Abstract
Background
Abusive head trauma (AHT) in young children usually has a severe outcome when associated with hypoxic-ischemic encephalopathy (HIE), which is best characterized by MRI in the acute or subacute phase utilizing diffusion-weighted imaging (DWI). HIE in this setting has been hypothesized to result from stretching of the spinal cord, brainstem, or vasculature.
Objective
To provide clinical correlation in patients with unilateral HIE and to postulate a mechanism in the setting of suspected AHT.
Materials and methods
IRB approval was obtained. Over a 5-year period, the medical records and images were reviewed of the 53 children ≤3 years of age who presented with acute head trauma according to the hospital registry. The children were subselected in order to determine how many suffered either HIE or AHT, and to detect those with unilateral HIE.
Results
In 11 of the 53 children, the etiology of the head trauma was highly suspicious for abuse. In 38 the head trauma was accidental and in 4 the trauma was of unknown etiology and at the time of this report was unresolved legally. Of the 53, 4 suffered HIE confirmed by CT or MRI. In three of these four with HIE the trauma was considered highly suspicious for AHT. Two of these three were the only patients with unilateral HIE, and both (7 months and 14 months of age) presented with early subacute phase HIE seen on DW MRI (range 4–7 days) and are described in detail with clinical correlation. The third child with AHT and HIE had bilateral findings. In the fourth patient the HIE was bilateral and was considered accidental. The work-up for both patients with unilateral HIE included head CT, craniocervical MRI, and craniocervical MR angiography (MRA). In both, there was mostly unilateral, deep white matter restricted diffusion, with subdural hematomas that were small compared to the extent of hypoxic-ischemic insult, and no skull fracture. Craniocervical MRA and axial thin-section fat-saturation images were negative for dissection, brainstem, or cord injury. Legal authorities obtained a confession of inflicted injury in one and a partial confession in the second (which did not fit the extent of injury). Five other children with HIE (based on DWI) were found during this period who had not suffered head trauma; all were bilateral insults.
Conclusion
HIE associated with AHT might present with largely unilateral white matter injury on DWI following extensive cortical infarction. We propose that unilateral HIE in a young child might be a sign of AHT and might result from cervical vascular compression, whether from kinking during hyperflexion/hyperextension or from direct strangulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypoxic-ischemic encephalopathy (HIE) in adults and in children generally reflects a global and bilateral insult, whether related to disrupted cerebral blood supply or hypoxia from insufficient perfusion. There are a multitude of potential causes, the most common of which are cardiac arrest and respiratory failure [1]. However, in a young child with HIE, abusive head trauma (AHT) is an additional, significant consideration, particularly if there are concomitant imaging findings including subdural hemorrhage (SDH), skull fractures or retinal hemorrhages, or an inconsistent clinical history [2–5]. Neurologic outcomes in children suffering AHT are generally poor relative to those of children suffering accidental trauma, particularly when HIE is involved [6–8]. Acute HIE (<3 days) in children and adults is demonstrated well by diffusion-weighted (DW) MRI. In the acute phase (<3 days) diffuse cortical restriction is seen, while in the subacute phase (>3 days) the findings are more extensive and can include diffuse white matter restricted diffusion [3–5, 8–11]. It has been hypothesized that HIE from AHT is caused by cervical cord, brainstem, or arterial injuries, presumably from hyperextension with stretching of the cervicomedullary junction [12–16].
Hence, after initially encountering a patient with unilateral HIE, we set out to retrospectively identify how many patients with HIE from AHT had been seen in our institution, and thereafter how many of these had unilateral HIE, by reviewing our acute head trauma registry of young children ≤3 years age. In this age group, HIE has been preliminarily shown to be more often related to AHT than to accidental trauma [17]. As acute or subacute HIE is readily detectable by MRI, the goal was to determine how many of these had largely unilateral HIE in the acute or subacute phase, as depicted by DW MRI. Out of this series of children with acute head trauma, we describe in detail two cases of largely unilateral HIE from AHT, both lacking cervicocranial dissection, brainstem, or spinal cord injury on MRI.
