Abstract
Purpose of Review
The purpose of this review is to identify and understand the incidence and implications of sugammadex-induced hypersensitivity and anaphylaxis. This review also focuses on the mechanistic causation of anaphylaxis with regard to sugammadex administration and the management of anaphylaxis.
Recent Findings
The overall incidence of sugammadex-induced anaphylaxis is low, approximately 1:3500, and is comparable with other medications commonly used during the intraoperative period. Several studies and case reports have demonstrated that the mechanism of anaphylaxis is likely IgE- or basophil-mediated and that a diagnosis of anaphylaxis to confirm the etiology of urticaria, hypotension, bronchospasm, and other symptoms is based on positive skin prick testing, elevated tryptase levels, and dose-dependent activation of basophils during in vitro analysis. Bradycardia is a poorly understood adverse effect of sugammadex; however, the risk of bradycardia does not appear to be increased when compared with neostigmine.
Summary
Sugammadex-induced anaphylaxis is a relatively rare complication but carries a risk of morbidity and mortality if inadequately identified and treated. Existing severity scales allow anesthesiologists to stratify the severity of an anaphylactic response and provide appropriate intraoperative treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Naturally occurring paralytics have been used for centuries to facilitate hunting by South American peoples. The first known use of a neuromuscular blocking agent (NMBA) to assist in surgery occurred in 1942 when Harold Griffith administered curare to a man undergoing an appendectomy. With this, the fields of surgery and anesthesiology were changed dramatically. By rendering patients immobile, NMBAs opened new doors to surgical procedures which were previously impossible [1]. However, as their use became more widespread, unintended consequences of their administration, such as residual paralysis, became more apparent. With inadequate strength, patients are at higher risk of postoperative respiratory complications, including higher rates of pneumonia, reintubation, and prolonged hospitalization. Traditionally, neuromuscular blockade has been reversed with an acetylcholinesterase inhibitor, such as edrophonium, pyridostigmine, or neostigmine. Anticholinesterases increase the concentration of acetylcholine at the neuromuscular junction increasing the binding of acetylcholine to the acetylcholine receptor and allowing recovery of neuromuscular function. Although neostigmine has been used for many decades, long-standing evidence indicates that many patients do not fully recover muscle strength following its administration. Inadequate recovery occurs for a host of reasons including administration of an inadequate dose of neostigmine, attempted reversal of a profound depth of neuromuscular blockade, and allowing an inadequate amount of time between administration of the anticholinesterase and tracheal extubation for the neostigmine to have had its maximal effect.
Sugammadex, first tested in humans in 2005, is a novel reversal agent that selectively binds aminosteroidal NMBAs and is effective in the rapid reversal of even profound depths of neuromuscular blockade induced with vecuronium or rocuronium. Since initial approval, its use has increased substantially throughout the world. In Japan alone, over 11 million vials were sold within 7 years of gaining regulatory approval [2]. Within the last 5 years, over 1000 articles referencing sugammadex have become available on PubMed.
Sugammadex is a modified gamma-cyclodextrin which was initially developed for clinical use in 1999 [3, 4••, 5, 6]. It consists of a negatively charged ring with eight concentric thioether-linked carboxylated glucopyranoside moieties and a lipophilic core that attracts the positively charged aminosteroidal structures of rocuronium and vecuronium in the plasma [7••, 8, 9]. Once the NMBA is enveloped within sugammadex, molecular changes occur which render the encapsulation essentially irreversible and, as a result of this, the NMBA is unable to diffuse into the neuromuscular junction. The decrease in the plasma concentration of rocuronium or vecuronium allows for the movement of NMBA down its concentration gradient and away from the neuromuscular junction, where it is in turn bound by available sugammadex. The inactivated and water-soluble complex of NMBA and sugammadex is excreted through the kidneys.
When compared with neostigmine, sugammadex has a few unique advantages in that, when administered in appropriate doses, it rapidly and consistently reverses deep neuromuscular blockade induced by aminosteroid NMBAs. In the first trial of sugammadex in volunteers, sugammadex, 8 mg/kg, 3 min after rocuronium, 0.6 mg/kg, resulted in recovery to a train-of-four ratio of 0.9 in 2 min [10]. A recent review [11] of the results of 17 trials of more than 1550 patients found that, when compared with neostigmine, administration of sugammadex eliminated signs of postoperative residual neuromuscular blockade. Numerous studies have demonstrated that complete recovery from even profound levels of vecuronium- or rocuronium-induced neuromuscular blockade can occur within minutes of administration of an appropriate dose of sugammadex and that recovery is both quicker and more predictable than following administration of neostigmine. For example, neostigmine will completely antagonize residual neuromuscular block from a train-of-four count of two to a train-of-four ratio > 0.90 in an average of 18.6 min compared with 1.5 min following administration of sugammadex. Moreover, recovery to a train-of-four ratio > 0.9 after neostigmine required as much as 107 min compared with 5 min in subjects receiving sugammadex [12]. Importantly, the range of time required for recovery to a TOFR > 0.9 is smaller after sugammadex than it is following administration of neostigmine. In one study of patients receiving sevoflurane anesthesia, neostigmine (50 mcg/kg) or sugammadex (2 mg/kg) was administered after spontaneous recovery to a train-of-four count of 2. Complete recovery to a TOFR of 0.9 occurred between 0.9 and 3.4 min following administration of sugammadex, a range of 2.5 min, and between 3.7 and 106.9 min in patients who had received neostigmine, a range of 103.2 min [12].
