Abstract
In the present work, an Al-Cu-Si-Ni-Mg brazing filler metal was developed for the joining of 6063 aluminum alloy and graphite metallized with Ag-Cu-Ti paste. The joint microstructures were studied by means of SEM and EDS. Results show that joints with a good quality can be produced at a brazing temperature of 550 °C for 10 min. TiC was formed at the graphite/active Ag-Cu-Ti paste interface due to the reaction between Ti and C, and thus, the wettability on the graphite was improved. Compared to the metallization stage, more diffusions of Al, Ag, Ti, and Cu from interlayer to graphite are observed. The bonding area mainly consists of α-Al, Ag rich phase, α + Al2Cu + Si, Al2Cu, and TiC. It is shown that joints with good quality can be produced and the average shear strength of the joints is 21 MPa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the size of the electron devices decreasing continuously, the characteristic of miniaturization and integration of steady system require great heat elimination capability. According to the reference, thermal reasons and related issues are responsible for 55% of contemporary microelectronic circuit failures [1]. There is an increasing interest in efficient heat transfer materials to cope with such problems. The aluminum alloys of 6000 group containing magnesium and silicon as major alloying elements are very attractive because of low density, good corrosion, and weldability [2, 3]. Graphite is an ideal candidate for manufacturing thermal conductive composites, due to its low cost, high thermal conductivity, and a unique layered nano structure. It can be seen that aluminum and graphite composites have wide prospects [4, 5]. The thermal conductivity of 6063 wrought aluminum alloy is 209 W/(m K) [6, 7]. It has been reported that natural crystalline flake graphite has a thermal conductivity of 2200 W/(m K) in its (002) crystal plane [8]. However, it is very difficult for metal to wet graphite surface because graphite has a covalently bonded structure with extreme physical and chemical stability. There is also a large difference in the thermal expansion coefficients and the mismatch of their physical properties [9]. As a result, the fusion-welding method can rarely provide a high-quality welding joint.
In the present study, the graphite was metallized by Ag-Cu-Ti paste and then bonded to 6063 aluminum alloy in a vacuum furnace. The interface evolution was analyzed and mechanical properties of joint were determined.
2 Materials and method
2.1 Starting materials
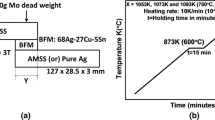
High density graphite plate (Beijing Sanye Carbon Co. Ltd, Beijing, China) and 6063 aluminum alloy (Northeast Light Alloy Co. Ltd, Shenyang, China) were used as parent metals. Ag-Cu-Ti alloy powder was prepared by gas atomization and AlCuSiNiMg20-6-1-1 foil was fabricated by rolling method in the present study. Graphite plate with a thickness of 5 mm and 6063 aluminum alloy with a thickness of 10 mm were used as parent materials. The average particle diameter of the gas atomized Ag-Cu-Ti powders was approximate 40 μm. The Ag-Cu-Ti powder was mixed with the nitrocotton solution in a 9 to 1 weight ratio and used as metallized film for graphite. The AlCuSiNiMg20-6-1-1 foil with a thickness of 0.1 mm was used as brazing filler metal.
The chemical compositions of the used aluminum alloys are given in Tables 1 and 2 based on the results of electron probe microanalysis test.
2.2 Joint sample preparation
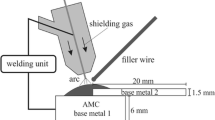
The samples for sealing tensile strength tests were cut into the proper size according to SJ/T 3326-2016 [7] and assembled as shown in Fig. 1. All the components were cut into 15 × 15 mm for the observation of the interface microstructure. The graphite substrate was coated with a 50–80 μm AgCuTi adhesion layer by screen print method and then sintered in vacuum furnace at 850 °C for 10 min. Subsequently, the metallized graphite and 6063 substrate were joined using the Al-Cu-Si-Ni-Mg brazing foils. The brazing joints were produced in the vacuum furnace (P = 3 × 10−3 Pa) at a temperature of 550 °C for 5 min, 10 min, and 15 min. After brazing, the specimens were cooled in the furnace with a cooling rate of 10 °C/min to 250 °C, followed by a fast cooling step with Ar shielding gas to room temperature.
The melting temperatures of the samples were determined by differential scanning calorimetry (DSC, STA 449 F5) at a heating rate of 10 °C min−1 under nitrogen atmosphere. The microstructure was observed by scanning electron microscopy (SEM, Hitachi SU-1510). An energy dispersive spectroscope (EDS) was also adopted to determine element composition of the microstructural characteristics and element distribution in the brazing seam. The tensile tests were carried out using a universal testing machine (CMT4105) to investigate the tensile properties of the joints. The loading speed was 0.5 mm/min. Three specimens were used for each test and the average value was taken into account.