Materials and methods
Initially, the neuroradiology and child abuse team staff physicians noted a patient with largely unilateral hypoxic-ischemic injury on DW MR images. It was then decided to review young children (≤3 years of age) with acute head trauma admitted to the hospital to discover any more with such an appearance, and whether they had undergone DW MRI. IRB approval was obtained. DW imaging (DWI) became a standard imaging sequence at our institution in late 2001, so the period of study was approximately 5.5 years from January 2002 until mid-2007. A list was obtained from the hospital’s trauma registry of all children ≤3 years of age who had suffered acute head trauma (n = 53). The medical records and CT (n = 50) and MRI images (n = 15) were reviewed. Patients were then categorized by the child abuse team’s clinical assessment (in combination with the legal assessment) as to whether they had suffered accidental head trauma, nonaccidental (abusive) head trauma (AHT), or as-yet-unresolved head trauma (unknown or case pending). A log was kept of the pertinent imaging findings, including the presence/absence of HIE, intracranial hemorrhage (and type), and skull fractures. These patients were reviewed by a neuroradiology staff physician and staff member of the child abuse team. After identifying those patients who had suffered HIE, the imaging of those with unilateral HIE (based on DW MRI and repeat CT or MRI scans) was then scrutinized further to evaluate the potential cause and postulate a mechanism for this uncommon appearance. Concurrently, a search was also performed of the neuroradiology case files and registry to identify any other children ≤3 years old with a nontraumatic HIE insult (excluding neonatal insults) based on DW MRI, in order to identify whether any had suffered unilateral injury.
MRI technique
The techniques for the brain MRI scans varied over the 5.5-year period, as both 1.5-T and 3.0-T scanners were utilized, but the standard protocol in the 15 patients with head trauma included axial T1-weighted (T1-W), T2-weighted (T2-W), FLAIR, T2* gradient recalled echo (GRE, to detect hemorrhage), and DWI without intravenous administration of contrast agent; imaging after contrast agent administration was not standard, being used in only one patient presenting in the subacute phase, and at our hospital it has not been traditionally utilized in the setting of trauma unless the diagnosis of trauma or HIE and the age of the insults are in question (Fig. 1). MRI parameters on the 1.5-T systems were TR/TE/TI 6,500–9,000/105–110/2,000–2,100 ms, NEX 1–2 and echo train length 15–23 with a 5-mm section thickness for FLAIR images, and TR/TE 3,300–4,000/71–120 ms with a 5-mm thickness for DWI. For the 3.0-T systems, parameters were TR/TE/TI 9,000–11,000/100–120/2,000–2,100 ms, NEX 1–2 and echo train length 10–25 with a 5-mm thickness for FLAIR, and TR/TE 2,800–3,000/70–90 ms with a 5-mm thickness for DWI. A gradient strength of b=1,000 s/mm2 was used for axial DWI.
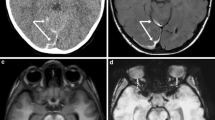
Unilateral HIE in suspected AHT (patient 1, a 7-month-old female infant). a, b CT scans obtained at an outside institution 4 days after a purported history of a sibling falling on her demonstrate subtle loss of gray–white differentiation in the posterior left temporal-parietal region (a) and a small interhemispheric SDH (b) without evidence of skull fracture on bone windows (not shown). c, d MRI performed at that institution 1 day later: the DW image (c) demonstrates a diffuse, acute cortical insult and the GRE image (d) confirms interhemispheric and convexity SDHs (arrows). On admission to our institution (7 days after the insult), a CT scan (not shown) showed progression of the brain swelling. e, f Repeat MRI to evaluate for vascular injury: the craniocervical MRA image (e) and the dedicated fat-saturation T2-W craniocervical image (f, arrows internal carotid artery) are unremarkable, as were T1-W craniocervical images (not shown). Cerebral swelling is confirmed on the FLAIR image (g), with diffuse cytotoxic injury visualized throughout the left cerebral white matter on the DW image and ADC map (h). i MR image (obtained 18 days after the insult) shows diffuse cortical enhancement consistent with late subacute cortical infarcts
Results
The mean age at presentation of the 53 children ≤3 years of age admitted with acute head trauma was 10.1 months (range 7 days to 36 months). A total of 15 of the children had undergone MRI, 50 had undergone CT, and 2 had outside head imaging not available for review but were presumed not to have suffered HIE because both were neurologically intact. In 11 of the 53 children the imaging findings were highly suspicious for AHT, in 38 the head trauma was considered accidental and in 4 the etiology was unknown and at the time of this report was still unresolved legally. Of the 53 children, 20 had intracranial hemorrhage. Of the 11 with suspected AHT, 6 had hemorrhage (all subdural or epidural); of the other 5, 3 had a discernible skull fracture on CT. Of these 11 with suspected AHT, three suffered HIE, which was largely unilateral in two who underwent extensive craniocervical MRI work-up (described further below, Figs. 1 and 2). Of the 38 children with acute head trauma thought to be accidental, one suffered HIE, which was bilateral. This child had suffered a severe crush-type head trauma with cardiac arrest and died shortly thereafter without time or hemodynamic stability to perform MRI. Hence, there were a total of four patients (out of the 53) suffering HIE based on the acute head trauma registry.