While sugammadex was approved for use in the European Union and Australia in 2008 and Asia in 2010, it was not approved for use in the USA by the Federal Drug Administration until 2015 because of concerns of reported hypersensitivity and anaphylactic reactions in some of the study subjects who had received it. This review focuses on defining and diagnosing hypersensitivity, hypersensitivity related to sugammadex, management of anaphylaxis, and its other poorly understood side effect: bradycardia.
Defining and Diagnosing Hypersensitivity and Anaphylaxis
While hypersensitivity refers to the constellation of symptoms related to an immune-type response, prior to 2006 there was no universally accepted definition of anaphylaxis. Without an accepted criterion, diagnosing and detecting anaphylaxis often involved subjective influence, making both research and patient care more challenging. In 2006, the National Institute of Allergy and Infectious Disease and Food Allergy and Anaphylaxis Network met to create an accepted definition of anaphylaxis. The Sampson criteria were created as a result of this meeting, providing a description of the distinct criteria to be used in diagnosing anaphylaxis. Anaphylaxis occurs in any of the following three scenarios:
-
1.
An acute onset of illness with involvement of the skin and/or mucosal tissue, and at least one of the following: Respiratory compromise, reduced blood pressure, or associated symptoms of end-organ dysfunction;
-
2.
Two or more of the following that occur rapidly after exposure to a likely allergen: Involvement of the skin-mucosal tissue, respiratory compromise, reduced blood pressure or associated symptoms, or persistent gastrointestinal symptoms;
-
3.
Reduced blood pressure after exposure to a known allergen defined as greater than a 30% decrease in systolic blood pressure from baseline or a systolic blood pressure of less than 90 mmHg in adults [13].
Familiarity with this focused classification allows for better and more rapid identification of anaphylaxis, treatment of life-threatening reactions, and adequate documentation of reactions. Additionally, it helps to avoid the unnecessary administration of prophylactic medications during future surgical procedures when anaphylaxis did not occur.
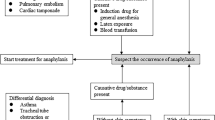
Previously, the classification of anaphylaxis was divided into anaphylactic and anaphylactoid reactions. Anaphylaxis was defined as an IgE-mediated reaction, while non-IgE-mediated reactions were defined as anaphylactoid. Since its redefinition in 2006, all IgE-mediated and non-IgE-mediated reactions are considered anaphylactic reactions [14]. As the definition of anaphylaxis has changed, it is also important to note that the severity of anaphylactic reactions varies greatly. As defined by Brown (Table 1), grade 1 is mild and involves skin and mucosal tissues, while grade 3 is severe, with symptoms involving cardiopulmonary collapse and neurologic symptoms, such as altered mental status and unresponsiveness [15]. Many of the severity scales require subjective observations or patient description and cannot be used in the perioperative period [16, 17]. Patients in the operating room may not present with cutaneous symptoms, such as itching, which can affect the interpretation and grading of severity of a reaction [18]. A more recent three-point grading system is more applicable for intraoperative diagnosis of anaphylaxis and directing treatment. Familiarity with and use of the severity scale developed by Rose [19••] (Table 1) is beneficial for the anesthesiologist because it allows for an objective measurement of symptoms, including blood pressure parameters and peak airway pressure cutoffs. In the grading scale described by Rose, anaphylactic reactions range from moderate, which are non-life-threatening, to severe, which are described as life-threatening, and complete cardiovascular collapse, requiring advance life support.
Identifying the Etiology of Anaphylaxis
Recognition of anaphylaxis-inducing mediators is important to prevent unnecessary avoidance of medications commonly used in anesthesia. Identification of allergens must be accessible, cost-effective, and precise. Currently, skin prick testing and intradermal testing (both involving injection of potential allergens) remain the gold standard for allergen testing [4••]. Current recommendations are to wait at least 4–6 weeks after hypersensitivity reactions to test potential allergens in order to avoid false negative reactions [20]. Newer methods of detecting hypersensitivity reactions are now being utilized more frequently. The basophil activating test has shown promise in helping to identify sugammadex as the etiology of an anaphylactic reaction without exposing a patient to the remote possibility of anaphylaxis during testing. The process involves incubating a patient’s basophils with dilutions of sugammadex and measuring the percentage of activated cell receptors. Currently, CD-63 and CD-203c markers have shown to have the most potential in identifying the causal agent of anaphylaxis with both a sensitivity and specificity greater than 90% [4••].
