Abstract
Purpose of Review
Maxillectomy for ablative surgery results in defects with significant functional and cosmetic morbidity. The hard or soft palate, dental arch, alveolus, nose, zygoma, malar process, or orbits may be involved, each with attendant considerations. Prosthodontic rehabilitation with a metallic and acrylic obturator has classically been used to replace missing teeth and separate the oral cavity from the nasal cavity and maxillary sinuses. Microvascular free tissue transfer has emerged as the current mainstay of treatment for patients needing composite reconstruction, especially where bony support is needed.
Recent Findings
Technical refinements in skin paddle design, muscle flap orientation, and bone fixation have dominated the literature over the past two decades. Recently, advances in microvascular techniques using virtual surgical planning and navigation-guided implant surgery have improved predictability of dental rehabilitation.
Summary
The current state of the art remains comprehensive functional and cosmetic rehabilitation, comprising facial support, definitive obturation of the oronasal and/or oroantral defect, and functional dental occlusion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The maxilla comprises a large portion of the facial skeleton and occupies the majority of the region termed the midface. The midface is made up of fifteen bones and provides support to the orbits, malar processes, external nose, and upper dentition. Due to its central location in the face, reconstruction of defects caused by trauma, congenital deformities, or neoplasms poses many challenges. The three vertical buttresses of the face—the nasomaxillary, zygomaticomaxillary, and pterygomaxillary—support mastication by directing chewing forces superiorly, toward the skull base and frontal bar [1].
Maxillectomy is a general term used to describe an operation performed on any portion of the midface for the purposes of neoplasm ablation. It may create a primarily oral cavity defect, a through-and-through skin defect with concomitant nasal, oral, or sinus mucosal defect, or involve orbital exenteration with anterior skull base defect. The use “maxillectomy” is a misnomer, as rarely does resection of a midfacial neoplasm result in a defect isolated only to the bony maxilla; rather, the frontal, nasal, lacrimal, ethmoid, vomer, or palatine bones may be involved. Regardless of the extent of the defect, the aim in maxillectomy reconstruction is to preserve—or restore to a premorbid state—ideal facial form and occlusal function. To accomplish this objective, a multispecialty team consisting of a head and neck reconstructive surgeon, maxillofacial prosthodontist, and speech language pathologist work together to optimize the patient’s reconstructive outcomes related to speech and swallowing.
General Goals of Maxillectomy Defect Reconstruction
-
Separate nasal cavity from oral cavity
-
Support globe, nose, cheek, upper lip, and facial soft tissues
-
Re-establish bony and soft-tissue foundation for ideal mastication
-
Maintain adequate mouth opening for chewing, speaking, and insertion/removal of a dental prosthesis
-
Provide a stable bony platform for dental implants if possible
-
Maintain patent nasal airway
-
Obliterate surgical dead space
-
Seal dura if violated during resection
-
Prepare the patient and wound bed for adjuvant radiotherapy when indicated [2, 3••].
Background
Classification of Maxillectomy Defects
Classification systems have been developed across disciplines (oral and maxillofacial surgery, otolaryngology, and prosthodontics) to aid in donor site selection and treatment planning. Attempts to clarify and classify maxillectomy defects help guide surgeons, ancillary staff, patients, and third-party payers in understanding the nature of the procedure and its implications. The number of classification systems in the literature speaks to the complex nature of cosmetic, functional, and social difficulties encountered following maxillectomy. All head and neck surgery patients experience some degree of social isolation, but maxillectomy patients who experience difficulties eating in public, speaking, and swallowing commonly report depression and anxiety as they recover from their disease [4••]. At conversational speaking distance, the deficits of the maxillectomy are highlighted even more, and the ability to socialize over meals or other regular social activities becomes impaired. Preoperative reconstructive planning has been shown to optimize postoperative speech, chewing, and swallowing outcomes. Various quality-of-life outcome assessments consistently cite these factors: speech, chewing, and swallowing—as the three most important components of a patient’s quality of life. By definition, each of these may be impaired in the maxillectomy when the defect involves the oral cavity [5–7].
The Functional Intraoral Glasgow scale (FIGS) is a method for predicting the degree of expected morbidity from ablative surgery based on the site of the oral cavity involved. Similar to the well-known Glasgow coma scale, a 3-part system is used to score a patient’s speech, chewing, and swallowing on a score of 1–5, yielding an overall score of 3–15. Based on the subsite included in the resection, an appropriate locoregional or free flap versus obturator may be selected to mitigate the anticipated functional losses. A correlation between resection size and functional outcome was identified, and patients who received postoperative radiotherapy fared worse overall [8]. Using these and other predictive models paired with specific reconstructive goals may help patients better understand their expected limitations postoperatively, and attempt to understand the need for intentional therapy interventions and social support.