Unilateral HIE in suspected AHT (patient 2). a, b CT scans obtained at an outside institution in an obtunded 14-month-old infant 1–2 h after a reported short fall demonstrate (a) a moderate-large left mixed-density SDH, cerebral swelling and midline shift, and (b) early blurring of the left gray–white interface near the vertex without skull fracture. Craniotomy was performed emergently. The right hemiparesis became more evident over the next 3 days. c T2*-W GRE cranial MR image shows diffuse left-side cerebral swelling without diffuse axonal injury, although a small SDH remains (arrows). d, e DW image (d) and ADC map (e) show severely restricted diffusion throughout the left cerebral white matter. Cranial MRA, MRV, cervical MRA, and dedicated craniocervical fat-saturation T1-W and T2-W images were unremarkable (not shown). CT images obtained 10 days after the insult showed early cortical hyperdensities consistent with cortical necrosis (not shown). f Follow-up CT image at 2 months shows multifocal cortical encephalomalacia with mild sparing of the opercular cortex
In three of the four patients with HIE confirmed by CT or MRI, the imaging findings were considered highly suspicious for AHT. In two of these three (ages 7 months and 14 months) the HIE was largely unilateral (Figs. 1 and 2); both presented in the early subacute phase as confirmed on DW MRI (range 4–7 days), which correlated with the clinical history (described in further detail below). The work-up for both patients with unilateral HIE included a noncontrast head CT scan, extensive MRI with thin fat-saturation images of the craniocervical region, and craniocervical MR angiography (MRA). In both, there was mostly unilateral deep white matter restricted diffusion, with subdural hematomas that were small compared to the extent of hypoxic-ischemic insult, and no skull fracture. Head and neck MRA and dedicated craniocervical MR images were negative in both for dissection, brainstem, and cord injury. Law enforcement officials obtained a confession of inflicted injury in both patients, but one was a partial confession in that the injury admitted to would have been less extensive than that seen. The third patient with HIE suspected to be from AHT had diffuse bilateral involvement, with some opercular sparing on one side, but did not undergo MRI (Fig. 3) because of concerns that he was too unstable for the procedure.
