Abstract
Abusive head trauma is the leading cause of severe traumatic brain injury in children <2 years of age. Because the clinical presentation of children with abusive head trauma can be nonspecific, CT and MR imaging play an important role in distinguishing these cases from episodes of accidental head trauma. In this article, we review the pathophysiology, imaging appearance, and specificity of patterns of traumatic brain injury associated with abusive head trauma, and highlight recent updates in the understanding of these injuries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abusive head trauma (AHT) is one of the commonest subtypes of non-accidental trauma (NAT). It accounts for a substantial proportion of NAT injuries in the first 2 years of life [1, 2] and is the leading cause of death among abused children [3]. AHT is responsible for the majority of severe traumatic brain injury in children <2 years of age [3–5], with case fatality rates above 20 % [6, 7]. Among children who survive, more than 75 % will have permanent neurologic impairment [8, 9].
AHT correlates with inconsolable crying behavior during infancy, which peaks in the second month of life [10]. Population-based studies in the UK and US suggest an annual incidence of 25–30 detected AHT cases per 100,000 children under the age of 1, with rapidly decreasing incidence over the first 5 years of life [6, 7, 11–13]. This is likely an underestimate due to the probability of missed and misdiagnosed cases [14].
Children with AHT can present with nonspecific signs and symptoms, making accurate recognition challenging. In a retrospective review of 173 children under the age of 3 diagnosed with AHT [14], 31 % of cases were initially missed by medical professionals. 28 % of these children sustained repeat injury prior to correct diagnosis. In 13 % of cases, missed AHT was due to misinterpretation of imaging studies. Improved awareness of the neuroimaging features of AHT is crucial for accurate diagnosis.
Imaging of AHT
Unenhanced CT of the head is the screening examination of choice for cases of suspected AHT [15•]. Multiplanar reformations and 3D volume rendering of the skull increase sensitivity for fracture and intracranial hemorrhage [16•]. Several studies have demonstrated an incidence as high as 27–39 % of unsuspected intracranial injury in neurologically asymptomatic patients with non-CNS injuries suspicious for NAT [3, 17, 18]. Many experts therefore recommend routine screening of the CNS in any child with sufficient clinical concern to warrant a skeletal trauma survey.
MRI of the brain is recommended for further evaluation of all abnormal screening examinations and in the cases of high clinical suspicion [19], generally between 3 and 5 after acute presentation. MRI in this time frame is sensitive for the detection of small volume extra-axial hemorrhage and for evolving parenchymal injury [20]. In addition to standard sequences, diffusion-weighted imaging (DWI) [21] and susceptibility-weighted imaging (SWI) [22] increase sensitivity for detection of parenchymal injury and microhemorrhage, and can provide prognostic information in AHT [23••]. Administration of gadolinium-based contrast material is not performed routinely but can be utilized in select cases to increase accuracy of dating extra-axial collections [24, 25].
MR of the cervical spine, including fat-suppressed fluid-sensitive sequences, should routinely be performed at the time of brain imaging, as unsuspected spinal injuries may be demonstrated in more than 75 % of cases [26, 27••, 28, 29•]. Whole spine imaging should be considered if there is suspicion for injury based on the trauma survey or an abnormal neurologic exam [30].
Terminology of AHT
Early publications [31–33] suggested the association of shaking-type abuse with a specific pattern of intracranial injuries, often in the absence of external signs of trauma. This association came to be known as “whiplash shaken infant syndrome” or simply “shaken baby syndrome,” terms that predominated in the literature for several decades and were considered synonymous with inflicted injury.
Over time, awareness of the diverse array of possible mechanisms of inflicted injury to the CNS has increased, particularly that of concurrent shaking and impact type injuries—the so-called “shaken impact syndrome” [5]. In addition, the contribution of less common mechanisms such as strangulation, suffocation, and penetrating trauma has been recognized.
In 2009, the term “abusive head trauma” was adopted by the American Academy of Pediatrics Committee on Child Abuse and Neglect, in an attempt to provide a more inclusive terminology for inflicted head injury that does not imply a specific mechanism of injury [34].
Mechanisms of Injury and Injury Patterns
AHT most commonly results from impulsive or impact loading [35]. Impulsive loading refers to nonimpact forces produced by rapid alternating angular acceleration and deceleration of the cranial contents, as with vigorous shaking. By contrast, impact loading refers to direct application of forces to the head. Both mechanisms can occur alone or in combination. Impulsive and impact loading are believed to result in distinct, but potentially overlapping, injury patterns.
Impulse loading produces differential angular acceleration of the intracranial contents, with risk for shearing injury to the brain and meninges [36]. The original reports of Guthkelch [31] and Caffey [32, 33] suggested the association of shaking-type injury with the triad of subdural hematoma (SDH), retinal hemorrhage (RH), and focal or diffuse parenchymal injury, often in the absence of external signs of injury. This association has been supported by numerous additional publications [37–40].
Impact loading typically results in soft tissue injury, skull fracture, subperiosteal hemorrhage, and parenchymal contusions [36]. In infants and young children, impact loading injury is less commonly observed in AHT than in accidental head trauma (nAHT) [41••, 42••, 43•]. When associated with AHT in young children, impact loading injuries are typically severe, and complex injury patterns can be present [44]. In older children, impact loading injuries becomes more common in AHT [44].
Goals of Imaging
The goals of imaging in suspected AHT are as follows: (1) detect pathology requiring emergent intervention; (2) assess the extent of the injury; (3) estimate timing of the injury; and (4) detect mimics or predisposing conditions.
Subdural Hematoma
SDH is the most commonly encountered imaging finding in AHT, present in 89 % of children on imaging in a recent prospective epidemiologic study [45]. This finding in isolation is only of moderate specificity for AHT, as it can be a feature of nAHT and non-traumatic conditions. The specificity of SDH for AHT is increased when associated with RH and underlying diffuse parenchymal injury [41••].
The typical SDH associated with AHT is thin and diffuse, without significant mass effect on underlying brain parenchyma [46]. The SDH is unlikely to be the cause of the clinical presentation but may serve as a marker for an impulsive mechanism of injury [47]. As discussed below, the prognosis is largely determined by the extent of associated parenchymal injury.
Pathophysiology
SDH has long been known to result from hemorrhage into the dural border cell layer—the innermost layer of the dura mater—and as such is actually an intradural hematoma [46, 48].
It has been considered axiomatic that traumatic SHD is the result of rupture of bridging veins [49]. Autopsy data on bridging vein rupture are sparse due to the technical difficulty of evaluating the bridging veins without introducing artifactual injury [50, 51]. Post-mortem imaging has occasionally been employed to demonstrate bridging vein rupture as the pathogenesis of SDH in fatal AHT cases [52–54], although it is not routinely used.
Recent imaging data suggests that this mechanism is actually more prevalent, with bridging vein thrombosis secondary to rupture demonstrated in between 40 and 45 % of AHT cases with SDH [55••, 56•]. When present, this confirms the traumatic (though not per se abusive) nature of the SDH [51, 57], and the vertex should be closely scrutinized in every case of unexplained SDH or suspected AHT.
In instances when bridging vein rupture is not demonstrable by imaging, bridging vein injury without thrombosis remains possible. Several authors have described a well-developed intradural vascular plexus, particularly prominent in young children, which may provide an alternative source for SDH [46, 48].
Imaging
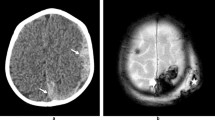
SDH is well demonstrated on screening CT, but detection and characterization both benefit from the improved spatial and contrast resolution of MR [19, 20, 58]. Associated bridging vein thrombosis may be evident as hyperdensity on CT or hypointensity on susceptibility-sensitive MR sequences (T2* or SWI), with linear intravascular thrombus extending into more globular extravascular clot at the site of vessel rupture near the vertex. This appearance has been termed the “tadpole” [55••] or “lollipop” [56•] sign (Fig. 1).
3-month-old male presented with seizures, bruising, subconjunctival hemorrhages, and multilayered RH. a Axial T2-weighted image shows focal enlargement of a parasagittal bridging vein near the vertex (white arrows), suggesting vein rupture. b Axial SWI image demonstrates marked hypointensity and blooming within the distal vein and at site of venous rupture (white arrows), confirming thrombosis. This appearance has been termed the “tadpole” or “lollipop” sign. Thin left SDH is evident as crescentic blooming artifact on the SWI image
The timing of SDH based on imaging should be approached with caution. A recent systematic review of studies evaluating the imaging appearance of SDH relative to time of trauma demonstrated considerable overlap—particularly in children, in AHT, and on MR [59••].