Description of Classification Systems
Several classification schemes attempt to stratify maxillectomy defects using language suitable for both the prosthodontist and head and neck surgeon. The Memorial Sloan-Kettering, MacGregor and MacGregor, and Aramany systems were initially used, but lacked the descriptive components meaningful to both groups [9–11]. For surgeons, maintenance of the orbital floor and globe and facial soft-tissue support influence decision making for reconstructive surgery and selection of an appropriate flap. Prosthodontists, on the other hand, are concerned primarily with the configuration of dentition present for anchorage and retention and available soft-tissue support. Cordeiro and Santamaria simplified the Spiro et al. classification system and developed an algorithm for reconstruction based on a four-part classification system, termed type I—limited, type II—subtotal, type IIIa—total maxillectomy with preservation of orbital contents, type IIIb—total maxillectomy with orbital exenteration, and type IV—orbitomaxillectomy. They described the maxilla as a six-walled hexahedrium, with the antrum occupying its center. Overall, the osteocutaneous radial forearm free flap was favored for reconstruction of type I and II defects, and a bulky myocutaneous free flap such as the rectus abdominus was preferred for type IIIa, IIIb, and IV [12••]. The algorithm was modified and validated in their 2012 retrospective reviews, demonstrating acceptable speech, diet, globe position, oral competence, microstomia, and esthetics in a fifteen-year period. Despite well recognized by head and neck surgeons, the Cordeiro system does not include prosthodontic facial or oral cavity restorations, limiting its usefulness among all members of the multispecialty head and neck oncology team [13, 14]. Okay et al. introduced a prosthodontically driven classification in their retrospective review of forty-seven patients treated at Mount Sinai Medical Center. Similar to the Brown system (see below), it focuses on the horizontal orientation of remaining sound teeth and bone for retention of a tooth and implant-borne prosthesis. As in the oromandibular classification system published by the same unit, the Okay system uses subclassifications and modifiers, which has limited its widespread adoption on multidisciplinary teams [15••].
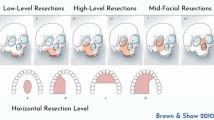
The Brown-modified maxillectomy classification is perhaps the system best known for bridging the “language barrier” between surgeons and prosthodontists. Introduced in 2000, it was reprised in 2010 as a six-subgroup schema comprising the vertical (numbered 1–6) and horizontal (a–d) extent of the resection, with the goal of predicting the functional and esthetic sequelae of ablative surgery [3••, 16] (Table 1).
Brown Class I: Subnasal/subantral alveolectomy (Ib–d) or central palatectomy (Ia) defects may be successfully treated with obturators, or fasciocutaneous flaps such as the radial forearm free flap or pedicled flaps such as the buccal fat pad, palatal island, or temporalis flap. The palatal island flap provides up to half of the hard palate mucosa for soft-tissue reconstruction based on the greater palatine artery. The denuded hard palate is allowed to heal by secondary intention for a period of 6–8 weeks, during which time a custom plastic surgical stent reduces postoperative morbidity and pain. Use of the palatal island flap is relatively contraindicated in reconstruction in postoperative radiotherapy defects because the exposed palatal bone is at increased risk of developing osteoradionecrosis [17]. The temporalis flap was introduced in 1895 and has been described for interpositional functional reconstruction of the articular disk of the temporomandibular joint as well as reconstruction of the palate, tongue, retromolar trigone, orbital floor (with pedicled coronoid process), and tongue defects [18]. Complications of flap harvest include temporal hollowing and injury to the temporal branch of the facial nerve in around 3 % of cases [12••]. Another simple pedicled flap option for defects <3 cm is the buccal fat pad flap, first described by Marie-Francois-Xavier Bichat as a circumscribed fatty appendage in 1801. The arterial supply to the buccal fat pad flap arises from the transverse facial artery, the buccal and deep temporal branches of the internal maxillary artery and, occasionally, inferiorly based branches of the facial artery [19]. The flap is mobilized by incising the periosteum overlying the zygomaticomaxillary buttress in the region of the maxillary tuberosity. Blunt dissection is then used to mobilize the flap into the oral cavity defect, and care is taken to avoid separating the fatty lobules from the intervening fascia and vascular stroma. Within 3–4 weeks epithelialization is noted, and the fat pad is typically well healed within 6–8 weeks.
