matrix. According to the results of a previous work [14], the volume fraction of P-nitrides in the central area was below 0.03 vol% (within the fields containing several dendrites); consequently, the nitrogen remaining in the matrix would be no less than 0.005 wt%. The temperature employed in this calculation was 1300 °C, the highest exposing temperature in this work. For the constants in Eq. (4), because reliable values for the solubility product of TiN in the nickel-based austenite were quite rare, the data for iron-based austenite were used instead [24,25,26,27]. Various TiN solubility product models and their corresponding calculated critical titanium contents are listed in Table 2.
As can be seen in Table 2, the published data on the solubility product of TiN in iron-based austenite differ by several orders of magnitude. Differences in the status of experimental materials, the design of experiments and the contents of Ti and N could all obviously affect the testing data. However, it is well acknowledged that the solubility of TiN in austenite is quite low even lower than that of any other nitride [27]. Because of the very low TiN solubility, all the calculated critical Ti contents are also quite low. Among these calculated values, even the highest one 0.012 wt% is apparently lower than the interdendritic Ti content of any part of the ingot. Therefore, the secondary precipitation of nitride is thermodynamically inevitable in the interdendritic region of the ESR ingot.
4.3 Future of the Alloy 690 with S-nitrides
Meng et al. [10] have investigated the corrosion behavior of different types of nitrides in Alloy 690. They claimed that stress corrosion cracking (SCC) could be initiated at micron-sized TiN particles. However, in contrast, no SCC initiation was found at submicron TiN inclusions. Fuchs et al. [28] also reported that submicron nitrides could restrict the growth of grains during solution annealing, thereby enhancing the yield and ultimate tensile strength of Alloy 690. Therefore, with the same total TiN volume, it is possible that Alloy 690 with a higher proportion of submicron-sized nitride would have better corrosion resistance and mechanical properties.
In order to generate S-nitrides in Alloy 690, the ESR process is necessary. The quantity of S-nitrides in Alloy 690 is closely correlated with the remelting process parameters. If the external cooling could be enhanced or if the remelting rate could be reduced, the precipitation of P-nitrides would be restricted, and consequently, the S-nitrides precipitation would be improved.
If S-nitrides are designed to be introduced into Alloy 690, we also hope that they could have a small size and be large in number. The ideal hot working temperature for Alloy 690 ESR ingots is around 1200 °C; therefore, during soaking, the ESR structure would experience a relatively long period of time between 1100 and 1200 °C. Figure 6 reveals that S-nitride particles would not grow significantly at temperatures below 1200 °C, and they could also remain in large quantities during the long-time exposure at 1100 °C and the short-time exposure at 1200 °C. As a consequence, the soaking procedure prior to deformation could be a good opportunity for S-nitrides to precipitate. Moreover, in subsequent deformations, generated S-nitrides could be dispersed uniformly in the matrix.
To summarize, the small size, large quantity and extensive distribution of S-nitrides make this kind of precipitate quite promising for improving the properties of Alloy 690. However, additional detailed investigations of S-nitrides still need to be performed in the future to fully validate this concept.
5 Conclusions
-
1.
Secondary nitrides (S-nitrides) can form in the interdendritic area of Alloy 690 ESR ingots at temperature higher than 1100 °C.
-
2.
These S-nitrides were much more numerous and finer than the primary nitrides and were identified as submicron-sized TiN particles with an FCC structure and a lattice constant of 0.424 nm.
-
3.
The precipitation of S-nitride in the ESR structure was controlled by the diffusion of nitrogen.
-
4.
S-nitride precipitation was determined to be thermodynamically inevitable in the ESR structure.