Although classically the density of SDH on CT has been correlated with age, it is clear that acute SDH can present with a variety of imaging appearances. Unclotted blood can result in a SDH isodense to cortex, reflecting either hyperacute bleeding (typically in the first 3 h) or delayed clotting in coagulopathy [24, 25]. (Hemato)hygroma related to meningeal tear or arachnoid cyst rupture can present as an acute iso- or hypodense subdural collection [60, 61, 62•]. Anemia (hemoglobin <8–10 g/dL) can result in hypoattenuation of acute hemorrhage [24]. SDH hyperdensity and acute bridging vein thrombosis are reliable indicators of acute hemorrhage (up to approximately 10 days), but their absence cannot be used to infer the opposite.
The specific case of mixed-density collections warrants discussion. Although mixed-density SDHs may reflect acute-on-chronic bleeding, they should not be taken as a priori evidence of injury of multiple ages. Hyperacute bleeding, acute hemorrhage with sedimentation levels, and inhomogeneous hematohygromas can all present as mixed-density collections [24, 25] (Fig. 2). A descriptive approach to the density or attenuation of SDH on CT is recommended, rather than a dogmatic assertion of timing.
5-week-old female presented with an acute onset of obtundation, fixed and dilated right pupil, and coagulation studies consistent with hemorrhagic disease of the newborn. Axial CT image demonstrates a right mixed-density SDH (white arrows), found to be admixed clotted and unclotted blood at subsequent surgical decompression. Note that unlike the typical SDH of AHT, there is significant associated mass effect with midline shift, right lateral ventricular effacement, and left lateral ventricular entrapment
More specific estimates of the timing of SDH may be possible with MR, but certain caveats apply. The timing of evolution of blood products on MR suggested by Bradley et al. were derived from longitudinal observation of intraparenchymal hematomas [63]. SDHs evolve over a generally longer time course with slightly different imaging characteristics [24, 64].
As recently emphasized by Wittshceiber et al. and others, the fate of acute SDH is to either quickly resolve or to rapidly evolve into subdural hygroma through interstitial fluid or CSF transudation, which in most cases will eventually undergo resorption [62•, 65]. As discussed above, subdural hygromas may present acutely as well, secondary to an arachnoid tear, and should not be considered markers of chronicity. Subdural hygromas are characterized by CSF-like signal intensity and absence of mass effect, enhancing neomembranes, and loculations.
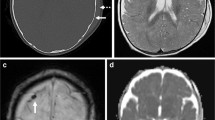
During their evolution, SDHs often demonstrate a layering sedimentation level, and the imaging characteristics of the serous fraction will typically approximate that of simple fluid, which can be erroneously interpreted as reflecting chronicity. Dating should be estimated from the dependent sediment layer [66] (Fig. 3).
4-month-old male presented with status epilepticus, facial bruising, and diffuse multilayered RH after fall from bed. a, b Axial T2-weighted and T1-weighted images obtained 1 day after presentation demonstrate bilateral holohemispheric subdural collections which approximate CSF signal intensity (white arrows), likely subdural hygromas. There is a dependent T2 hypointense, T1 isointense sediment layer on the left (black arrows), suggesting recent hemorrhage. Watershed HII was also present, not shown
In the presence of negative intradural pressure related to parenchymal volume loss or intracranial shunting, a small subset of hygromas may persist and form enhancing vascular neomembranes along the outer then inner margins [24] (Fig. 4). Repeated spontaneous hemorrhage from these membranes transforms the collections into chronic SDHs [46, 62•]. They may be relatively simple in appearance initially, but can assume an increasingly loculated configuration with increasing mass effect over time. Enhancing neomembranes are estimated to become apparent between 10 days and 3 weeks after injury [46, 65], and are one of the few reliable indicators of non-acuity in subdural collections.
4-month-old male imaged 1 month following acute bilateral SDH related to AHT. a Coronal T2-weighted image shows bilateral subdural collections. The dura can be identified as a thin hypointense line (white arrows) between the collections and the subarachnoid space. A loculation is present on the right (black arrows) containing blood products with different signal intensity. Note the mass effect on the subjacent brain surface. b Coronal contrast-enhanced T1-weighted image shows enhancing neomembranes (white arrows) in continuity with the loculation, confirming chronic SDH with superimposed rebleeding. Such rebleeding can occur with minimal or no trauma and does not in itself suggest repeated abuse
The possibility that acute SDH may represent rebleeding into a chronic subdural collection, spontaneously or with trivial trauma, is a common dilemma in imaging of AHT. Based on the pathophysiology proposed by Whittscheiber and others, spontaneous hemorrhage into pre-existing subdural hygromas can be inferred to occur [62•, 64]. Identification of enhancing subdural membranes and associated parenchymal volume loss or shunting are supportive imaging features (Fig. 4). Acute neurological deterioration, new parenchymal injury, new subarachnoid hemorrhage, and new discontinuous SDH should not be present, however, and are suspicious for repeat AHT [65, 67].
Controversies
SDH has traditionally been considered a marker of possible traumatic injury. Several recent publications have postulated that a variety of relatively common non-traumatic pathologies may result in SDH and RH, thereby mimicking AHT. Proposed theories include hypoxic-ischemic injury (HII), elevated central venous pressures, and primary dural venous sinus thrombosis (DVST).
Geddes et al. have suggested that SDH and RH seen in the setting of AHT might be the result of the HII rather than a direct consequence of trauma [68]. This was based on the observation that microscopic intradural bleeding was noted in 36/50 children in their series who died of non-traumatic causes, including HII and intracranial infection. This line of reasoning has been coopted to support the hypothesis that HII from any cause—including choking events, viral lung infections, and sudden infant death syndrome (SIDS) [69, 70]—can result in the classic imaging features of AHT.
It is important to note that the intradural hemorrhage that was described by Geddes et al. in their article was in all cases microscopic [68]. The extrapolation to cases of macroscopic SDH such as those seen on imaging studies in AHT is not supported by current evidence. In fact, large case series examining imaging studies of children with severe HII related to drowning [71] and cardiac arrest [72] did not report macroscopic SDH in any case.
Dysphagic choking acute life threatening events (ALTEs) resulting in paroxysmal cough and elevated central venous pressures have also been postulated to result in SDH and RH in two reports [70, 73]. Scant clinical data exist to evaluate this contention. Supporting evidence is based on computer modeling and two reported cases of pertussis-related SDH that predate neuroimaging [74–76]. No recent well-documented clinical cases exist to support this association, however [77].
DVST has been suggested to result in non-traumatic SDH. Case reports [78] and a small case series [79] in adults support this possibility. However, a cases series of 36 consecutive infants and young children with DVST found no association with SDH [80••]. A second case series of 9 consecutive infants with DVST demonstrated only 2 SDH, one 8 days after forceps delivery and another in a child after MVA [81]. The evidence base does not support DVST as a cause of spontaneous SDH in young children at this time. Nonetheless, some centers routinely include a 2-D time of flight MRV through the head in cases of suspected abuse to excluded DVST for medicolegal purposes.
Diffuse Parenchymal Injury
In isolation, diffuse parenchymal injury is not specific for trauma of any etiology. In the presence of SDH, RH, and/or cervicomedullary injury, diffuse parenchymal injury is highly associated with AHT [27••, 29•, 41••, 42••, 43•, 44]. Thomas et al. recently demonstrated in a large series of nAHT victims that parenchymal injury was not present in the absence of a high-force mechanism, essentially excluding a history of trivial trauma as an explanation for diffuse parenchymal injury [82].
Excepting with pure asphyxiation, diffuse parenchymal injury is rarely present in the absence of SDH and RH in AHT [83]. Diffuse parenchymal injury is noted in less than a third of patients with suspected AHT, but it is the most important imaging feature predictive of outcome [23••, 43•, 83].
Pathophysiology
The etiology of parenchymal injury associated with impulsive-type AHT is incompletely understood. It was initially presumed to reflect traumatic diffuse axonal injury (DAI) [84, 85], based to some extent on extrapolation from the neuropathology of adult traumatic parenchymal injury [36, 86, 87]. Subsequent studies have suggested based on patterns of immunoreactivity to beta-amyloid precursor protein (BAPP) that the predominant parenchymal injury in children with AHT is more likely HII rather than traumatic DAI [86–89].
These studies further described focal cervicomedullary traumatic axonal injury, which the authors suggested may have resulted in apnea and consequent HII. This association is supported by both animal models [90] and clinical series of suspected AHT [91–93]. Traumatic brain injury may also directly result in ischemic injury secondary to altered excitotoxicity and oxidative stress [94, 95], possibly mediated by clinical or subclinical seizure activity [96].
Several authors have reported unilateral HII [83, 97] as a characteristic injury pattern in AHT. The etiology of unilateral HII is difficult to understand from the mechanistic standpoint of cervicomedullary injury but has been explained by transient unilateral vascular occlusion [93]. Another possibility is “second impact syndrome”—an injury pattern described primarily in adolescents subject to repeated head trauma—characterized by catastrophic brain edema with a unilateral HII pattern [98]. The etiology of this syndrome is unknown but is hypothesized to reflect disordered cerebral autoregulation.