Brown Class II: Oronasal or oroantral fistulae are reconstructed with vascularized bone flaps if the defect is anterior to or including the canine tooth, or if dental implants are planned. Of 147 cases, Brown and Shaw reported the use of bone-containing free flaps in the majority of cases [16]. Options for bone-containing free flaps include the deep circumflex iliac artery (DCIA), scapula, and fibula free flap (FFF), all of which provide bone for vertical facial support and a platform for a dental implant-supported prosthesis. Class IIb defects are reconstructed with a straight segment of bone, with the primary goal of implant placement. Support of the nasal tip and alar base is key in class IIc and IId defects. To support the fibula neoalveolus, struts of nonvascularized fibula or cadaveric rib may be used where inadequate support at the pyriform rims remains. Another option is the DCIA flap, which may be contoured to precisely fit the defect without the need for additional vertical support at the facial buttresses. The scapular tip is also well suited, in particular for premaxillary reconstruction, supporting the upper lip by reproducing the effect of the anterior nasal spine [20]. The submental artery island perforator flap was described by Patel et al. [21] for use in IIc defects, where harvest of the lingual cortex of the inferior mandible provides vascularized bone and soft tissue (Figs. 1, 2).
a Infrastructure maxillectomy for treatment of T4aN0M0 squamous cell carcinoma of the right alveolar ridge results in Brown 3b defect. b Osteoseptocutaneous fibula free flap is used to support nasal floor and midface and provide platform for implant-supported dental prosthesis. c, d Facial support and symmetry is maintained in early postoperative views
Brown Class III: Defects including the orbital floor, malar process, and alveolus are termed class III. The primary goal in reconstruction of class III defects is to maintain globe position and prevent diplopia resulting from hypoglobus or enophthalmos. Rarely can a class III defect be reconstructed completely with a single flap alone, rather, orbital floor reconstruction using titanium mesh (covered by vascularized soft tissue), cranial bone grafts, or pedicled mandibular coronoid process is employed with an osseous flap. Fibula, osteocutaneous radial forearm, DCIA, and scapula have all been used extensively, each with unique features. Despite limited in its application (short pedicle length, donor site morbidity including abdominal hernia formation), the DCIA may be contoured to fit to replace the orbital rim, support the cheek, and reconstruct the alveolus with adequate bone for dental implant placement [16]. Defects involving the zygomaticomaxillary buttress were reconstructed successfully in 24 patients using the osteocutaneous radial forearm free flap with mesh used for the orbital floor when indicated. Excellent speech and swallowing function (80 %) was noted, and most patients were able to resume normal daily activities without restriction [22].
Overall, in terms of versatility, the subscapular system of flaps provides the greatest number of options for head and neck reconstruction: fasciocutaneous (scapular/parascapular/thoracodorsal artery perforator), osteofasciocutaneous (scapular/parascapular with lateral scapular border or scapular tip), musculocutaneous (latissimus dorsi/serratus anterior), and musculo-osteocutaneous (latissimus dorsi/serratus anterior + rib). Vascularized bone, muscle, fat, skin, and fascia may be transferred as individual components or combined as a chimeric “mega flap” on a single vascular pedicle, with each component able to be placed on disparate axes [20]. Swartz et al. first described using the lateral border and lateral border + tip of the scapula for reconstruction in 26 cases of maxillectomy and composite mandibular defects [23]. The angular artery and its relationship with the scapular tip were later described by Deraemaecker [24], with Coleman and Sultan describing use of the scapular tip for midface and mandibular reconstruction [25].
Brown Class IV: When orbital exenteration is performed, there is less concern for bony reconstruction and facial support; emphasis is shifted to preparing the patient for adjuvant therapy, sealing any potential cerebrospinal fluid leaks, and minimizing postoperative contracture within the orbit. Free flap options include bipaddled latissimus dorsi, rectus abdominus, anterolateral thigh, and DCIA. In the long term, fasciocutaneous perforator flaps with minimal muscle bulk demonstrate less contracture and contour deformity than myocutaneous or myofascial options. Yetzer and Fernandes reported 21 patients with orbitomaxillary defects, 85 % of which were reconstructed with vascularized free flaps. Composite flaps were used less frequently than soft-tissue flaps, even when the hard palate was absent as in class IV defects. Prosthetic rehabilitation was carried out in two of these patients using zygomatic implants [26•, 27].
Brown Class V: Solitary orbitomaxillary defects are among the most straightforward to reconstruct, though concern for cerebrospinal fluid leakage may necessitate a bulkier myocutaneous or fasciocutaneous perforator flap. A concave external surface is needed for the prosthodontist to fabricate an orbital prosthesis, which may require one or more debulking procedures to achieve the desired contour.
Brown Class VI: The isolated nasoethmoid defect, with intact overlying skin, may not require reconstruction unless a high risk of cerebrospinal fluid leak is present. Nasomaxillary defects, however, require reconstruction either by a prosthesis or a bone-supported vascularized flap. Composite osteocutaneous radial forearm [13, 14, 16] and fasciocutaneous forearm covering a calvarial strut [26•] may be used to reconstruct the nasal dorsum and sidewalls and appropriate position the new nasal radix.