3 Results and discussion
3.1 Melting characteristic of filler metals
The melting property of brazing alloy is one of the key performance indicators for filler metals, and the temperature determines the bonding temperature. Figure 2 shows the DSC curves of Ag-Cu-Ti powder and Al-Cu-Si-Ni-Mg alloy. It is shown that a single endothermic peak appears in each DSC curve. The solidus and liquidus temperatures are marked in the DSC curve. The melting temperature range of AgCuTi powder is 786.10 to 792.19 °C. The solidus and liquidus temperatures of Al-Cu-Si-Ni-Mg are 506.92 and 516.73 °C respectively.
3.2 Microstructures of metallized graphite
The metalization layer is composed of Ag, Cu, Ti, and graphite is made of C. In these metallurgical systems, the Ag-Ti system has a weak compound formation tendency compared to the Cu-Ti-C system [10]. According to the references [11, 12], the Gibbs energies of formation in the Cu-Ti-C system can be expressed by the following formulae:
It can be seen that the Gibbs free energy formations of TiC and CuxTiy are negative and TiC forms more easily than CuxTiy compounds.
To further study metallurgical reaction, the interface morphology of metallized graphite is given in Fig. 3. According to the SEM-EDS results, it can be inferred that part of Ti in the liquid Ag-Cu-Ti alloy tends to diffuse to the graphite substrate. A reactive wetting takes place during the metallization by the reaction of Ti and C and TiC compound is formed. It seems that the Ag-Cu-Ti layer is divided into two zones. The top layer of the metallization layer is composed of Ag-rich, Cu-rich and Cu-Ti compounds (zone І in Fig. 6(a)). Adjacent to the TiC layer beside the substrate, an Ag-Cu eutectic microstructure is observed (zone II in Fig. 6(a)).
3.3 Microstructure of the brazed joint
After brazing at 550 °C for 10 min, the bonding area microstructures of the 6063-graphite samples is displayed in Fig. 4(a). There are no micro defects such as crevices and cracking on the interface. The interface between Ag-Cu-Ti and Al-Cu-Si-Ni-Mg filler metal disappears after brazing. The microstructures of the interlayer adjacent to the different substrates are obviously different. In order to determine the element distribution, the elemental line scan of the brazing joint is shown in Fig. 4(b). The Ag and Al atoms are enriched in the bonding area; Ti is concentrated at the boundary of graphite. Additionally, Si aggregation is detected inside the filler metal and graphite. Moreover, Mg is distributed evenly in the filler metals.
To evaluate the phase composition of the interface, XRD is conducted on the fracture surface of joints brazed at 550 °C for 10 min after tensile tests and the result is given in Fig. 5. In accordance with the partial enlarged drawing shown in Fig. 6, diffusion of Ti towards graphite continues and increases during the brazing compared with the metallization stage, and a TiC layer forms continuously. Table 3 shows the chemical compositions of the points indicated in Fig. 6. CuTi2 formation is found adjacent to TiC when Ti diffusion reaches saturation. No AgTi compound is detected according to the XRD result because most Ti reacts with C. Mg and Ni are uniformly distributed in the interlayer.
High magnification image of the microstructure and EDS of joint in Fig. 4. (a) Graphite-filler metal. (b) 6063-filler metal
Compared to the metallization stage, more diffusions of Al, Ag, Ti, and Cu from interlayer to graphite are observed. From the XRD result, it is believed that the white phase is mainly Ag-rich phase; the light gray and the gray phase are Al2Cu and α-Al, respectively. The dendritic Al2Cu is scattered and solid solution α-Al has a large proportion of total. This is beneficial to the strength and ductility of the brazed joint and also counters balance for the brittleness from CuAl2. In addition, Ni in brazing alloy compensates for brittleness of Al2Cu. A large number of α-Al + Al2Cu + Si are found to be embedded in the filler metals. Moreover, the Ag2Al are formed adjacent to Ag-rich phase.
The microstructure near 6063 substrate is obviously different. The α-Al + Al2Cu + Si are distributed evenly and the size of this phase decreased gradually from interlayer to substrate. The gray Al2Cu phase increased as well as the white Ag rich phase decreased. A small number of Si is embedded in Al2Cu. Tiny amounts of Ni segregations are detected, as seen from Figs. 5 and 6.