Although the ability of the basophil-activating test to detect allergens has increased greatly, its cost is prohibitive. While skin prick and intradermal tests can be performed in an office setting in a relatively cost-effective manner, the basophil-activating test requires blood sampling, off-site processing, and laboratory equipment, which significantly increases both the cost and the time of diagnosis. Additionally, despite having a high sensitivity for detection of anaphylaxis, there has been no consensus on how to standardize interpretation of the results of the basophil-activating test, allowing varied testing methodologies [21]. What may amount to a positive basophil activation test at certain institutions may be deemed indeterminate at others. Because of cost, relative inaccessibility to testing equipment, and varied testing processes of the basophil-activating test, the skin prick, and intradermal tests remain the first line of testing to determine if a substance is an allergen.
Tryptase, a marker of mast cell degranulation, and histamine are indicators that can be measured to assist in the diagnosis after a presumed anaphylactic reaction [22••, 23]. To be useful, tryptase levels should be drawn within 2 hours of the event. It is important to note that although tryptase and histamine levels can be helpful in supporting a diagnosis, levels within normal limits do not rule out anaphylaxis. In fact, tryptase levels can be normal, while prostaglandins and leukotrienes, other markers of anaphylaxis which are often not measured, can be elevated [24].
Common Causes of Hypersensitivity and Anaphylaxis in the Operating Room
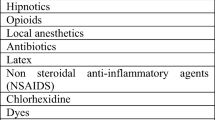
Perioperative hypersensitivity-related reactions and anaphylaxis are rare but serious events, and aminosteroidal NMBAs are the most common cause of intraoperative anaphylaxis in most countries. Antibiotics are the second most common cause of anaphylaxis in the USA, France, and Western Australia [25,26,27]. Anaphylaxis caused by aminosteroidal NMBAs is likely related to its quaternary ammonium structure, which is the probable cause of IgE cross-linking and basophil activation [25, 28••]. Interestingly, women are 2.5 times more likely than men to have an anaphylactic reaction to aminosteroidal NMBAs [29]. Two theories exist to potentially explain the higher incidence of anaphylaxis in women. It may be due to either chronic skin sensitization related to beauty products such as hair dye and shampoos, many of which have allergenic amines, or due to hormonal changes during puberty. Both theories are supported by what is a relatively equal rate of anaphylaxis between males and females in the pediatric population [29, 30]. Overall, anaphylaxis to steroidal NMBAs occurs in approximately 1 in 2500 individuals receiving either vecuronium or rocuronium [31].
Sugammadex approval by the US FDA was initially delayed due to concern of hypersensitivity and anaphylactic reactions; however, recent studies have found that sugammadex-associated anaphylaxis occurs infrequently. In fact, it appears that sugammadex has an equal, if not lower overall incidence of anaphylaxis than aminosteroidal NMBAs and antibiotics [4••, 18, 31, 32••, 33••]. Additionally, there seems to be a geographical variation of hypersensitivity and anaphylactic reactions to sugammadex [25]. In the USA, anaphylaxis to sugammadex occurs at a rate of approximately 1 in 3500–10,000, while in Japan, a country with high rates of sugammadex administration is estimated to be as low as 1 in 35,000 [31]. This reported low incidence may actually be somewhat higher, as there are likely cases that have either not been reported or where sugammadex is not recognized as the anaphylactic agent. The exact reason for this geographic variability in the incidence of anaphylaxis is unclear; one leading theory is a difference in diet. Cyclodextrin is used worldwide as a food preservative and ingestion of cyclodextrin may lead to sensitization to sugammadex [5, 25]. This theory is supported by the finding that almost all reported cases of hypersensitivity and anaphylaxis to sugammadex have occurred in patients who have never received the selective-relaxant binding agent in the past [5]. Individuals living in the USA generally consume more than 4 g of cyclodextrins per day. In Japan and other Asian countries where food preservatives are used less frequently, preoperative exposure to cyclodextrins is lower, decreasing the potential risk of hypersensitivity and anaphylaxis when compared with what has been observed in Western countries [8].