Obturator Reconstruction
For decades, the standard in maxillectomy defect reconstruction has been the prosthetic obturator. Obturators are removable appliances comprised of two main parts: a horizontally oriented, rigid metallic framework with a lightweight, smooth (often hollow) acrylic bulb that separates the oral cavity from the nasal cavity and antrum. In the short term, obturators are relatively inexpensive when compared to free flaps and are advantageous for patients with medical comorbidities precluding microvascular reconstruction. Prior to the advent of advanced CT and PET-CT imaging, it was felt an obturator provided better oncologic surveillance, as removal provided unrestricted examination of the tumor bed to aid in detection of an early recurrence; however, this has been refuted [28, 29]. Other advantages include lower cost, the ability to modify the device easily and quickly, and shortened surgical and hospital time [30]. Disadvantages include lack of access to a qualified prosthodontist in many regions, ongoing lifetime costs associated with obturator repair, remake, and revision, the need for manual dexterity to remove the device, and the need for adequate oral opening to place and remove the device. Impressions must also be made. When comparing quality of life in patients reconstructed with a free flap versus an obturator, outcomes are similar but tend to favor reconstruction [31–33]. As for every prosthetic device, retention, stability, and support must be present for an obturator to function properly [34]. The Obturator Functioning Scale (OFS) was introduced by Kornblith et al. to characterize speech, eating, and cosmetic outcomes in patients who had a maxillectomy (Figs. 3, 4).
In 47 patients, the most important predictor of quality of life and of the patient’s perception of their adjustment to the socioeconomic impact of cancer on their lives was good fit and function of the device. Specific qualities of the obturator function cited were the ability to chew and swallow, maintenance of voice quality, and little difficulty in pronouncing words [35]. Irish et al. cited leakage with oronasal regurgitation when attempting to swallow foods as the most common problem reported with obturators. When poor seal, excessive weight and bulk of the bulb component, or lack of retention resulted in obturator dysfunction, patients tended to avoid social encounters, a powerful part of regaining one’s premorbid mental and emotional health [36]. Kreeft et al. evaluated 32 patients with respect to mouth opening, masticatory function, and swallowing. Mastication with a good-fitting obturator was felt to be similar to a standard complete denture used for acquired edentulism. Trismus secondary to surgical scarring and radiotherapy impacted self-reported speech and swallowing problems [37]. Schmidt and colleagues also confirmed radiation therapy to be the single most important prognostic factor in quality of life after maxillectomy, based on reduced quantity of saliva and speech and appearance [38]. These and other studies confirm that a well-functioning obturator is the most significant factor contributing to quality of life in this patient population subset, and that inability to achieve retention, stability, and support may influence the choice of reconstruction used [33].
Dental implant-supported prosthetic rehabilitation remains the comprehensive and ideal rehabilitation following ablative oral cavity surgery [39, 40, 41]. When an osseous free flap is used, implants provide a foundation for a precision-retained prosthesis that provides the highest degree of masticatory function and facial soft-tissue support. Paramount to success is establishing an adequate bony foundation into which titanium dental implants may be placed and successfully osseointegrate. Contemporary dental implants are endosseous “root form” implants, though historically subperiosteal, endosteal blade, and transmandibular staple designs have been used, with variable success. Subperiosteal implants were introduced in the 1940s and became popular until the 1970s. The procedure involved a prosthodontist making a surgical impression of the native mandible after elevation of mucoperiosteal flaps. A vitallium frame prosthesis was fabricated overnight, to which prosthetic teeth could be affixed and occlusion loaded. The frame was integrated with surrounding fibrous scar tissue and bone eventually grew over the implant, providing a secure platform for mastication. Unfortunately, a small mucosal dehiscence often resulted in exposure of the framework and osteomyelitis, with bone destruction and subsequent defect formation. Endosseous implants, introduced by Branemark in 1978, are the most common type placed in the current era [42••]. Root form implants, may be vertical or angulated. Typical dental implants, such as those used in the restoration of teeth lost to periodontal disease or caries, are vertical and approximate the position and length of the tooth. An intact alveolus is required with at least 10 mm of bone for the titanium dioxide surface to irreversibly osseointegrate to the alveolus. Longstanding edentulism with alveolar atrophy and pneumatization of the maxillary sinuses often necessitates a sinus lift procedure to create the required bone volume in the posterior maxilla. For a prosthodontic framework to be stable, adequate anterioposterior spread of the implants is needed. This also avoids oblique occlusal forces to the implant fixture, which leads to early bone resorption and implant loss. A sinus lift involves placing allogenic bone graft material between the elevated Schneiderian membrane and the bony floor of the sinus [43]. Patients who have undergone maxillectomy have lost their alveolar support and other available sites of cortical bone must be engaged to provide stability. These include the zygoma [44] or nasal floor/pyriform rim (All on Four, Nobel Biocare, Zurich). The various prosthetic designs and materials available exceed the scope of this article, but are evolving rapidly as 3D imaging and CAD-CAM rendering supplants change the field of maxillofacial prosthodontics. Implants may be placed into vascularized free flaps, but donor sites must be compared on the basis offer variable bone stock (Table 2) [45]. Moscoso and colleagues assessed suitability of four common bone flaps to receive dental implants in their study of 28 cadavers. Serial cross sections of the lateral border of the scapula show greater implantability distal from the glenohumeral joint, where bone stock is thickest. Overall, 78 % of harvested scapular bone segments were deemed fit for implant placement, defined as those with a neomandibular height of 10 mm and a width of 5 mm or more. This quantity of available bone was significantly more consistent than the fibula free flap [46••].