References
J.J. Kai, G.P. Yu, C.H. Tsai, M.N. Liu, S.C. Yao, Metall. Trans. A 20, 2057–2067 (1989)
C. Leroy, T. Czerwiec, C. Gabet, T. Belmonte, H. Michel, Surf. Coat. Technol. 142, 241–247 (2001)
W.S. Lee, C.Y. Liu, T.N. Sun, J. Mater, Process Technol. 153, 219–225 (2004)
J.D. Busch, J.J. deBarbadillo, M.J.M. Krane, Metall. Mater. Trans. A 44A, 5295–5303 (2013)
J.J. Burke, M.C. Flemings, A.E. Gorum, Solidification Technology, 1st edn. (Brook Hill, Chestnut Hill, 1972), p. 154
Y.S. Lim, J.S. Kim, H.P. Kim, H.D. Cho, J. Nucl. Mater. 335, 108–114 (2004)
K. Stiller, J.O. Nilsson, K. Norring, Metall. Mater. Trans. A 27, 327–341 (1996)
W.J. Ma, Y.P. Bao, L.H. Zhao, M. Wang, Int. J Min. Met. Mater. 21, 234–239 (2014)
B. Chen, S. Li, Y.C. Ma, M. Gao, K. Liu, Acta Metall. Sin. 47, 816–822 (2011). (in Chinese)
F. Meng, J. Wang, E.-H. Han, W. Ke, Corros. Sci. 52, 927–932 (2010)
R.S. Dutta, A. Lobo, R. Purandare, S.K. Kulkarni, G.K. Dey, Metall. Mater. Trans. A 33, 1437–1447 (2002)
R.S. Dutta, R. Tewari, P.K. De, Corros. Sci. 49, 303–318 (2007)
R. Jiang, B. Chen, X. Hao, Y. Ma, S. Li, K. Liu, J. Mater. Sci. Technol. 28, 446–452 (2012)
M. Wang, X.D. Zha, M. Gao, Y.C. Ma, K. Liu, Y.Y. Li, Metall. Mater. Trans. A 46, 5217–5231 (2015)
S.K. Michelic, D. Loder, T. Reip, A.A. Barani, C. Bernhard, Mater. Charact. 100, 61–67 (2015)
P. Ferguson, J.H. Driver, A. Hendry, J. Mater. Sci. 18, 2951–2956 (1983)
I.M. Lifshitz, V.V. Slyozov, J. Phys. Chem. Solids 19, 35 (1961)
C. Wagner, Z. Elektrochem. 65, 581 (1961)
S. Zhao, X. Xie, G.D. Smith, S.J. Patel, Mater. Des. 27, 1120–1127 (2006)
J. Cormier, X. Milhet, J. Mendez, J. Mater. Sci. 42, 7780–7786 (2007)
S.H. Moll, R.E. Ogilvie, Trans. Am. Inst. Min. Metall. Eng. 215, 613–618 (1959)
P. Grieveson, E.T. Turkdogan, Trans. Metall. Soc. Aime 230, 407 (1964)
R.S. Moshtaghin, S. Asgari, Mater. Des. 24, 325–330 (2003)
H. Wada, R.D. Pehlke, Metall. Trans. B 16, 815–822 (1985)
K. Inoue, I. Ohnuma, H. Ohtani, K. Ishida, T. Nishizawa, ISIJ Int. 38, 991–997 (1998)
K.J. Irvine, F.B. Pickerin, T. Gladman, ISIJ 205, 161 (1967)
J. Kunze, Met. Sci. 16, 217–218 (1982)
G.E. Fuchs, S.Z. Hayden, Scr. Mater. 25, 1483–1488 (1991)
Acknowledgements
This work was supported by the Chinese Academy of Sciences Innovation Foundation under Grant No. CXJJ-14-M44.
Author information
Authors and Affiliations
Corresponding author
Additional information
Available online at http://springerlink.bibliotecabuap.elogim.com/journal/40195.
Rights and permissions
About this article
Cite this article
Wang, M., Chen, B., Hao, XC. et al. Secondary Precipitation Behavior of Nitride in the Electro-slag Remelted Structure of Alloy 690. Acta Metall. Sin. (Engl. Lett.) 30, 771–780 (2017). https://doi.org/10.1007/s40195-017-0541-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40195-017-0541-3