Hypersensitivity-Mechanism and Severity of Reactions
While there has been mixed evidence on the exact mechanism of anaphylaxis with regard to sugammadex, most anaphylactic reactions to intraoperative medications are either IgE- or mast cell-mediated. De Kam [34] randomized 382 healthy, non-anesthetized volunteers to receive either sugammadex (4 or 16 mg/kg) or placebo to assess the frequency with which non-anesthetized individuals developed hypersensitivity. Of the 298 volunteers who received sugammadex, eight developed hypersensitivity reactions. One met the criteria for a grade 3 anaphylactic reaction, the most severe level of anaphylaxis on the scale developed by Brown [15]. Those with hypersensitivity symptoms were assessed with skin testing, anti-sugammadex antibodies, and tryptase levels to evaluate for mast cell degranulation. On analysis of the volunteer records at the conclusion of the study, it appeared that protocol deviations in treatment of reactions to sugammadex may have introduced bias. De Kam’s conclusion was that the reaction was neither IgE- nor basophil/mast cell-mediated. Because of concern of study bias, a follow-up study, similar in design [22••] was conducted. In this multicenter study, Min [22••] looked at the impact of three repeated administrations of 4 mg/kg sugammadex, 16 mg/kg sugammadex, or placebo in 375 healthy, non-anesthetized individuals at 5-week intervals. Out of 299 volunteers receiving sugammadex, 25 exhibited grade I hypersensitivity reactions, according to Brown’s classification [15]. One volunteer developed grade III criteria. Follow-up testing found that no volunteers who experienced hypersensitivity either tested positive for IgE antibodies or had elevated tryptase levels immediately after the reactions. The tests for IgE antibodies were done 4–6 weeks after the hypersensitivity reactions, which do not allow sufficient time for antibodies to form, decreasing the possibility of a positive finding. Similar to De Kam’s study, hypersensitivity occurred in volunteers who had no prior history of exposure to sugammadex, the likelihood of developing a hypersensitivity reaction was not related to the dose of sugammadex, and there was no sensitization with repeated administration of sugammadex. In contrast to these two studies, a study by Horiuchi [4••] analyzed patients who had episodes of confirmed sugammadex-induced anaphylaxis and found that all tested positive for skin pick test or intradermal prick test with sugammadex. These investigators found that there was a dose-dependent up-regulation of basophil activity, specifically CD-63 and CD-203c when basophils were exposed to varying dilutions of sugammadex. Other clinical trials have validated these results, finding positive tryptase levels and skin prick and intradermal tests in patients who presented with hypersensitivity and anaphylactic reactions. A review of case studies of intraoperative anaphylactic reactions to sugammadex finds that almost every patient who has had an anaphylactic reaction had both a positive skin prick or intradermal test when assessed postoperatively and elevated tryptase levels [4••, 32••, 33••, 35, 36]. In contrast to the findings of studies in volunteers, the reported clinical cases of anaphylaxis and response to the basophil-activating test indicate that the hypersensitivity reactions to sugammadex are likely IgE- and/or basophil-mediated.
It is possible that the presence of rocuronium may also play a role in immunoglobulin formation and the development of anaphylaxis. Numerous studies have shown that while a patient may test negative for sugammadex or rocuronium alone, some patients who experienced reactions to sugammadex may test positive to the rocuronium–sugammadex complex [37,38,39,40]. Additionally, a likely hypersensitivity reaction to the rocuronium–sugammadex complex has been suggested as the cause of coronary vasospasm triggered by anaphylaxis, a phenomenon known as Kounis syndrome. In the case report, the patient who developed this reaction tested positive for the rocuronium–sugammadex complex but negative for either rocuronium or sugammadex alone, further supporting the hypothesis that the sugammadex–rocuronium complex may be an allergen [41••]. There have also been reports of individuals testing positive for photo-denatured sugammadex after an earlier negative skin prick or intradermal test for sugammadex. The denatured complex, without its side chains, shares a closer resemblance to the gamma-cyclodextrin structure used in food emulsification than non-denatured sugammadex, causing it to be a more potent allergen [36].
Sugammadex Anaphylaxis Management
Although sugammadex has a favorable safety profile and low incidence of anaphylaxis compared with other frequently used intraoperative medications, delayed recognition of anaphylaxis and inadequate treatment is a cause for significant intraoperative morbidity. Intraoperative mortality of patients experiencing anaphylaxis can be as high as 1–9% and morbidity, including prolonged hospitalization and postoperative cardiopulmonary complications, can be as great as one third of patients who have had anaphylaxis [25, 30, 35, 42]. In all case reports of anaphylaxis after sugammadex, the anaphylaxis consistently presented within 5–10 min of its administration, similar to the timeframe of anaphylaxis caused by other medications [43].
While sugammadex-induced anaphylaxis should be relatively rapidly recognized and treated, since it is most often given upon emergence and within moments of extubation, it can be difficult to diagnose in the patient emerging from anesthesia. Subtle indicators of anaphylaxis are more likely to be rapidly identified in an awake patient. Emergence from anesthesia presents a unique challenge in diagnosing anaphylaxis. Hypertension and variability in heart rate that are likely to be present on emergence can confound the diagnosis of anaphylaxis. Additionally, increased airway pressures associated with anaphylaxis can be misidentified as inadequate anesthesia and coughing. Post-anesthesia, awake patients who have had an unrecognized hypersensitivity response to sugammadex may present with wheezing and airway edema requiring medical management and possibly reintubation.