Current State of the Art and Controversies
New directions in therapy focus primarily on early dental implant restoration with minimal surgical interventions. Recent reports suggest a growing number of young patients develop oral cancer, despite an overall decline in incidence worldwide [47, 48]. These younger patients are more likely to have a need to return to work and family responsibilities, and most have healthy dentition with increased esthetic and functional demands. Currently, dental implant placement is performed after 9–12 months of flap healing, to provide adequate recovery from microvascular surgery and radiotherapy [49]. A two-stage process is usually advocated, where the implants are again buried under soft tissue for an additional 6 months before second-stage uncovering and loading. Overall, the process to have an implant-borne process may take up to a year or more after microvascular reconstruction. Regardless of the type of osteocutaneous flap used, excessive soft-tissue bulk usually mandates one or more debulking and lipectomy or liposuction procedures before dental implants may be placed so they emerge through a thin, immobile band of keratinized tissue. This is because soft-tissue thickness of 4 mm or greater creates a peri-implant pocket that is noncleansable, resulting in trapping of periodontal pathogens, peri-implantitis, bone loss, and eventual implant loss [34]. To minimize the need for these additional preprosthetic surgeries, Santamaria et al. proposed the prelaminated fibula free flap for anterior maxillectomy (Brown IIc) defects. Oral mucosa is transferred to the fibula donor site and placed over the lateral and anterior surface of the bone after removing all but the usual 3 mm of the lateral compartment muscles. After 2–3 weeks, the predominated flap is transferred as a mucosa-osseous flap, with the soft-tissue mucosa paddle oriented into the oral cavity. Dental implants may be placed after 4–6 months of healing, theoretically reducing the number of debulking surgeries needed prior to impression making by the prosthodontist. In the author’s (A.M.) experience with this flap modification, some debulking remains necessary to provide firm, immobile mucosa prior to implant placement [50].
Virtual 3D surgical planning has replaced traditional stone cast model surgery in many areas of maxillofacial surgery, including orthognathic and trauma surgery [51•]. Patient-specific data are used to generate three-dimensional surgical models to simultaneously pair flap and dental reconstruction with the anticipated defect. There are increased preoperative costs and time involved, but intraoperative time is reduced when precision cutting jigs and custom surgical guides are used [26•]. When a bone flap segment is placed into an unusable position, however, a functional prosthesis may be impossible regardless of available bone stock. Pre-emptive planning offers a high degree of reliability compared to conventional surgery in bony reconstruction of the maxilla (Wang et al. [52] mandible [53•]). Following trends in implant dentistry and prosthodontics, where “Teeth in a Day” are marketed for immediate implant placement and prosthesis delivery are offered to patients with typical edentulism, Hirsch and colleagues introduced “Jaw in a Day” in 2013, presenting 3 patients with benign tumors, two undergoing mandibular continuity resections, and one maxillectomy with immediate fibular flap reconstruction loaded with dental implants. Using precise CAD-CAM modeling and custom surgical guides, all three patients were able to receive immediate temporary (nonload-bearing) dental prostheses before leaving the operating room [54••]. Because of the additional preoperative planning time involved, immediate implant placement is at this time used largely for benign neoplasms or osteonecrosis, but an increasing number of trained surgeon–prosthodontist teams may expedite the process for future use across the spectrum of head and neck reconstructive procedures.