In adolescent cases of second impact syndrome, thin ipsilateral SDH is a universal associated feature, not unlike AHT cases. This suggests that SDH itself may have a direct or compounding effect on the subsequent development of HII. Animal studies have shown altered metabolism underlying SDH [99]. These findings suggest that the HII seen in AHT is likely multifactorial.
Imaging
The parenchymal injury associated with AHT may be occult on early screening CT, or may be seen as diffuse or hemispheric hypodensity with evidence of mass effect. Thin SDH associated with disproportionate ipsilateral hemispheric mass effect on screening CT should raise the possibility of early unilateral HII and prompt consideration of AHT and further evaluation with MRI (Fig. 5).
Two and half-year-old male presented with seizure and decreased level of consciousness following unwitnessed fall down 2 stairs. Initially misdiagnosed as stroke at OSH and transferred to tertiary care hospital for rehabilitation, patient was noted to have multiple healing fractures and diffuse multilayered RH on further evaluation. a Initial CT from OSH demonstrated thin right SDH with disproportionate mass effect resulting in right to left midline shift. b Axial FLAIR image from subsequent MR demonstrates diffuse unilateral cortical hyperintensity on the right. c Axial ADC map demonstrates a predominately subcortical pattern of diffusion restriction
Early injury is better demonstrated on MRI. T2 hyperintensity reflecting tissue edema may be subtle in the unmyelinated brain, and DWI will dramatically increase conspicuity of cytotoxic injury [100]. Studies examining the patterns of diffusion restriction in AHT have suggested a watershed pattern of HII in a majority of cases [93, 100, 101]. However, multiple patterns of parenchymal injury are potentially consistent with AHT (Fig. 6).
A recent study that examined white matter microstructural changes in AHT using diffusion tensor imaging demonstrated reductions in mean diffusivity largely as a result of decreased axial diffusivity, with preserved radial diffusivity and fractional anisotropy [102•]. These findings are presumed to reflect hypoxic-ischemic axonopathy and were found to be of prognostic value in stratifying functional outcome.
The time course of the evolution of HII on DWI is variable, but may be delayed relative to that seen in closed-vessel ischemic injury typical in adults. This reflects variability in the underlying pathologic substrate of HII in the immature brain, ranging from acute tissue necrosis to necrosis-like cell death to delayed apoptosis [103], depending on multiple factors including the severity of the initial insult, contribution of inflammation and excitotoxicity, and superimposed seizures. This variability must be considered when attempts are made at dating parenchymal injury related to AHT.
Controversies
There is continued medicolegal debate regarding the feasibility of isolated shaking as a viable etiology for parenchymal injury in pediatric patients. A study by Duhaime et al. using a 1-month-old baby model found that shaking injury without impact was unable to generate sufficient angular acceleration to meet expected thresholds for parenchymal injury including concussion and DAI [104].
Other authors [105] have called into questions this conclusion, pointing out problems with the biofidelity of the model used. They further noted that injury thresholds were scaled from single high-frequency impulsive loading studies in adult primates, and their comparability to repeated low-frequency shaking injury in infant children is unknown.
In light of the evolving understanding of the nature of parenchymal injury in abused children and the diversity of probable underlying mechanisms, the continued relevance of early estimates of the biomechanical feasibility of shaking alone as a cause for traumatic DAI and concussion is of questionable importance.
Focal Parenchymal Injury
Focal parenchymal injuries in AHT may result from impulsive or impact loading injury and are less common and less well described than diffuse injury. The pattern of focal parenchymal injury secondary to impulsive loading evolves with increasing age, likely due to progressive myelination and changes in brain parenchymal viscosity.
In children under 5–6 months of age, shear injury can result in very distinctive parenchymal lacerations or contusion clefts [36]. First described by Lindenberg and Freytag in 1969 [106], these lacerations have received very little attention in the imaging literature, largely limited to an older cases series using cranial ultrasound [107]. A recent report by Palifka et al. reaffirmed that these lesions are highly associated with AHT and reported them on MR in children up to 11 months of age [108•].
In older children, multifocal parenchymal lesions may uncommonly occur with severe trauma, likely representing classic DAI [101]. At any age, focal hemorrhagic contusions may be present reflecting an impact loading mechanism, typically with overlying skull fracture and occasionally with a coup-contrecoup pattern.
Imaging
Parenchymal lacerations or contusional clefts appear as linear tears in the brain parenchyma, predominately within the cortex or at the gray-white junction of the gyral crests. They predominate in the frontal and temporal lobes. They frequently demonstrate layering sedimentation levels on both CT and MR (Fig. 7). When superficial, they can be associated with overlying subpial hemorrhage.
6-week-old female presented with vomiting, seizures, abnormal breathing, abdominal bruising, and facial abrasions. a Axial-unenhanced head CT image shows hyperdense (acute) right frontal subdural hemorrhage (black arrows) and a hypodense left frontal cleft containing dependent hyperdense blood (white arrows). b, c Axial T2-weighted and SWI images show the acute blood products as T2 hypointense material in the subdural space (black arrows) and layering within the contusional tear (white arrows)
Focal parenchymal lesions in older children may occur and are presumed to be related to DAI. They are characterized by multifocal T2 prolongation, most common in the white matter of the frontal lobes and splenium. FLAIR is incrementally more sensitive for demonstration of lesions than T2, while SWI is substantially more sensitive [22, 109]. Presence of parenchymal microhemorrhage on SWI in AHT has been shown to correlate with poor clinical outcome, particularly when associated with underlying diffuse parenchymal abnormality [23••]. Occasionally, a pattern of multifocal punctate diffusion restriction is evident [101].
Retinal Hemorrhage
RH is estimated to occur in up to 85 % of AHT, but similar to SDH is of only moderate specificity [110]. Specificity is increased when RH is bilateral, multilayered (involving the pre-, intra-, and subretinal layers), and when it extends peripherally to the ora serrata on fundoscopic examination [111]. A recent report by Binenbaum et al. found that RH severity was correlated with severity of HII in children with traumatic brain injury [112].
Pathophysiology
RH in AHT is believed to result from traumatic retinoschisis or splitting of the retinal layers by shearing forces [113]. This mechanism can sometimes be suggested on fundoscopic examination by identification of retinal folds at the periphery of the hemorrhage, though this is inconstant [111].
Imaging
RH has not traditionally been considered an imaging diagnosis, although it has occasionally been reported [114]. It presents as high-attenuation foci at the posterior globes on CT, and low-signal foci on MR sequences (Fig. 8).
4-month-old male presented with seizures and decreased level of consciousness. Dilated fundoscopic examination demonstrated multilayered retinal hemorrhage with a macular schisis cavities in the bilateral eyes. a Axial T2-weighted image demonstrates retinoschisis of the posterior globes bilaterally (white arrows). b Axial SWI images demonstrate increased conspicuity due to blooming artifact (white arrows), confirming RH
Data recently published by Beavers et al. demonstrated a 61 % sensitivity for confirmed RH [115••] using concurrent analysis of T2*, T2, FLAIR, T1, and T1 post-contrast sequences. They demonstrated decreasing sensitivity of sequences in the order listed. They went on to correlate MR detection rates with fundoscopic severity, concluding that high-grade hemorrhage was significantly more likely to be detected by MR than low-grade hemorrhage, at 76 versus14 %.
With the clinical implementation of SWI, MR has become more sensitive for the detection of RH. Zuccoli et al. demonstrated a sensitivity of 62 % for the detection of RH with this single sequence using a gold standard of dilated fundoscopy [116•]. Sensitivity increased to 80 % when a dedicated high-resolution orbital SWI sequence was employed, and all missed cases were of mild severity on fundoscopic evaluation.
Given that increasing severity of RH has been shown to correlate with increasing risk for AHT, MR-visible RH must be viewed as suspicious. Detection of signal abnormality in the posterior globes in children absent adequate clinical history of severe trauma should prompt close scrutiny for additional intracranial abnormalities and consideration of dilated fundoscopic examination.
Controversies
Many alternate theories of causation for RH and SDH have been proposed, many of which have already been discussed in the section on SDH.
Skull Fractures
Skull fractures are not specific for AHT. Non-displaced linear skull fractures and associated small volume subperiosteal hemorrhage are common findings in low-impact nAHT, such as with short-distance falls [42••]. Contradictory data exist regarding which features, if any, may increase the specificity of skull fractures for AHT [117–119]. Fractures which are multiple, complex, diastatic, or growing suggest a high-energy mechanism and are concerning in the absence of appropriate history (Fig. 9).