Bronchospasm has been reported to occur in patients with a history of pulmonary disease after the administration of sugammadex [44]. Patients without a history of pulmonary disease may also develop bronchospasm after the administration of sugammadex at the conclusion of a desflurane anesthetic [45, 46]. While it is possible that irritant properties of desflurane may exacerbate bronchospasm caused by a hypersensitivity reaction to sugammadex, the cause of this response has not yet been explored.
As with all other presumed cases of anaphylaxis, treatment of anaphylaxis after administration of sugammadex is mainly supportive with the goal of temporizing cardiopulmonary symptoms and decreasing the progression of symptoms due to ongoing release of mediators. Epinephrine is the first line of therapy in suspected anaphylaxis [47], administered in 5–10 mcg boluses and titrated to effect. Its alpha-adrenergic activity increases peripheral vascular tone, reverses peripheral vasodilation, and increases blood pressure. Its beta-1 activity increases the rate and strength of cardiac contraction, and its beta-2 adrenergic stimulation inhibits further inflammatory mediator release from mast cells and basophils [48], in addition to causing bronchodilation [49]. Beta-2 adrenergic agonists, such as albuterol, may also be administered for treatment of bronchospasm. In the setting of persistent hypotension, fluid administration and additional vasopressors are often necessary depending on the clinical picture and severity of the reaction. While not proven to improve outcomes in placebo-controlled trials, steroid administration at time of presentation has been suggested to help prevent delayed recurrence of anaphylaxis [13]. A combination of H1 and H2 antagonists may also have a secondary role in the treatment of anaphylaxis [50].
Sugammadex-Related Bradycardia and Cardiopulmonary Collapse
This review would not be complete without mentioning an additional adverse reaction to sugammadex. Bradycardia was first noted as a side effect of administration during pre-market trials. Since receiving drug approval there have been continued case reports of patients having both transient and severe bradycardic episodes, occasionally resulting in asystole requiring CPR and utilization of a transient pacemaker [51, 52]. Between 2009 and 2017, 138 adverse cardiac events, including bradycardia and transient heart block, and nine deaths were reported after administration of sugammadex [7••, 52]. The majority of these patients had no prior history of cardiopulmonary disease that would have rendered them more susceptible to develop transient heart block. The cause of sugammadex-induced bradycardia remains unknown. One case report noted that severe bradycardia occurred after the administration of sugammadex in a 10-year old boy who previously had an orthotopic heart transplantation [53••]. Since transplantation denervates the heart, sugammadex-induced bradycardia is likely not to be parasympathetic-mediated. Bradycardia may be induced by complement activation-related cardiac anaphylaxis by C5a, a mechanism described by Szebeni [54]; however, this has not been proven in patients or volunteers or with sugammadex. The response occurs sporadically and no evidence exists of measures to avoid it, including pretreatment with antimuscarinics. Bradycardia is typically successfully treated with the administration of atropine.
Although bradycardia carries a significant risk of morbidity and mortality if inadequately managed, the overall incidence of bradycardia after sugammadex is very low. A review by Hristovska [6••] of the safety of different methods of reversal of neuromuscular blockade found that patients who received sugammadex had significantly fewer episodes of bradycardia when compared with those who received neostigmine. This is likely due to the administration of an inadequate dose of antimuscarinic with the anticholinesterase, mistiming of its administration, or an exaggerated response to neostigmine. The incidence of critical adverse events associated with the administration of sugammadex or neostigmine was similar.
Sugammadex has been used safely in patients with a history of heart transplantation. Reinnervation of the heart is known to occur after transplantation; however, it is dependent on the time since transplantation and extent of dissection. Sugammadex has been used successfully in surgeries after transplantation to assist in preventing unpredictable responses of hemodynamic changes which can occur with neostigmine [55, 56]. Kizilay [57] found that the use of sugammadex in cardiac patients undergoing noncardiac surgery caused less hemodynamic variability when compared with similar patients receiving neostigmine. In general, sugammadex appears to have a more favorable hemodynamic profile than reversal of neuromuscular blockade with an anticholinesterase—which can be beneficial in critically ill patients undergoing complex surgical procedures.
Conclusion
Sugammadex, a gamma-cyclodextrin, provides a novel means of reversing the action of NMBAs by binding rocuronium and vecuronium so that they are unable to gain access to the acetylcholine receptors of the neuromuscular junction. Since its introduction into clinical practice, sugammadex has transformed the anesthesiologist’s approach to reversal of neuromuscular blockade. While it allows for quicker and more predictable reversal of paralysis, its administration, like that of many other medications, may have unintended consequences and drug-induced anaphylaxis remains a concern. The incidence of sugammadex-induced anaphylaxis is low, approximately 1 in 3500 in the USA. Based upon case reports and post-operative testing, including basophil-activating test, anaphylaxis is likely IgE- or basophil-mediated. Skin prick testing remains the gold standard for confirming hypersensitivity related to sugammadex.