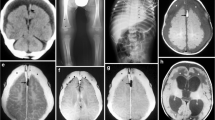
Bilateral HIE in suspected AHT. A 9-month-old boy suffered head trauma after an in-home day-care provider claimed that the infant was dropped from waist level onto a carpeted floor. The discordant history, along with other clinical findings, was considered highly suspicious for AHT. a, b CT images show (a) diffuse cerebral edema, with bilateral loss of gray–white matter differentiation, and (b) a moderately sized mixed-density SDH (arrows). c CT image obtained 4 months later demonstrates diffusely shrunken gyri as a sequela of severe HIE, with a small area of relatively spared cortex in the right frontal-temporal opercular region (arrows). Craniectomy was performed acutely to relieve the HIE-related edema, and the left skull depression is a result of subsequent sinking in of the cranioplasty bone to fill the space created by liquefactive necrosis of the cerebrum
Regarding nonacute head trauma, five additional nonneonatal pediatric patients (<3 years old) were identified during this time period who suffered HIE confirmed by MRI. In two of these the HIE was caused by congenital heart disease with cardiopulmonary arrest or surgical complications of the repair, in one by a hypotensive episode during a bout of infectious pneumonia, in one due to a prolonged febrile seizure, and one child died after admission with multiple injuries including liver and splenic lacerations and truncal and facial bruises (lacking head injury). In the last of these children the HIE was clinically suspected to represent abuse, with a discordant reported height of fall. Forced submersion was thought to account for the HIE, although the legal outcome of the case was still pending at the time of this report (Fig. 4). All had suffered bilateral diffuse insults as seen on DW images (as in Fig. 4). Additionally, none of these 5 had a demonstrable SDH on MR images.
Bilateral HIE from suspected abuse in a 29-month-old girl without external signs of head trauma. She suffered multiple solid organ lacerations (seen on thoracoabdominal CT) from a reported fall. a CT image of the head obtained the same day does not show hemorrhage or skull fracture and was interpreted as negative; however, the girl remained unresponsive. b MR FLAIR image obtained the following day on a 3.0-T scanner shows a nearly normal appearance with only possibly mildly hyperintense caudate nuclei without sulcal effacement. c The suboptimally windowed DW image demonstrates a pseudonormal appearance. d Appropriately windowed DW image, however, shows more pronounced gray-white differentiation e The ADC map confirms the findings. This case demonstrates how severe bilateral HIE might not be detectable on acute noncontrast CT and can even present some difficulties in diagnosis with MRI, necessitating careful assessment of the DW images along with the ADC maps
The two patients with unilateral HIE from suspected AHT are described in the following sections.
Case 1 of unilateral HIE in suspected AHT
A 7-month-old girl presented from an outside institution with multiple bruises and acute right-side seizures after a reported history of a 2-year-old sibling falling on her 5 days previously, with the infant’s head arching back sharply and subsequent decreased responsiveness. A CT scan on the day of presentation (4 days after the initial insult) to the outside institution (Fig. 1) demonstrated diffuse left-side cerebral swelling and suspected early infarcts with relative sparing of the cerebellum and brainstem, a small (2-mm) interhemispheric SDH (Fig. 1), and no evidence of a skull fracture. The tiny SDH was mostly homogeneous but whether there was an internal mixed density was difficult to determine due to its small size. The brain MRI from the outside institution performed 1 day later (5 days after the insult) demonstrated diffuse cortical infarction on DW images (Fig. 1), with confirmed left-side cerebral swelling and a small interhemispheric and convexity SDH on GRE images (Fig. 1). There was no evidence of diffuse axonal injury (DAI). MRA of the cervical and cranial vessels was normal.
The child was clinically deteriorating, so was transported to our institution while intubated and sedated. Head CT scan and brain MRI were repeated, at that point approximately 7 days after the insult. Lumbar puncture was not performed. The MRI included dedicated fat-saturation axial cranial and cervical T1-W and T2-W sequences as well as head and neck MRA to evaluate for arterial injury. The MRI and MRA were negative for vascular injury, brainstem and spinal cord injury (Fig. 1). However, the cranial MRI demonstrated left cerebral swelling with restricted diffusion throughout the deep and subcortical white matter (Fig. 1), consistent with subacute HIE, while the SDH was bright on T1-W and T2-W images, consistent with subacute hemorrhage and timing of the insult [18]. EEG also demonstrated abnormalities originating from the left cerebral hemisphere.