1-year-old male presented with head injury after mother’s boyfriend “fell on him” while removing him from his car seat. a Axial-unenhanced CT image shows impact injury pattern with scalp swelling and cephalohematoma (white arrows), diastatic right occipital fracture, and hemorrhagic contusion (black arrows). b Volume-rendered CT image demonstrates complex occipitoparietal fractures crossing sutures (white arrows), with fracture diastasis (black arrows). c Volume-rendered CT image at 2-month follow-up redemonstrates complex fractures (black arrows), with increasing diastasis of the displaced fracture suggesting a “growing fracture.” d Volume-rendered T2-weighted MR image after skull stripping demonstrates a large posterior pseudomeningocele or “leptomeningeal cyst” as the cause of the growing fracture
Pathophysiology
Skull fractures occur when impact forces result in deformation of the calvarium in excess of the failure strength of the bone. The calvarium of young children is considerably more pliable than that of older children and adults, and significant deformation can occur without consequent fracture. In addition, a plastic “ping-pong” type fracture can occur.
Imaging
Skull fractures in children are best evaluated on CT reconstructed at submillimeter slice thickness. Multiplanar reconstructions and volume-rendered images of bone should be routinely performed and are helpful for identifying “in plane” fractures which are easily missed on axial images only.
Dating of skull fractures on CT can be difficult, as expected imaging findings associated with healing long bone fractures are not typical. In our experience, blurring of the fracture margins and new bone formation by the intact periosteal layer of dura are features of subacute healing fractures and are inconsistent with recent trauma (Fig. 10). Absence of these features cannot reliably be used to conclude acuity, however. Soft tissue swelling and subgaleal hemorrhage are suggestive of more recent injury but are not universally present in acute injury.
6-month-old male presented with head injury after being stuck with a hammer. a Axial-unenhanced CT image shows an acute diastatic right parietal fracture (black arrows) with associated scalp swelling (white arrows). b Axial-unenhanced CT image performed after 4 weeks shows linear periosteal reaction consistent with healing (black arrows), as well as decreased scalp swelling (white arrows)
Spine Injuries
Until recently, spine injury had been considered rare in NAT, with a reported incidence of <2 % [120, 121]. Incidence of spine fracture is increased to almost 10 % in the setting of a positive skeletal survey and is significantly associated with intracranial injury [122]. Occult spine injuries are often not routinely sought in evaluation of AHT, however, and their prevalence is likely significantly underestimated.
Indeed, recent evidence supports that spine injury in children with AHT is not uncommon. Ligamentous injury at the craniocervical junction (CCJ) and spinal SDH are reported in 36–78 % [27••, 29•] and 44–63 % [24, 26] of AHT cases, respectively, in several recent retrospective studies. Fracture [122], bone contusion [122], epidural hematoma [123], and cord injury have also been reported [29•].
CCJ injury in particular is highly correlated with abusive HII and can be helpful in establishing the traumatic etiology of SDH [27••, 29•].
Pathophysiology
The mechanisms of spine injury in AHT can be diverse. Of particular importance in children under 12 months of age is ligamento-osseous injury at the CCJ, as it may be expected to result from the whiplash-like motion of the cervical spine associated with impulsive loading of the cranium [124]. Pathologic studies have demonstrated CCJ injury in >75 % of fatal AHT cases [86, 87, 91, 125–127]. Specific injuries included subdural and epidural hemorrhage; cervical cord contusion, laceration, and hemorrhage; cervical nerve root avulsions; and ligamentous or soft tissue injuries.
Imaging
MR imaging of the cervical spine is recommended routinely along with MR brain imaging in all cases of suspected AHT. Although an early report demonstrated low sensitivity for detection of CCJ injury with MR in cases of severe AHT [126], this study predated the widespread clinical availability of STIR and other fat saturation techniques that increase the conspicuity of soft tissue and subtle bony injury. Recent studies have demonstrated improved rates of detection of abnormalities at the CCJ with MR [27••, 29•, 120], with ligamentous injury the most commonly reported finding (Fig. 11). Routine utilization of fat-saturated fluid-sensitive sequences is therefore recommended.
6-week-old male found down at home. MR of the brain revealed diffuse HII, bilateral SDH, and bridging vein thrombosis. Left intraretinal RH on dilated fundoscopy. a Sagittal STIR image shows hyperintense signal in the nuchal ligament and underlying posterior cervical fat (white arrows), consistent with ligamentous injury. b Sagittal T1-weighted image shows mildly hyperintense retroclival and thoracolumbar extra-axial collections (white arrows), consistent with subdural hemorrhage
A recent report by Koshy et al. highlighted the association of head trauma and retroclival hematoma, both epidural and subdural [128]. Retroclival epidural hematoma occurs anterior to the tectorial membrane and is associated with ligamentous injury at the CCJ [128, 129]. It should be differentiated if possible from SDH at the CCJ which occurs posterior to the tectorial membrane, as this latter finding likely represents redistribution of posterior fossa SDH. Although classically associated with severe nAHT, Silvera et al. demonstrated that retroclival hematomas were present in a third of cases with AHT in their series [123] (Fig. 11).
The value of routine screening of the whole spine in suspected AHT is being increasingly recognized. Studies of MR limited to the cervical spine have demonstrated a relatively low incidence of spinal SDH [29•, 126]; detection is significantly increased when imaging is extended through the thoracolumbar spine [26, 28] (Fig. 11). Although detection of spinal SDH is rarely associated with a change in management, Choudhary et al. have reported that the presence of spinal SDH is highly associated with an inflicted rather than accidental mechanism (present in 46 % AHT vs. 1 % nAHT in their cohort), and therefore, may be of diagnostic value.
Routine MR screening may also help to identify occult bony injury. Barber et al. found that spine fractures were present in up to 10 % of children with positive skeletal surveys [122]; spine injuries were reported at all levels in a recent review of spine injuries in AHT by Kemp et al. [30]. As it is known that MR increases the yield for detection of spine fractures in AHT [130], whole spine MR may have added value for identifying subtle bony injury in this population. For these reasons, some authorities now suggest that routine screening of the whole spine be performed in any child receiving an MRI of the brain for suspicion of AHT [28, 30, 120, 121].
Differential Considerations of AHT
Accidental HT
The primary differential consideration for AHT is nAHT. A number of studies have analyzed the imaging injury patterns present in children with AHT and nAHT in an attempt to establish the association of particular injuries or patterns of injury with AHT.
Two recent comprehensive systematic reviews [42••, 43•] demonstrated that SDH, HII, RH, and skull fracture associated with intracranial injury were associated with AHT; epidural hematoma, isolated skull fracture, and scalp swelling were associated with nAHT; subarachnoid hemorrhage, focal contusion, and DAI were not consistently associated with either etiology of trauma.
These findings were corroborated by Roach et al. who recently published the largest single cases series of AHT to date [131]. Among 2015 children with traumatic head injury under age 5, they found that patients with AHT were significantly less like to have skull fracture and epidural hematoma, and significantly more likely to have SDH and parenchymal injury.
Another recent case series of 345 children with traumatic head injury published by Kelly et al. [44] found that patients with AHT were significantly more likely to have absence of impact injuries, SDH, and HII. They found that this association held only for children under 2 years of age, suggesting that older abused children suffer more impact-related AHT. When AHT was associated with impact injuries in younger children, however, they were more likely to be severe with associated intracranial injury (Fig. 9).
It is important to address the potential effect of ‘circularity of reasoning’ as it impacts the data on patterns of injury with AHT and nAHT. To the extent that presence of certain injuries (e.g., SDH) or particular patterns of injury (e.g., SDH, RH, and HII) themselves led to the initial categorization of head trauma as abusive or accidental, an analysis of the association of these same features with abuse will be spuriously strengthened.
In an attempt to control for this, Vinchon et al. published an analysis of prospectively identified, corroborated cases of head trauma in children under age 2 (45 confessed AHT and 39 publicly witnessed nAHT) [132••]. Identical to the aforementioned studies, they reported that SDH, HII, RH, and absence of scalp injury were strongly associated with AHT, while extracranial hemorrhage, fracture, and contusion were highly associated with nAHT. These findings suggest that the relationship between impulsive injury patterns and AHT in children under age 2 is robust.
Birth-Related Trauma
A subset of nAHT is related to birth trauma. In a series of 41 consecutive patients with symptomatic birth injury, Pollina et al. reported 73 % SDH, 25 % extracalvarial hemorrhage, 20 % subarachnoid hemorrhage, 20 % intraparenchymal hemorrhage, and 5 % fracture [133]. There was an increased risk of birth trauma with vacuum- and forceps-assisted vaginal delivery.