Conclusion
Numerous advanced in maxillectomy reconstruction are due to recent application of navigation, virtual surgical planning, and complete dental rehabilitation. The revised Brown–Shaw classification provides a common platform for prosthodontists and surgeons to communicate. Obturator rehabilitation of maxillectomy defects is acceptable for patients with posterior, lateral, and smaller limited defects. In cases of aggressive or high-grade malignancies (e.g., osteosarcoma) improved surveillance of the tumor bed may enhance detection of an early recurrence. An obturator is also ideal for the patient with significant medical comorbidities precluding lengthier microvascular flap procedures. For patients undergoing reconstructive surgery, microvascular reconstruction of a maxillectomy defect may involve soft tissue alone or a composite flap of bone, skin, fascia, fat, and/or muscle. If prosthodontic tooth replacement is anticipated, bone reconstruction should ideally provide adequate height and width of the neoalveolus to accommodate root form dental implants. In selected cases additional autogenous bone may be grafted secondarily for dental implants. The choice of a bone-containing free flap donor site depends on pedicle length, bone stock required, and the option for a two-team simultaneous harvest. The DCIA flap provides ideal bone volume and long-term stability due to multiple osseous surfaces, which form bony union with the osteotomized maxilla; however, pedicle length and excessive bulk are drawbacks. Cutaneous flaps in the oral cavity must nearly always undergo one or more secondary debulking and vestibuloplasty procedures to provide a thin, immobile soft-tissue base prior to implant placement. Myo-osseous flaps or prelaminated mucosa-osseous flaps may be used to expedite dental implant placement by eliminating the need for secondary debulking. Recently, virtual surgical planning using three-dimensional modeling has allowed for precise positioning of bone flaps and can be used to provide simultaneous, one-step implant placement at the time of flap insetting.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Louis PJ. Management of panfacial fractures. In: Miloro M, Ghali G, Larsen PE, Waite PD, editors. Peterson’s principles of oral and maxillofacial surgery. Hamilton: BC Decker; 2004. p. 547–59.
Lenox ND, Kim DD. Maxillary reconstruction. Oral Maxillofac Surg Clin N Am. 2013;25(2):215–22.
•• Brown JS, et al. A modified classification for the maxillectomy defect. Head Neck. 2000;22(1):17–26. Brown et al. developed the first translational classification system, using language common to both prosthodontists and head and neck surgeons. A four-part numeric scale describes the extent of the vertical defect (globe, nasal, and upper lip support—important to surgeons), and a three-part, lettered horizontal system characterizes the horizontal component (dentoalveolar structures—important to dentists).
•• Andrades P, et al. Current strategies in reconstruction of maxillectomy defects. Arch Otolaryngol Head Neck Surg. 2011;137(8):806–12. This review covers in detail the breadth and complexity of the topic of maxillectomy defect reconstruction, with emphasis on the Cordeiro classification system.
Laraway DC, Rogers SN. A structured review of journal articles reporting outcomes using the University of Washington Quality of Life Scale. Br J Oral Maxillofac Surg. 2012;50(2):122–31.
Kumar P, et al. Assessment of the quality of life in maxillectomy patients: a longitudinal study. J Adv Prosthodont. 2013;5(1):29–35.
List MA, Ritter-Sterr C, Lansky SB. A performance status scale for head and neck cancer patients. Cancer. 1990;66(3):564–9.
Nicoletti G, et al. Chewing and swallowing after surgical treatment for oral cancer: functional evaluation in 196 selected cases. Plast Reconstr Surg. 2004;114(2):329–38.
Aramany MA. Basic principles of obturator design for partially edentulous patients. Part II: design principles. J Prosthet Dent. 1978;40(6):656–62.
Aramany MA. Basic principles of obturator design for partially edentulous patients. Part I: classification. J Prosthet Dent. 1978;40(5):554–7.
Spiro RH, Strong EW, Shah JP. Maxillectomy and its classification. Head Neck. 1997;19(4):309–14.
•• Cordeiro PG, Santamaria E. A classification system and algorithm for reconstruction of maxillectomy and midfacial defects. Plast Reconstr Surg. 2000;105(7):2331–46; discussion 2347–8. Cordeiro and Santamaria describe the maxilla as a six-walled hexahedrium, and classify maxillectomy defects based on the walls involved in tumor resection. For surgeons, this algorithm provides an important approach to the three-dimensional challenges of maxillectomy defects. Free tissue transfer is advocated; this is one of few references where the “folded” osteocutaneous forearm flap is advocated, with excellent cosmetic and functional results.
Cordeiro PG, Chen CM. A 15-year review of midface reconstruction after total and subtotal maxillectomy: part II. Technical modifications to maximize aesthetic and functional outcomes. Plast Reconstr Surg. 2012;129(1):139–47.
Cordeiro PG, Chen CM. A 15-year review of midface reconstruction after total and subtotal maxillectomy: part I. Algorithm and outcomes. Plast Reconstr Surg. 2012;129(1):124–36.