Although the overall risk of anaphylaxis is similar to other commonly used drugs, failure to diagnose an anaphylactic reaction can be a cause of increased morbidity. Familiarity with the Sampson criteria for anaphylaxis and rapid recognition of the development of an anaphylactic response to sugammadex is paramount for appropriate and effective treatment of the adverse medication reaction. Understanding the different anaphylaxis severity scales may aid in selecting appropriate interventions based on symptoms and presentation.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Griffith HRJ, G.E. The use of curare in general anesthesia. J Anesth. 1942;3(4):418–20.
Takazaka TM, Miyasaka K, Sawa T, Iida H. The current status of sugammadex usage and the occurrence of sugammadex-induced anaphylaxis in Japan. APSF Newsletter. 2018;33 at: www.apsf.org/newsletter/june-2018 (accessed February 2020).
Godai K, Hasegawa-Moriyama M, Kuniyoshi T, Kakoi T, Ikoma K, Isowaki S, et al. Three cases of suspected sugammadex-induced hypersensitivity reactions. Br J Anaesth. 2012;109(2):216–8.
•• Horiuchi T, Yokohama A, Orihara M, Tomita Y, Tomioka A, Yoshida N, et al. Usefulness of Basophil Activation Tests for Diagnosis of Sugammadex-Induced Anaphylaxis. Anesth Analg. 2018;126(5):1509–16 This article discusses the use of basophil activation tests for identification and diagnosis of anaphylactic mediators, as well as provide laboratory evidence that there is upregulation of basophil cell receptors, supporting that reactions to sugammadex are likely anaphylactic in nature.
Hotta E, Tamagawa-Mineoka R, Masuda K, Taura M, Nakagawa Y, Kanehisa F, et al. Anaphylaxis caused by gamma-cyclodextrin in sugammadex. Allergol Int. 2016;65(3):356–8.
•• Hristovska AM, Duch P, Allingstrup M, Afshari A. Efficacy and safety of sugammadex versus neostigmine in reversing neuromuscular blockade in adults. Cochrane Database Syst Rev. 2017;8:Cd012763 This Cochrane database review summarizes the safety profile of sugammadex in relation to patient safety, critical events, hemodynamic changes, and overall safety versus neostigmine. The article provides evidence that sugammadex has an overall favorable profile and similar rates of critical events and lower overall risk of bradycardia.
•• Hunter JM, Naguib M. Sugammadex-induced bradycardia and asystole: how great is the risk? Br J Anaesth. 2018;121(1):8–12 In this report the authors summarize the data regarding either anaphylaxis or bradycardia after the administration of sugammadex and note that the reporting of adverse events is more common after the release of a new medication than it is for one that has been used for years. This practice may have resulted in the apparently greater incidence of reported serious cardiac events observed with sugammadex since 2016.
Ue KL, Kasternow B, Wagner A, Rutkowski R, Rutkowski K. Sugammadex: an emerging trigger of intraoperative anaphylaxis. Ann Allergy Asthma Immunol. 2016;117(6):714–6.
Khirwadkar R, Hunter JM. Neuromuscular physiology and pharmacology: an update. Continuing Education in Anaesthesia, Critical Care & Pain. 2012;12(5):237–44.
Gijsenbergh F, Ramael S, Houwing N, van Iersel T. First human exposure of org 25969, a novel agent to reverse the action of rocuronium bromide. Anesthesiology. 2005;103(4):695–703.
Abad-Gurumeta A, Ripolles-Melchor J, Casans-Frances R, Espinosa A, Martinez-Hurtado E, Fernandez-Perez C, et al. A systematic review of sugammadex vs neostigmine for reversal of neuromuscular blockade. Anaesthesia. 2015;70(12):1441–52.
Blobner M, Eriksson LI, Scholz J, Motsch J, Della Rocca G, Prins ME. Reversal of rocuronium-induced neuromuscular blockade with sugammadex compared with neostigmine during sevoflurane anaesthesia: results of a randomised, controlled trial. Eur J Anaesthesiol. 2010;27(10):874–81.
Sampson HA, Munoz-Furlong A, Campbell RL, Adkinson NF Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report--second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006;47(4):373–80.
Simons FE, Ardusso LR, Bilo MB, Cardona V, Ebisawa M, El-Gamal YM, et al. International consensus on (ICON) anaphylaxis. World Allergy Organ J. 2014;7(1):9.
Brown SG. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004;114(2):371–6.
Kroigaard M, Garvey LH, Gillberg L, Johansson SG, Mosbech H, Florvaag E, et al. Scandinavian clinical practice guidelines on the diagnosis, management and follow-up of anaphylaxis during anaesthesia. Acta Anaesthesiol Scand. 2007;51(6):655–70.
Ring J, Messmer K. Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet. 1977;1(8009):466–9.
Matsumura T, Mitani S, Fukayama H. Sugammadex-induced anaphylaxis involving sudden onset of severe abdominal pain. J Clin Anesth. 2019;57:119–20.