Rooks et al. reported SDH in 46 % of asymptomatic newborns MR within 3 days of birth, with lowest rates reported with caesarian section and the highest rates reported in assisted vaginal deliveries [134]. Lower rates of detection have been reported in other studies with decreased field strength [135] and longer time to initial imaging [136]. Birth-related SDH in these studies were generally dependent and thin (< 3 mm) (Fig. 12). All resolved within the first postnatal month [134, 136]. Evolution to chronic SDH was not demonstrated, as expected given lack of predisposing factors [62•]. One patient in the study by Rooks et al. had a new separate SDH incidentally noted on a 2-week follow-up examination in the context of benign enlargement of the subarachnoid space, which subsequently resolved by 3 months.
Laghmari et al. reviewed over 2000 children born by spontaneous vaginal delivery and demonstrated that nearly one-third of children had RH [137]. These tended to be few in number and localized to the optic disks and posterior pole of the retina. There is currently no imaging data to suggest that such RH is routinely visible by MR. Similar to SDH, all cases of birth-related RH resolved within 1 month.
Non-Traumatic SDH
SDH is reported to occur in the context of numerous underlying medical conditions, though these are rare. Potential etiologies include vascular malformations, hematologic malignancies, coagulopathy (Fig. 2), and metabolic disorders such as Menkes kinky hair syndrome and glutaric aciduria type I (Fig. 13). Awareness of underlying medical conditions that might present with spontaneous hemorrhage that can mimic AHT is crucial for accurate diagnosis, and reinforces the notion that the evaluation of AHT must be accomplished in a multi-disciplinary fashion on a case-by-case basis.
6-month-old male with developmental delay and macrocephaly, subsequently confirmed to have glutaric aciduria type I. Axial T2-weighted image shows bilaterally enlarged perisylvian subarachnoid spaces secondary to frontal and temporal opercular hypoplasia and a left subdural collection (black arrows)
Mimics
Normal Neonatal Imaging Features
Care must be taken not to interpret normal imaging features of neonates and young infants as evidence of AHT. Normal sutural prominence in the neonatal period, hypodense or T2 hyperintense unmyelinated white matter, and dense dural venous sinus blood are potential pitfalls that can mimic sutural diastasis, parenchymal edema, and sinovenous thrombosis or layering SDH, respectively. Accessory sutures can occasionally mimic fractures in children but can usually be distinguished based on their frequent bilaterality, sclerotic borders, and serpiginous course [138].
Benign Enlargement of the Subarachnoid Space (BESS)
From 0.5 to 0.8 in 1000, children will demonstrate mild to moderate enlargement of the subarachnoid spaces in the first 18 months of life, resulting in macrocephaly [139]. This appearance can easily be confused with hypodense bifrontal SDHs, particularly on CT. A key discriminating feature is the presence of normal subarachnoid vessels traversing the enlarged fluid space in BESS; these vessels are notably absent in SDH, in which case the subarachnoid space is compressed over the cerebral convexities (Fig. 14). Slight differences in signal intensity between the different fluid spaces is often accentuated on the FLAIR sequence, and the displaced dural-arachnoid membrane can be well visualized on high-resolution steady-state free precession sequences (CISS, FIESTA, and b-FFE) in unclear cases.
It is worth noting that some publications suggest that BESS can itself predispose to SDH in the setting of minimal or no trauma, and that the two can occasionally co-exist. In two retrospective reviews of children diagnosed with BESS on imaging, asymptomatic SDH was identified in between 3 and 4 % [140••, 141].
Several retrospective case reviews have reported the existence of acute symptomatic SDH in the setting of trivial trauma in young children, with a majority occurring in the setting of BESS [142–144]. In a prospective evaluation of 164 children under age 2 presenting with symptomatic SDH, 16 (10 %) were categorized as spontaneous, among which 75 % were associated with BESS and macrocephaly [41••]. Additional risk factors included severe dehydration and lumbar puncture with intracranial hypotension.
These data should be interpreted with caution. 25 % of patients with SDH in the setting of BESS in the series by McKeag et al. manifested additional injuries suggestive of physical abuse [140••]. In addition, many cases of SDH deemed secondary to trivial trauma in retrospective case reviews were associated with RH or went on to develop diffuse encephalomalacia [142, 143], suggesting a likelihood of missed AHT in some cases. Given this, thorough clinical, ophthalmologic, laboratory, and radiologic evaluation of all children with unexplained SDH is warranted.
Conclusion
AHT is a common cause of morbidity and mortality in the pediatric population, and imaging evaluation is crucial for accurate diagnosis and prognostication. A comprehensive review of the imaging of AHT has been provided. Recent advances in the field of imaging of AHT have been emphasized, including limitations of imaging for dating AHT, emerging concepts in the evolution of SDH over time, the importance of parenchymal lacerations as high-specificity injuries in AHT, and the increasing utility of MR for the detection of RH and spine injuries in AHT. Understanding of the common patterns of abusive and accidental injury can help increase accuracy of diagnosis, both by increasing recognition of high-specificity injuries and by preventing unwarranted concern in cases of clearly concordant history and injuries.
Abbreviations
- AHT:
-
Abusive head trauma
- NAT:
-
Non-accidental trauma
- CCJ:
-
Craniocervical junction
- DWI:
-
Diffusion-weighted imaging
- SWI:
-
Susceptibility-weighted imaging
- SDH:
-
Subdural hematoma
- RH:
-
Retinal hemorrhage
- SIDS:
-
Sudden infant death syndrome
- ALTE:
-
Acute life threatening event
- DVST:
-
Dural venous sinus thrombosis
- DAI:
-
Diffuse axonal injury
- HII:
-
Hypoxic-ischemic injury
- nAHT:
-
Accidental (non-abusive) head trauma
- BESS:
-
Benign enlargement of the subarachnoid space
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sibert JR, Payne EH, Kemp AM, et al. The incidence of severe physical child abuse in Wales. Child Abuse Negl. 2002;26(3):267–76.
Gessner BD, Moore M, Hamilton B, et al. The incidence of infant physical abuse in Alaska. Child Abuse Negl. 2004;28(1):9–23.
Keenan HT, Runyan DK, Marshall SW, et al. A population-based comparison of clinical and outcome characteristics of young children with serious inflicted and noninflicted traumatic brain injury. Pediatrics. 2004;114(3):633–9.
Billmire ME, Myers PA. Serious head injury in infants: accident or abuse? Pediatrics. 1985;75(2):340–2.
Bruce DA, Zimmerman RA. Shaken impact syndrome. Pediatr Ann. 1989;18(8):482–94.
Kesler H, Dias MS, Shaffer M, et al. Demographics of abusive head trauma in the Commonwealth of Pennsylvania. J Neurosurg Pediatr. 2008;1(5):351–6.
Keenan HT, Runyan DK, Marshall SW, et al. A population-based study of inflicted traumatic brain injury in young children. JAMA. 2003;290(5):621–6.
Barlow K, Thompson E, Johnson D, et al. The neurological outcome of non-accidental head injury. Pediatr Rehabil. 2004;7(3):195–203.
Barlow KM, Thomson E, Johnson D, et al. Late neurologic and cognitive sequelae of inflicted traumatic brain injury in infancy. Pediatrics. 2005;116(2):e174–85.
Barr RG. Crying as a trigger for abusive head trauma: a key to prevention. Pediatr Radiol. 2014;44(4):S559–64.
Barlow KM, Minns RA. Annual incidence of shaken impact syndrome in young children. Lancet. 2000;356(9241):1571–2.
Selassie AW, Borg K, Busch C, et al. Abusive head trauma in young children: a population-based study. Pediatr Emerg Care. 2013;29(3):283–91.
Ellingson KD, Leventhal JM, Weiss HB. Using hospital discharge data to track inflicted traumatic brain injury. Am J Prev Med. 2008;34(4):S157–62.
Jenny C, Hymel KP, Ritzen A, et al. Analysis of missed cases of abusive head trauma. JAMA. 1999;281(7):621–6.
• Ryan ME, Palasis S, Saigal G, et al. ACR appropriateness criteria head trauma child. J Am Coll Radiol. 2014;11(10):939–47. Updated, evidence-based guidelines issued by the American College of Radiology to assist physicians in making imaging decisions for pediatric head trauma, including suspected AHT.
• Langford S, Panigrahy A, Narayanan S, et al. Neuroradiology. Multiplanar reconstructed CT images increased depiction of intracranial hemorrhages in pediatric head trauma. 2015 (Epub ahead of print). Study demonstrating increased sensitivity of multiplanar reconstruction of volumetric CT data for detection of traumatic intracranial hemorrhage.
Rubin DM, Christian CW, Bilaniuk LT, et al. Occult head injury in high-risk abused children. Pediatrics. 2003;111(6 Pt 1):1382–6.
Laskey AL, Holsti M, Runyan DK, et al. Occult head trauma in young suspected victims of physical abuse. J Pediatr. 2004;144(6):719–22.
Kemp AM, Rajaram S, Mann M, et al. What neuroimaging should be performed in children in whom inflicted brain injury (iBI) is suspected?: a systematic review. Clin Radiol. 2009;64(5):473–83.