Based on the SEM and XRD results, an interface evolution model of the brazed joint is proposed in Fig. 7. Most of Ti in the Ag-Cu-Ti paste diffuses to the graphite through the interface reaction between Ti and C atoms. A few of Ti reacted with Cu and in the form of Cu-Ti intermetallic compounds. Ag-Cu eutectic structure is formed with the decrease of brazing temperature. It seems that small amounts of Al diffuse from the filler metal to the graphite surface. Moreover, Ag and Cu diffuse to both sides of the parent materials.
3.4 Mechanical properties
The effect of holding time on the joint strength is shown in Fig. 8. It reveals that when the holding time is 5 min, the tensile strength is 12 MPa. This means the holding time is too short for element diffusion and interface reactions. When the holding time is 10 min, the tensile strength reaches the maximum value of 21 MPa. However, the tensile strength declines to 15 MPa when the holding time is prolonged to 15 min. This is due to the formation of much more brittle intermetallics in the interlayer.
The tensile broken joint and the fracture microstructures of the joint brazed at 550 °C for 10 min are shown in Fig. 9. It can be observed that most of the fractures developed in the graphite matrix near the metallization layer. The fractured surface reveals a brittle fracture mode.
4 Conclusions
The microstructure and strength of 6063 aluminum alloy and metallized graphite using Al-Cu-Si-Ni-Mg filler metal are studied. Sound joints were achieved and the tensile strength of the joints reached 21 MPa under the brazing condition of 550 °C for 10 min. The interface structure mainly consisted of α-Al, Ag rich phase, α + Al2Cu + Si, Al2Cu, and TiC. TiC was formed by the reaction between Ag-Cu-Ti paste and the graphite. This played a key role in the brazing process by improving the wettability of the graphite.
References
Janicki M, Napieralski A (2000) Modelling electronic circuit radiation cooling using analytical thermal model. Microelectron J 31(9-10):781–785
Shaji MC, Ravikumar KK, Ravi M, Sukumaran K (2013) Development of a high strength cast aluminium alloy for possible automotive applications. Mater Sci Forum 765:54–58
Jiang L, Wang P, Xiu Z, Chen G, Lin X, Dai C, Wu G (2015) Interfacial characteristics of diamond/aluminum composites with high thermal conductivity fabricated by squeeze-casting method. Mater Charact 106:346–351
Hirsch J, Al-Samman T (2013) Superior light metals by texture engineering: optimized aluminum and magnesium alloys for automotive applications. Acta Mater 61(3):818–843
Cui B, Huang JH, Xiong HJ et al (2013) Reaction-composite brazing of carbon fiber reinforced SiC composite and TC4 alloy using Ag–Cu–Ti–(Ti+C) mixed powder. Mater Sci Eng A 562:203–210
Chen ZB, Bian H, Hu SP, Song XG, Niu CN, Duan XK, Cao J, Feng JC (2018) Surface modification on wetting and vacuum brazing behavior of graphite using AgCu filler metal. Surf Coat Technol 348:104–110
Electronics Industry Standard (2016) Sealing tensile strength test method for cermic-metal. SJ/T 3326-2016. China Electronics Technology Standardization Institute, Beijing
Zhou SX, Chiang S, Xu JZ et al (2012) Modeling the in-plane thermal conductivity of a graphite/polymer composite sheet with a very high content of natural flake graphite. Carbon 50:5052–5061
Yu WY, Liu SH, Liu XY, Liu MP, Shi WG (2015) Interface reaction in ultrasonic vibration-assisted brazing of aluminum to graphite using Sn-Ag-Ti solder foil. J Mater Process Technol 221:285–290
Guo W, Zhu Y, Wang L, Qu P, Kang H, Chu PK (2013) Microstruture evolution and mechanical properties of vacuum-brazed C/C composite with AgCuTi foil. Mater Sci Eng A 564:192–198
Arroyave R, Eagar W, Kaufman L (2003) Thermodyanmic assessment of the Cu-Ti-Zr system. J Alloys Compd 351:158–170
Zhang XY (2012) Interficial stress analysis and structural design for composite and Cu joints. Dissertation, Central South University, Changsha
Funding
This study was financially supported by the National Key R&D Program of China (2017YFB0305702)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Recommended for publication by Commission XVII - Brazing, Soldering and Diffusion Bonding
Rights and permissions
About this article
Cite this article
Yuan, L., Wang, W., Chen, X. et al. Brazing of graphite to 6063 aluminum alloy with Al-Cu-Si-Ni-Mg filler metal and Ag-Cu-Ti paste. Weld World 65, 1211–1216 (2021). https://doi.org/10.1007/s40194-021-01094-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40194-021-01094-3