•• Okay DJ, et al. Prosthodontic guidelines for surgical reconstruction of the maxilla: a classification system of defects. J Prosthet Dent. 2001;86(4):352–63. Okay, a well-known maxillofacial prosthodontist, describes a complex system similar to the other Urken/Buchbinder systems used in classification of the mandibulectomy defect. Emphasis is placed on the ability of the prosthodontist to reconstruct the alveolar defect using the remaining available teeth, and the impact of midline involvement and excursive occlusal forces on prosthetic stability.
Brown JS, Shaw RJ. Reconstruction of the maxilla and midface: introducing a new classification. Lancet Oncol. 2010;11(10):1001–8.
Urken M, Cheney ML. Atlas of regional and free flaps for head and neck reconstruction. Philadelphia PA: Wolters-Kluwer LWW; 2012.
Yu M, et al. A modified technique for reconstruction of a total maxillary defect. Br J Oral Maxillofac Surg. 2016;54(1):106–8.
Arce K. Buccal fat pad in maxillary reconstruction. Atlas Oral Maxillofac Surg Clin North Am. 2007;15(1):23–32.
Morlandt A, Fernandes R, Ramirez C. Scapula free flap. In: Kademani D, Tiwana P, editors. Atlas of oral and maxillofacial surgery. Missouri: Elsevier Saunders; 2016. p. 1211–21.
Patel UA, Bayles SW, Hayden RE. The submental flap: a modified technique for resident training. Laryngoscope. 2007;117(1):186–9.
Andrades P, et al. Zygomatic-maxillary buttress reconstruction of midface defects with the osteocutaneous radial forearm free flap. Head Neck. 2008;30(10):1295–302.
Swartz WM, et al. The osteocutaneous scapular flap for mandibular and maxillary reconstruction. Plast Reconstr Surg. 1986;77(4):530–45.
Deraemaeker R, Tienen CV, Lejour M, Dor P. The serratus anterior-scapular free flap: a new osteomuscular unit for reconstruction after radical head and neck surgery (abstract). In: Proceedings of the Second Intl Conf on Head and Neck Cancer. 1988.
Coleman JJ 3rd, Sultan MR. The bipedicled osteocutaneous scapula flap: a new subscapular system free flap. Plast Reconstr Surg. 1991;87(4):682–92.
• Yetzer J, Fernandes R. Reconstruction of orbitomaxillary defects. J Oral Maxillofac Surg. 2013;71(2):398–409. Fernandes and Yetzer outline 21 patients reconstructed with a variety of free and local flaps, including some with obturators. Brown class III to VI defects were overall happier with free tissue transfer versus obturation. The authors propose that perforator flaps will offer greater dimensional stability and improved facial contour in the long term, in contrast to myocutaneous flaps which undergo denervation atrophy.
Boyes-Varley JG, et al. A protocol for maxillary reconstruction following oncology resection using zygomatic implants. Int J Prosthodont. 2007;20(5):521–31.
Brown JS. Deep circumflex iliac artery free flap with internal oblique muscle as a new method of immediate reconstruction of maxillectomy defect. Head Neck. 1996;18(5):412–21.
Futran ND. Primary reconstruction of the maxilla following maxillectomy with or without sacrifice of the orbit. J Oral Maxillofac Surg. 2005;63(12):1765–9.
Sakuraba M, et al. Simple maxillary reconstruction using free tissue transfer and prostheses. Plast Reconstr Surg. 2003;111(2):594–8; discussion 599–600.
Rogers SN, et al. Health-related quality of life after maxillectomy: a comparison between prosthetic obturation and free flap. J Oral Maxillofac Surg. 2003;61(2):174–81.
Moreno MA, et al. Microvascular free flap reconstruction versus palatal obturation for maxillectomy defects. Head Neck. 2010;32(7):860–8.
Genden EM, et al. Comparison of functional and quality-of-life outcomes in patients with and without palatomaxillary reconstruction: a preliminary report. Arch Otolaryngol Head Neck Surg. 2003;129(7):775–80.
Holmes JD, Aponte-Wesson R. Dental implants after reconstruction with free tissue transfer. Oral Maxillofac Surg Clin N Am. 2010;22(3):407–18, vii.
Kornblith AB, et al. Quality of life of maxillectomy patients using an obturator prosthesis. Head Neck. 1996;18(4):323–34.
Seignemartin CP, et al. Understandability of speech predicts quality of life among maxillectomy patients restored with obturator prosthesis. J Oral Maxillofac Surg. 2015;73(10):2040–8.
Kreeft AM, et al. Oral function after maxillectomy and reconstruction with an obturator. Int J Oral Maxillofac Surg. 2012;41(11):1387–92.