Datta S, Stoodley N, Jayawant S, et al. Neuroradiological aspects of subdural haemorrhages. Arch Dis Child. 2005;90(9):947–51.
Biousse V, Suh DY, Newman NJ, et al. Diffusion-weighted magnetic resonance imaging in shaken baby syndrome. Am J Ophthalmol. 2002;133(2):249–55.
Beauchamp MH, Ditchfield M, Babl FE, et al. Detecting traumatic brain lesions in children: CT versus MRI versus susceptibility weighted imaging (SWI). J Neurotrauma. 2011;28(6):915–27.
•• Colbert CA, Holshouser BA, Aaen GS, et al. Value of cerebral microhemorrhages detected with susceptibility-weighted MR imaging for prediction of long-term outcome in children with nonaccidental trauma. Radiology. 2010;256(3):898–905. Retrospective case review demonstrating that presence of HII and parenchymal microhemorrhages at presentation are the two most important independent imaging predictors of poor functional outcome following AHT.
Vezina G. Assessment of the nature and age of subdural collections in nonaccidental head injury with CT and MRI. Pediatr Radiol. 2009;39(6):586–90.
Hedlund GL. Subdural hemorrhage in abusive head trauma: imaging challenges and controversies. J Am Osteopath Coll Radiol. 2012;1(1):23–30.
Choudhary AK, Bradford RK, Dias MS, et al. Spinal subdural hemorrhage in abusive head trauma: a retrospective study. Radiology. 2012;262(1):216–23.
•• Choudhary AK, Ishak R, Zacharia TT, et al. Imaging of spinal injury in abusive head trauma: a retrospective study. Pediatr Radiol. 2014;44(9):1130–40. Retrospective case control study of cervical spine imaging in AHT and nAHT patients, demonstrating a significantly higher prevalence of CCJ ligamentous injury in AHT than has previously been reported and than was present in control nAHT cases, and further demonstrating an association between CCJ injury and HII.
Koumellis P, McConachie NS, Jaspan T. Spinal subdural haematomas in children with non-accidental head injury. Arch Dis Child. 2009;94(3):216–9.
• Kadom N, Khademian Z, Vezina G. Usefulness of MRI detection of cervical spine and brain injuries in the evaluation of abusive head trauma. Pediatr Radiol. 2014;44(7):839–48. Retrospective review of cervical spine MR in suspected AHT demonstrating CCJ ligamentous injury in a substantial number of AHT cases, and demonstrating a correlation between cervical injury and HII.
Kemp AM, Joshi AH, Mann M, et al. What are the clinical and radiological characteristics of spinal injuries from physical abuse: a systematic review. Arch Dis Child. 2010;95(5):355–60.
Guthkelch AN. Infantile subdural haematoma and its relationship to whiplash injuries. Br Med J. 1971;2(5759):430–1.
Caffey J. On the theory and practice of shaking infants: its potential residual effects of permanent brain damage and mental retardation. Am J Dis Child. 1972;124(2):161–9.
Caffey J. The whiplash shaken infant syndrome: manual shaking by the extremities with whiplash-induced intracranial and intraocular bleedings, linked with residual permanent brain damage and mental retardation. Pediatrics. 1974;54(4):396–403.
Christian CW, Block R, Jenny C. Abusive head trauma in infants and children. Pediatrics. 2009;123(5):1409–11.
Case ME. Distinguishing accidental from inflicted head trauma at autopsy. Pediatr Radiol. 2014;44(4):S632–40.
Case ME, Graham MA, Handy TC, et al. Position paper on fatal abusive head injuries in infants and young children. Am J Forensic Med Pathol. 2001;22(2):112–22.
Smith SM, Hanson R. 134 battered children: a medical and psychological study. Br Med J. 1974;3(5932):666–70.
Gilliland MG, Folberg R. Shaken babies: some have no impact injuries. J Forensic Sci. 1996;41(1):114–6.
Lazoritz S, Baldwin S, Kini N. The whiplash shaken infant syndrome: has Caffey’s syndrome changed or have we changed his syndrome? Child Abuse Negl. 1997;21(10):1009–14.
DiScala C, Sege R, Li G, et al. Child abuse and unintentional injuries: a 10-year retrospective. Arch Pediatr Adolesc Med. 2000;154(1):16–22.
•• Vinchon M, Delestret I, DeFoort-Dhellemmes S, et al. Subdural hematoma in infants: can it occur spontaneously? Data from a prospective series and critical review of the literature. Childs Nerv Syst. 2010;26(9):1195–205. Prospective evaluation of case series of suspected AHT demonstrating that SDH can occur spontaneously or with trivial trauma, typically in the setting of enlarged subarachnoid spaces.
•• Piteau SJ, Ward MG, Barrowman NJ, et al. Clinical and radiographic characteristics associated with abusive and nonabusive head trauma: a systematic review. Pediatrics. 2012;130(2):315–23. Systematic review of 24 studies evaluating the radiographic characteristics of AHT and nATH, demonstrating association of impulsive-type injuries and AHT.
• Kemp AM, Jaspan T, Griffiths J, et al. Neuroimaging: what neuroradiological features distinguish abusive from non-abusive head trauma? A systematic review. Arch Dis Child. 2011;96(12):1103–12. Systematic review of 21 studies evaluating the radiographic characteristics of AHT and nATH, demonstrating association of impulsive-type injuries and AHT.
Kelly P, John S, Vincent AL, et al. Abusive head trauma and accidental head injury: a 20-year comparative study of referrals to a hospital child protection team. Arch Dis Child. 2015 (Epub ahead of print).
Fanconi M, Lips U. Shaken baby syndrome in Switzerland: results of a prospective follow-up study, 2002–2007. Eur J Pediatr. 2010;169(8):1023–8.
Squier W, Mack J. The neuropathology of infant subdural haemorrhage. Forensic Sci Int. 2009;187(1–3):6–13.
Duhaime AC, Christian CW, Rorke LB, et al. Nonaccidental head injury in infants: the “shaken-baby syndrome”. N Engl J Med. 1998;338(25):1822–9.
Hanna JA. The aetiology of subdural hematoma (an anatomical and pathological study). J Nerv Ment Dis. 1936;84(2):169–86.
Miller JD, Nader R. Acute subdural hematoma from bridging vein rupture: a potential mechanism for growth. J Neurosurg. 2014;120(6):1378–84.
Maxeiner H. Detection of ruptured cerebral bridging veins at autopsy. Forensic Sci Int. 1997;89(1–2):103–10.
Rambaud C. Bridging veins and autopsy findings in abusive head trauma. Pediatr Radiol. 2015;45(8):1126–31.
Maxeiner H. Demonstration and interpretation of bridging vein ruptures in cases of infantile subdural bleedings. J Forensic Sci. 2001;46(1):85–93.
Ehrlich E, Maxeiner H, Lange J. Postmortem radiological investigation of bridging vein ruptures. Leg Med (Tokyo). 2003;5(1):S225–7.
Stein KM, Ruf K, Ganten MK, et al. Representation of cerebral bridging veins in infants by postmortem computed tomography. Forensic Sci Int. 2006;163(1–2):93–101.
•• Hahnemann ML, Kinner S, Schweiger B, et al. Imaging of bridging vein thrombosis in infants with abusive head trauma: the “Tadpole Sign”. Eur Radiol. 2015;25(2):299–305. Retrospective case review demonstrating an association of bridging vein thrombosis with traumatic SDH.
• Choudhary AK, Bradford R, Dias MS, et al. Venous injury in abusive head trauma. Pediatr Radiol. 2015 (Epub ahead of print). Retrospective case review demonstrating the association of isolated bridging vein thrombosis with AHT, and differentiating this pattern of venous abnormality from that seen in primary DSVT.
Adamsbaum C, Rambaud C. Abusive head trauma: don’t overlook bridging vein thrombosis. Pediatr Radiol. 2012;42(11):1298–300.
Vázquez E, Delgado I, Sánchez-Montañez A, et al. Imaging abusive head trauma: why use both computed tomography and magnetic resonance imaging? Pediatr Radiol. 2014;44(4):S589–603.
•• Sieswerda-Hoogendoorn T, Postema FA, Verbaan D, et al. Age determination of subdural hematomas with CT and MRI: a systematic review. Eur J Radiol. 2014;83(7):1257–68. A systematic review of 22 studies evaluating the age of SDH based on CT and MR imaging features, demonstrating broad overlap in imaging appearance at different stages of evolution with particular variation seen in children, in AHT, and on MR.
Joy HM, Anscombe AM, Gawne-Cain ML. Blood-stained, acute subdural hygroma mimicking a subacute subdural haematoma in non-accidental head injury. Clin Radiol. 2007;62(7):703–6.