•• Rose MA, Green SL, Crilly HM, Kolawole H. Perioperative anaphylaxis grading system: 'making the grade'. Br J Anaesth. 2016, 117;(5):551–3 This article outlines a grading system to assist anesthesiologists in perioperative diagnosis of anaphylaxis and its severity. The grading system uses objective measurements such as respiratory and cardiovascular parameters to identify and categorize the severity of a reaction.
Heinzerling L, Mari A, Bergmann KC, Bresciani M, Burbach G, Darsow U, et al. The skin prick test - European standards. Clin Transl Allergy. 2013;3(1):3.
McGowan EC, Saini S. Update on the performance and application of basophil activation tests. Curr Allergy Asthma Rep. 2013;13(1):101–9.
•• Min KC, Bondiskey P, Schulz V, Woo T, Assaid C, Yu W, et al. Hypersensitivity incidence after sugammadex administration in healthy subjects: a randomised controlled trial. Br J Anaesth. 2018;121(4):749–57 In this trial, hypersensitivity occurring in response to sugammadex administration did not require previous exposure to sugammadex and was unrelated to the dose of sugammadex.
Payne V, Kam PC. Mast cell tryptase: a review of its physiology and clinical significance. Anaesthesia. 2004;59(7):695–703.
Colak A, Yilmaz E, Kiray BK. Sugammadex-induced hypersensitivity reaction in a pediatric patient. Turk J Anaesthesiol Reanim. 2018;46(1):66–8.
Mertes PM, Ebo DG, Garcez T, Rose M, Sabato V, Takazawa T, et al. Comparative epidemiology of suspected perioperative hypersensitivity reactions. Br J Anaesth. 2019;123(1):e16–28.
Tacquard C, Collange O, Gomis P, Malinovsky JM, Petitpain N, Demoly P, et al. Anaesthetic hypersensitivity reactions in France between 2011 and 2012: the 10th GERAP epidemiologic survey. Acta Anaesthesiol Scand. 2017;61(3):290–9.
Sadleir PH, Clarke RC, Bunning DL, Platt PR. Anaphylaxis to neuromuscular blocking drugs: incidence and cross-reactivity in Western Australia from 2002 to 2011. Br J Anaesth. 2013;110(6):981–7.
•• Harper NJN, Cook TM, Garcez T, Farmer L, Floss K, Marinho S, et al. Anaesthesia, surgery, and life-threatening allergic reactions: epidemiology and clinical features of perioperative anaphylaxis in the 6th National Audit Project (NAP6). Br J Anaesth. 2018;121(1):159–71 In this retrospective analysis the incidence of perioperative anaphylaxix was found to be approximately 1/10,000 anesthetics.
Mertes PM, Alla F, Trechot P, Auroy Y, Jougla E. Groupe d'Etudes des reactions Anaphylactoides P. anaphylaxis during anesthesia in France: an 8-year national survey. J Allergy Clin Immunol. 2011;128(2):366–73.
McDonnell NJ, Pavy TJ, Green LK, Platt PR. Sugammadex in the management of rocuronium-induced anaphylaxis. Br J Anaesth. 2011;106(2):199–201.
Binczak M, Fischler M, Le Guen M. Efficacy of Sugammadex in preventing skin test reaction in a patient with confirmed Rocuronium anaphylaxis: a case report. A A Pract. 2019;13(1):17–9.
•• Miyazaki Y, Sunaga H, Kida K, Hobo S, Inoue N, Muto M, et al. Incidence of Anaphylaxis Associated With Sugammadex. Anesth Analg. 2018;126(5):1505–8 This retrospective analysis found the incidence of sugammadex-associated anaphylaxix to be approximately 0.039% - similar to that for succinylcholine or rocuronium.
•• Min KC, Woo T, Assaid C, McCrea J, Gurner DM, Sisk CM, et al. Incidence of hypersensitivity and anaphylaxis with sugammadex. J Clin Anesth. 2018;47:67–73 This study found that the incidence of hypersensitivity or anaphylaxis to sugammadex in patients receiving general anesthesia was the same as that t placebo or neostigmine.
de Kam PJ, Nolte H, Good S, Yunan M, Williams-Herman DE, Burggraaf J, et al. Sugammadex hypersensitivity and underlying mechanisms: a randomised study of healthy non-anaesthetised volunteers. Br J Anaesth. 2018;121(4):758–67.
Jeyadoss J, Kuruppu P, Nanjappa N, Van Wijk R. Sugammadex hypersensitivity-a case of anaphylaxis. Anaesth Intensive Care. 2014;42(1):89–92.
Yamada T, Suzuki T, Murase R, Nagata H, Kosugi S. Anaphylactic reactions to native and light-exposed Sugammadex suggested by basophil activation test: a report of 2 cases. A A Pract. 2018;11(7):181–3.