Chigurupati R, et al. Quality of life after maxillectomy and prosthetic obturator rehabilitation. J Oral Maxillofac Surg. 2013;71(8):1471–8.
Urken ML, et al. Functional evaluation following microvascular oromandibular reconstruction of the oral cancer patient: a comparative study of reconstructed and nonreconstructed patients. Laryngoscope. 1991;101(9):935–50.
Tang JA, Rieger JM, Wolfaardt JF. A review of functional outcomes related to prosthetic treatment after maxillary and mandibular reconstruction in patients with head and neck cancer. Int J Prosthodont. 2008;21(4):337–54.
Cuesta-Gil M, et al. Oral rehabilitation with osseointegrated implants in oncologic patients. J Oral Maxillofac Surg. 2009;67(11):2485–96.
•• Branemark PI, et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl. 1977;16:1–132. Dr. Per Ingvar Branemark is considered the father of osseointegrated implant technology, and is responsible for the worldwide shift from subperiosteal and transmandibular implants to the contemporary root form and zygomatic titanium implants used in dentistry today.
Carrao V, DeMatteis I. Maxillary sinus bone augmentation techniques. Oral Maxillofac Surg Clin North Am. 2015;27(2):245–53.
Vega LG, Gielincki W, Fernandes RP. Zygoma implant reconstruction of acquired maxillary bony defects. Oral Maxillofac Surg Clin N Am. 2013;25(2):223–39.
Frodel JL Jr, et al. Osseointegrated implants: a comparative study of bone thickness in four vascularized bone flaps. Plast Reconstr Surg. 1993;92(3):449–55; discussion 456–8.
•• Moscoso JF, et al. Vascularized bone flaps in oromandibular reconstruction. A comparative anatomic study of bone stock from various donor sites to assess suitability for enosseous dental implants. Arch Otolaryngol Head Neck Surg. 1994;120(1):36–43. In a series of 28 cadavers, a variety of vascularized bone flap sites were assessed for implantability. The most consistent and reliable bone stock for dental implant stability was found in the DCIA, followed by scapula, fibula, and radius respectively.
Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20-44 years. Cancer. 2005;103(9):1843–9.
Goldstein DP, Irish JC. Head and neck squamous cell carcinoma in the young patient. Curr Opin Otolaryngol Head Neck Surg. 2005;13(4):207–11.
Jacobsson M, et al. Integration of titanium implants in irradiated bone. Histologic and clinical study. Ann Otol Rhinol Laryngol. 1988;97(4 Pt 1):337–40.
Santamaria E, et al. A shift from the osteocutaneous fibula flap to the prelaminated osteomucosal fibula flap for maxillary reconstruction. Plast Reconstr Surg. 2012;130(5):1023–30.
• Hirsch DL, et al. Use of computer-aided design and computer-aided manufacturing to produce orthognathically ideal surgical outcomes: a paradigm shift in head and neck reconstruction. J Oral Maxillofac Surg. 2009;67(10):2115–22. Hirsch and colleagues have introduced virtual surgical planning (“VSP”) into contemporary maxillofacial literature. VSP is now widely used in orthognathic and tumor surgery.
Wang YY, et al. Virtual surgical planning in precise maxillary reconstruction with vascularized fibular graft after tumor ablation. J Oral Maxillofac Surg. 2016;74(6):1255–64.
• Roser SM, et al. The accuracy of virtual surgical planning in free fibula mandibular reconstruction: comparison of planned and final results. J Oral Maxillofac Surg. 2010;68(11):2824–32. After VSP gained popularity, Roser et al. confirmed that indeed, preoperative planning using VSP technology in complex mandibular reconstruction (multiple fibula osteotomies) is a precise, and time-saving effort.
•• Levine JP, et al. Jaw in a day: total maxillofacial reconstruction using digital technology. Plast Reconstr Surg. 2013;131(6):1386–91. “Jaw in a Day” is analogous to the “Teeth in a Day” advertised by many implant dentists, where a patient may undergo dental extractions, have immediate implants placed, and a temporary prosthesis in the same day. In an era of increasing public awareness, the “Jaw in a Day” multi-specialty approach offers the most efficient and comprehensive method of rehabilitating a patient’s facial and dental status to their premorbid condition. The traditional methods of dental implant rehabilitation after microvascular free tissue transfer take up to a year or longer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Anthony B. Morlandt declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Head and Neck Reconstruction.
Rights and permissions
About this article
Cite this article
Morlandt, A.B. Reconstruction of the Maxillectomy Defect. Curr Otorhinolaryngol Rep 4, 201–210 (2016). https://doi.org/10.1007/s40136-016-0130-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40136-016-0130-4