Offiah C, St Clair Forbes W, Thorne J. Non-haemorrhagic subdural collection complicating rupture of a middle cranial fossa arachnoid cyst. Br J Radiol. 2006;79(937):79–82.
• Wittschieber D, Karger B, Niederstadt T, et al. Subdural hygromas in abusive head trauma: pathogenesis, diagnosis, and forensic implications. AJNR Am J Neuroradiol. 2015;36(3):432–9. Review article synthesizing the current understanding of the evolution of acute SDH to chronic SDH by way of subdural hygroma, related to neomembrane formation and repeated rebleeding in select cases with predisposing factors, including parenchymal volume loss or shunting.
Bradley WG Jr. MR appearance of hemorrhage in the brain. Radiology. 1993;189(1):15–26.
Duhem R, Vinchon M, Tonnelle V, et al. Principaux aspects évolutifs du signal des hématomes sous-duraux en IRM et intérêts pratiques dans la datation des traumatismes crâniens. Neurochirurgie. 2006;52(2–3 Pt 1):93–104.
Hymel KP, Jenny C, Block RW. Intracranial hemorrhage and rebleeding in suspected victims of abusive head trauma: addressing the forensic controversies. Child Maltreat. 2002;7(4):329–48.
Vinchon M, Noulé N, Tchofo PJ, et al. Imaging of head injuries in infants: temporal correlates and forensic implications for the diagnosis of child abuse. J Neurosurg. 2004;101(1):44–52.
Adamsbaum C, Morel B, Ducot B, et al. Dating the abusive head trauma episode and perpetrator statements: key points for imaging. Pediatr Radiol. 2014;44(4):S578–88.
Geddes JF, Tasker RC, Hackshaw AK, et al. Dural haemorrhage in non-traumatic infant deaths: does it explain the bleeding in ‘shaken baby syndrome’? Neuropathol Appl Neurobiol. 2003;29(1):14–22.
Barnes PD. Imaging of nonaccidental injury and the mimics: issues and controversies in the era of evidence-based medicine. Radiol Clin N Am. 2011;49(1):205–29.
Barnes PD, Galaznik J, Gardner H, et al. Infant acute life-threatening event: dysphagic choking versus nonaccidental injury. Semin Pediatr Neurol. 2010;17(1):7–11.
Rafaat KT, Spear RM, Kuelbs C, et al. Cranial computed tomographic findings in a large group of children with drowning: diagnostic, prognostic, and forensic implications. Pediatr Crit Care Med. 2008;9(6):567–72.
Hurley M, Dineen R, Padfield CJ, et al. Is there a causal relationship between the hypoxia-ischaemia associated with cardiorespiratory arrest and subdural haematomas?: an observational study. Br J Radiol. 2010;83(993):736–43.
Talbert DG. Paroxysmal cough injury, vascular rupture and ‘shaken baby syndrome’. Med Hypotheses. 2005;64(1):8–13.
Geddes JF, Talbert DG. Paroxysmal coughing, subdural and retinal bleeding: a computer modelling approach. Neuropathol Appl Neurobiol. 2006;32(6):625–34.
Marshall JN. Aphasia and cerebral haemorrhage complicating whooping-cough. Glasg Med J. 1885;23:24–7.
Watts CC, Acosta C. Pertussis and bilateral subdural hematomas. Am J Dis Child. 1969;118(3):518–9.
Greeley CS. Letter to the editor. Semin Pediatr Neurol. 2010;17:275–8.
Sahoo RK, Tripathy P, Praharaj HN. Cerebral venous sinus thrombosis with nontraumatic subdural hematoma. Int J Crit Illn Inj Sci. 2015;5(1):59.
Akins PT, Axelrod YK, Ji C, et al. Cerebral venous sinus thrombosis complicated by subdural hematomas: case series and literature review. Surg Neurol Int. 2013;4:85.
•• McLean LA, Frasier LD, Hedlund GL. Does intracranial venous thrombosis cause subdural hemorrhage in the pediatric population? AJNR Am J Neuroradiol. 2012;33(7):1281–4. Retrospective review of cases of intracranial venous thrombosis in children demonstrating no association with SDH.
Kwong Y, Jaspan T. Venous sinus thrombosis and subdural haematomas in infants: is there a causal link? Arch Dis Child. 2013;98(8):650–1.
Thomas AG, Hegde SV, Dineen RA, et al. Patterns of accidental craniocerebral injury occurring in early childhood. Arch Dis Child. 2013;98(10):787–92.
Foster KA, Recker MJ, Lee PS, et al. Factors associated with hemispheric hypodensity after subdural hematoma following abusive head trauma in children. J Neurotrauma. 2014;31(19):1625–31.
Calder IM, Hill I, Scholtz CL. Primary brain trauma in non-accidental injury. J Clin Pathol. 1984;37(10):1095–100.
Vowles GH, Scholtz CL, Cameron JM. Diffuse axonal injury in early infancy. J Clin Pathol. 1987;40(2):185–9.
Geddes JF, Hackshaw AK, Vowles GH, et al. Neuropathology of inflicted head injury in children I. Patterns of brain damage. Brain. 2001;124(Pt 7):1290–8.
Geddes JF, Vowles GH, Hackshaw AK, et al. Neuropathology of inflicted head injury in children. II. Microscopic brain injury in infants. Brain. 2001;124(Pt 7):1299–306.
Shannon P, Smith CR, Deck J, et al. Axonal injury and the neuropathology of shaken baby syndrome. Acta Neuropathol. 1998;95(6):625–31.
Matschke J, Büttner A, Bergmann M, et al. Encephalopathy and death in infants with abusive head trauma is due to hypoxic-ischemic injury following local brain trauma to vital brainstem centers. Int J Leg Med. 2015;129(1):105–14.
Atkinson JL, Anderson RE, Murray MJ. The early critical phase of severe head injury: importance of apnea and dysfunctional respiration. J Trauma. 1998;45(5):941–5.
Johnson DL, Boal D, Baule R. Role of apnea in nonaccidental head injury. Pediatr Neurosurg. 1995;23(6):305–10.
Kemp AM, Stoodley N, Cobley C. Apnoea and brain swelling in non-accidental head injury. Arch Dis Child. 2003;88(6):472–6.
Ichord RN, Naim M, Pollock AN, et al. Hypoxic-ischemic injury complicates inflicted and accidental traumatic brain injury in young children: the role of diffusion-weighted imaging. J Neurotrauma. 2007;24(1):106–18.
Wagner AK, Bayir H, Ren D, et al. Relationships between cerebrospinal fluid markers of excitotoxicity, ischemia, and oxidative damage after severe TBI: the impact of gender, age, and hypothermia. J Neurotrauma. 2004;21(2):125–36.
Bayir H, Kochanek PM, Kagan VE. Oxidative stress in immature brain after traumatic brain injury. Dev Neurosci. 2006;28(4–5):420–31.
Greiner MV, Greiner HM, Caré MM, et al. Adding insult to injury: nonconvulsive seizures in abusive head trauma. J Child Neurol. 2015 (Epub ahead of print).
McKinney AM, Thompson LR, Truwit CL, et al. Unilateral hypoxic-ischemic injury in young children from abusive head trauma, lacking craniocervical vascular dissection or cord injury. Pediatr Radiol. 2008;38(2):164–74.
Cantu RC, Gean AD. Second-impact syndrome and a small subdural hematoma: an uncommon catastrophic result of repetitive head injury with a characteristic imaging appearance. J Neurotrauma. 2010;27(9):1557–64.
Kuroda Y, Bullock R. Failure of cerebral blood flow-metabolism coupling after acute subdural hematoma in the rat. Neurosurgery. 1992;31(6):1062–71.
Suh DY, Davis PC, Hopkins KL, et al. Nonaccidental pediatric head injury: diffusion-weighted imaging findings. Neurosurgery. 2001;49(2):309–18.
Zimmerman RA, Bilaniuk LT, Farina L. Non-accidental brain trauma in infants: diffusion imaging, contributions to understanding the injury process. J Neuroradiol. 2007;34(2):109–14.
• Imagawa KK, Hamilton A, Ceschin R, et al. Characterization of microstructural injury: a novel approach in infant abusive head trauma-initial experience. J Neurotrauma. 2014;31(19):1632–8. Retrospective review of DTI data from AHT cases demonstrating that reductions in mean diffusivity that occur in diffuse parenchymal injury related to decreased axial diffusivity, with preserved radial diffusivity and fractional anisotropy, supportive of diffuse ischemic axonopathy as the etiology of the signal abnormality.
Chavez-Valdez R, Martin LJ, Northington FJ. Programmed necrosis: a prominent mechanism of cell death following neonatal brain injury. Neurol Res Int. 2012;2012:257563.
Duhaime AC, Gennarelli TA, Thibault LE, et al. The shaken baby syndrome: a clinical, pathological, and biomechanical study. J Neurosurg. 1987;66(3):409–15.