Baldo BA. Anaphylaxis caused by sugammadex- rocuronium inclusion complex: what is the basis of the allergenic recognition? J Clin Anesth. 2019;54:48–9.
Orihara M, Takazawa T, Saito S. Is sugammadex alone sufficient to cause anaphylaxis? J Anesth. 2018;32(2):307.
Takazawa T, Mitsuhata H, Mertes PM. Sugammadex and rocuronium-induced anaphylaxis. J Anesth. 2016;30(2):290–7.
Yamaoka M, Deguchi M, Ninomiya K, Kurasako T, Matsumoto M. A suspected case of rocuronium-sugammadex complex-induced anaphylactic shock after cesarean section. J Anesth. 2017;31(1):148–51.
•• Okuno A, Matsuki Y, Tabata M, Shigemi K. A suspected case of coronary vasospasm induced by anaphylactic shock caused by rocuronium-sugammadex complex. J Clin Anesth. 2018;48:7 The authors describe an allergic response to the sugammadex-rocuronium complex that resulted in coronary vasospasm. The diagnosis of Kounis syndrome was delayed because when the vasospasm occurred 2 years prior to the second administration of sugammadex, the allergenic potential of the complex had not been appreciated.
Yamada Y, Yamamoto T, Tanabe K, Fukuoka N, Takenaka M, Iida H. A case of anaphylaxis apparently induced by sugammadex and rocuronium in successive surgeries. J Clin Anesth. 2016;32:30–2.
Tsur A, Kalansky A. Hypersensitivity associated with sugammadex administration: a systematic review. Anaesthesia. 2014;69(11):1251–7.
Amao R, Zornow MH, Cowan RM, Cheng DC, Morte JB, Allard MW. Use of sugammadex in patients with a history of pulmonary disease. J Clin Anesth. 2012;24(4):289–97.
Baronos S, Selvaraj BJ, Liang M, Ahmed K, Yarmush J. Sugammadex-induced bronchospasm during desflurane anaesthesia. Br J Anaesth. 2019;123(1):e155–e6.
Eskander JP, Cornett EM, Stuker W, Fox CJ, Breehl M. The combination of sugammadex and desflurane may increase the risk of bronchospasm during general anesthesia. J Clin Anesth. 2017;41:73.
Barach EM, Nowak RM, Lee TG, Tomlanovich MC. Epinephrine for treatment of anaphylactic shock. JAMA. 1984;251(16):2118–22.
Stoloff R, Adams SL, Orfan N, Harris KE, Greenberger PA, Patterson R. Emergency medical recognition and management of idiopathic anaphylaxis. J Emerg Med. 1992;10(6):693–8.
Brown AF. Anaphylactic shock: mechanisms and treatment. J Accid Emerg Med. 1995;12(2):89–100.
Brown AF. Therapeutic controversies in the management of acute anaphylaxis. J Accid Emerg Med. 1998;15(2):89–95.
Bhavani SS. Severe bradycardia and asystole after sugammadex. Br J Anaesth. 2018;121(1):95–6.
Saito I, Osaka Y, Shimada M. Transient third-degree AV block following sugammadex. J Anesth. 2015;29(4):641.
•• King A, Naguib A, Tobias JD. Bradycardia in a Pediatric Heart Transplant Recipient: Is It the Sugammadex? J Pediatr Pharmacol Ther. 2017;22(5):378–81 This report describes the occurrence of profound bradycardia in a 10 year-old heart transplant recipient who had received sugammadex at the conclusion of surgery. The bradycardia responded to a small dose of epinephrine (2 mcg/kg).
Szebeni J, Baranyi L, Savay S, Bodo M, Milosevits J, Alving CR, et al. Complement activation-related cardiac anaphylaxis in pigs: role of C5a anaphylatoxin and adenosine in liposome-induced abnormalities in ECG and heart function. Am J Physiol Heart Circ Physiol. 2006;290(3):H1050–8.
Tezcan B, Saylan A, Bolukbasi D, Koculu R, Karadeniz U. Use of Sugammadex in a heart transplant recipient: review of the unique physiology of the transplanted heart. J Cardiothorac Vasc Anesth. 2016;30(2):462–5.
Varela N, Golvano M, Perez-Pevida B. Safety of Sugammadex for neuromuscular reversal in cardiac transplant patients. J Cardiothorac Vasc Anesth. 2016;30(4):e37.
Kizilay D, Dal D, Saracoglu KT, Eti Z, Gogus FY. Comparison of neostigmine and sugammadex for hemodynamic parameters in cardiac patients undergoing noncardiac surgery. J Clin Anesth. 2016;28:30–5.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neuromuscular Blockade
Rights and permissions
About this article
Cite this article
Gregory, R.J., Woehlck, H. & Lien, C.A. Sugammadex and Hypersensitivity-Related Reactions: a Review. Curr Anesthesiol Rep 10, 123–130 (2020). https://doi.org/10.1007/s40140-020-00390-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40140-020-00390-w