Cory CZ, Jones BM. Can shaking alone cause fatal brain injury? A biomechanical assessment of the Duhaime shaken baby syndrome model. Med Sci Law. 2003;43(4):317–33.
Lindenberg R, Freytag E. Morphology of brain lesions from blunt trauma in early infancy. Arch Pathol. 1969;87(3):298–305.
Jaspan T, Narborough G, Punt JA, et al. Cerebral contusional tears as a marker of child abuse: detection by cranial sonography. Pediatr Radiol. 1992;22(4):237–45.
• Palifka LA, Frasier LD, Metzger RR, et al. Parenchymal brain lacerations in abusive head trauma. American Society of Neuroradiology Meeting. May 2014, Montreal. AJNR Am J Neuroradiol. 2015 (In Press). Retrospective case control study of ATH and nAHT cases demonstrating that parenchymal brain lacerations are characteristic injuries in AHT.
Sigmund GA, Tong KA, Nickerson JP, et al. Multimodality comparison of neuroimaging in pediatric traumatic brain injury. Pediatr Neurol. 2007;36(4):217–26.
Levin AV, Cordovez JA, Leiby BE, et al. Retinal hemorrhage in abusive head trauma: finding a common language. Trans Am Ophthalmol Soc. 2014;112:1–10.
Maguire SA, Watts PO, Shaw AD, et al. Retinal haemorrhages and related findings in abusive and non-abusive head trauma: a systematic review. Eye (Lond). 2013;27(1):28–36.
Binenbaum G, Christian CW, Ichord RN, et al. Retinal hemorrhage and brain injury patterns on diffusion-weighted magnetic resonance imaging in children with head trauma. J AAPOS. 2013;17(6):603–8.
Greenwald MJ, Weiss A, Oesterle CS, et al. Traumatic retinoschisis in battered babies. Ophthalmology. 1986;93(5):618–25.
Altinok D, Saleem S, Zhang Z, et al. MR imaging findings of retinal hemorrhage in a case of nonaccidental trauma. Pediatr Radiol. 2009;39(3):290–2.
•• Beavers AJ, Stagner AM, Allbery SM, et al. MR detection of retinal hemorrhages: correlation with graded ophthalmologic exam. Pediatr Radiol. 2015;45(9):1363–71. Retrospective review demonstrating high detection rate of RH on standard MR sequences, with increased sensitivity for higher grade hemorrhages confirmed by dilated fundoscopy.
• Zuccoli G, Panigrahy A, Haldipur A, et al. Susceptibility weighted imaging depicts retinal hemorrhages in abusive head trauma. Neuroradiology. 2013;55(7):889–93. Retrospective review demonstrating improved sensitivity for detection of RH on SWI over standard MR protocols, with the highest sensitivity related to use of a dedicated high resolution SWI orbital protocol.
Hobbs CJ. Skull fracture and the diagnosis of abuse. Arch Dis Child. 1984;59(3):246–52.
Meservy CJ, Towbin R, McLaurin RL, et al. Radiographic characteristics of skull fractures resulting from child abuse. AJR Am J Roentgenol. 1987;149(1):173–5.
Reece RM, Sege R. Childhood head injuries: accidental or inflicted? Arch Pediatr Adolesc Med. 2000;154(1):11–5.
Knox JB, Schneider JE, Cage JM, et al. Spine trauma in very young children: a retrospective study of 206 patients presenting to a level 1 pediatric trauma center. J Pediatr Orthop. 2014;34(7):698–702.
Barber I, Perez-Rossello JM, Wilson CR, et al. The yield of high-detail radiographic skeletal surveys in suspected infant abuse. Pediatr Radiol. 2015;45(1):69–80.
Barber I, Perez-Rossello JM, Wilson CR, et al. Prevalence and relevance of pediatric spinal fractures in suspected child abuse. Pediatr Radiol. 2013;43(11):1507–15.
Silvera VM, Danehy AR, Newton AW, et al. Retroclival collections associated with abusive head trauma in children. Pediatr Radiol. 2014;44(4):S621–31.
Kemp A, Cowley L, Maguire S. Spinal injuries in abusive head trauma: patterns and recommendations. Pediatr Radiol. 2014;44(4):S604–12.
Hadley MN, Sonntag VK, Rekate HL, et al. The infant whiplash-shake injury syndrome: a clinical and pathological study. Neurosurgery. 1989;24(4):536–40.
Feldman KW, Weinberger E, Milstein JM, et al. Cervical spine MRI in abused infants. Child Abuse Negl. 1997;21(2):199–205.
Brennan LK, Rubin D, Christian CW, et al. Neck injuries in young pediatric homicide victims. J Neurosurg Pediatr. 2009;3(3):232–9.
Koshy J, Scheurkogel MM, Clough L, et al. Neuroimaging findings of retroclival hemorrhage in children: a diagnostic conundrum. Childs Nerv Syst. 2014;30(5):835–9.
Tubbs RS, Griessenauer CJ, Hankinson T, et al. Retroclival epidural hematomas: a clinical series. Neurosurgery. 2010;67(2):404–6.
Roach JP, Acker SN, Bensard DD, et al. Head injury pattern in children can help differentiate accidental from non-accidental trauma. Pediatr Surg Int. 2014;30(11):1103–6.
Drubach LA, Johnston PR, Newton AW, et al. Skeletal trauma in child abuse: detection with 18F-NaF PET. Radiology. 2010;255(1):173–81.
•• Vinchon M, de Foort-Dhellemmes S, Desurmont M, et al. Confessed abuse versus witnessed accidents in infants: comparison of clinical, radiological, and ophthalmological data in corroborated cases. Childs Nerv Syst. 2010;26(5):637–45. Prospective evaluation of patterns of injury in AHT and nAHT with inclusion based on perpetrator confession or witnessed nAHT to minimize circularity bias, confirming the association of impulsive-type injuries in AHT in children under age 2.
Pollina J, Dias MS, Li V, et al. Cranial birth injuries in term newborn infants. Pediatr Neurosurg. 2001;35(3):113–9.
Rooks VJ, Eaton JP, Ruess L, et al. Prevalence and evolution of intracranial hemorrhage in asymptomatic term infants. AJNR Am J Neuroradiol. 2008;29(6):1082–9.
Whitby EH, Griffiths PD, Rutter S, et al. Frequency and natural history of subdural haemorrhages in babies and relation to obstetric factors. Lancet. 2004;363(9412):846–51.
Looney CB, Smith JK, Merck LH, et al. Intracranial hemorrhage in asymptomatic neonates: prevalence on MR images and relationship to obstetric and neonatal risk factors. Radiology. 2007;242(2):535–41.
Laghmari M, Skiker H, Handor H, et al. Hémorragies rétiniennes liées à l’accouchement chez le nouveau-né: fréquence et relation avec les facteurs maternels, néonataux et obstétricaux. Étude prospective de 2031 cas. J Fr Ophtalmol. 2014;37(4):313–9.
Sanchez T, Stewart D, Walvick M, et al. Skull fracture vs. accessory sutures: how can we tell the difference? Emerg Radiol. 2010;17(5):413–8.
Marino MA, Morabito R, Vinci S, et al. Benign external hydrocephalus in infants: a single centre experience and literature review. Neuroradiol J. 2014;27(2):245–50.
•• McKeag H, Christian CW, Rubin D, Daymont C, Pollock AN, Wood J. Subdural hemorrhage in pediatric patients with enlargement of the subarachnoid spaces. J Neurosurg Pediatr. 2013;11(4):438–44. Retrospective review demonstrating that spontaneous SDH can rarely occur in the setting of BESS, but that AHT should still be ruled out, as some percentage of patients may have signs of abuse notwithstanding BESS as a possible etiology.
Greiner MV, Richards TJ, Care MM, et al. Prevalence of subdural collections in children with macrocrania. AJNR Am J Neuroradiol. 2013;34(12):2373–8.
Aoki N, Masuzawa H. Infantile acute subdural hematoma: clinical analysis of 26 cases. J Neurosurg. 1984;61(2):273–80.
Nishimoto H, Kurihara J. Re-estimation of acute subdural hematoma in children caused by trivial household head trauma. Shoni No Noshinkei. 2006;31(3):215–23.
McNeely PD, Atkinson JD, Saigal G, et al. Subdural hematomas in infants with benign enlargement of the subarachnoid spaces are not pathognomonic for child abuse. AJNR Am J Neuroradiol. 2006;27(8):1725–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Pediatrics.
Rights and permissions
About this article
Cite this article
Nixon, J.N., Soares, B.P. Imaging of Abusive Head Trauma: A Review and Update. Curr Radiol Rep 4, 8 (2016). https://doi.org/10.1007/s40134-015-0136-6
Published:
DOI: https://doi.org/10.1007/s40134-015-0136-6