On clinical examination, unilateral left-side retinal hemorrhages were noted by a neurologist on the day of admission (5 days after the insult), and following an ophthalmology consultation (7 days after the insult). According to the medical record, the perinatal and postnatal history was unrevealing up until 2 months of age, when the baby presented to the local emergency room with a left downward gaze and listlessness, right-side seizures, and bruises on her cheek and back. At 6 months of age, she presented with rib fractures and a history of falling off a bed. A skeletal survey at that time showed healing fractures of the left posterior sixth and seventh ribs. The skeletal survey and a subsequent thoracoabdominal CT scan on admission to our institution did not reveal evidence of skeletal dysplasias. There was no available information about family history of such. Child protection authorities were involved, with the child subsequently placed in protective custody for presumed AHT. Law enforcement officials obtained a confession from her mother, an alcoholic, that she had punched the child in the back of her head, and knocked her down from a sitting position so that the infant hit her head on a television stand as she fell backward. Also, the mother admitted to covering her mouth sometime afterward to keep her from crying. MRI performed 18 days after the initial insult demonstrated no further restricted diffusion, diffuse cortical enhancement on postcontrast T1-W images consistent with late subacute phase insults and cortical necrosis (Fig. 1), and another unremarkable head MRA. There was residual right hemiparesis at the time of discharge, and the child maintained a left gaze preference. She was extubated and feeding orally at that point.
Case 2 of unilateral HIE in suspected AHT
A 14-month-old obtunded, previously healthy boy presented from a referring institution with a CT scan showing a left-side moderately sized mixed-density SDH, moderate mass effect and midline shift, and a small amount of traumatic subarachnoid hemorrhage, but no definite skull fracture (Fig. 2). However, subtle areas of cortical low attenuation were present, with blurring of the gray–white matter interface near the vertex, suspicious for small acute infarcts. The child was in the care of his mother’s boyfriend when he was reported to have fallen from a bed. On physical examination performed on admission to our institution, a large area of bruising consistent with a hand print was noted over his buttocks. The neurosurgeon noted intermittent left third nerve palsy, and, given the loss of gray–white differentiation and the mass effect, became concerned about the size and potential for growth of the subdural hematoma; hence, the hematoma was evacuated via craniotomy emergently. Underlying the SDH, a small focus of left frontal cortex was biopsied, and demonstrated acute petechial hemorrhage but no underlying inflammatory or neoplastic process. The dura itself was not submitted for analysis.
Multiple serial CT scans during the next 3 days demonstrated progression of the diminished gray–white matter differentiation, suspicious for diffuse infarcts, with a small residual SDH. Given the suspected infarcts, as well as a worsening right hemiparesis after evacuation of the SDH, brain MRI and MR venography (MRV) as well as head and neck MRA were performed 4 days after the insult, with additional axial fat-saturation cranial and cervical T1-W and T2-W imaging. The MRI, MRA, and MRV were negative regarding craniocervical vascular injury, brain stem edema, DAI, spinal cord edema, and venous thrombosis, but demonstrated diffuse left-side cerebral swelling and white matter restricted diffusion involving nearly all of the left cerebral hemisphere (Fig. 2). The residual SDH was bright on T1-W images and isointense on T2-W images, consistent with early subacute phase hematoma and the timing of the insult [18]. Additionally, there was involvement of a small portion of the right orbitofrontal region, in the apparent distribution of the right anterior cerebral artery.
On the admission clinical examination, bilateral severe retinal hemorrhages and bruises to the buttocks consistent with a hand print were noted, without neck or scalp bruises. Two lumbar punctures with culture (4 and 7 days after admission) were performed, but yielded only elevated RBCs in the CSF. EEG was not performed. Further history elicited by the clinicians discovered that the mother’s boyfriend had been babysitting the child for about 2 hours (approximately 1–2 hours before the outside CT scan was performed), during which the boyfriend stated that the child had fallen off a one-foot-high toddler bed. The perinatal and postnatal history prior to presentation was unremarkable, with normal growth and development up to the time of presentation. The plain film skeletal survey did not demonstrate any fractures. Given the discordant history and the nature of the injuries, the child abuse team determined that the situation was highly suspicious for AHT, and child protection and law enforcement authorities were involved. A restraining order was placed against the boyfriend, who admitted to hitting the child over the back area using his hand. Subsequent CT scans over the next 10 days demonstrated evolving dense cortical infarcts, with decreased swelling. On discharge, he still had signs of right hemiparesis, which had slightly improved. A CT scan performed 2 months after the injury (Fig. 2) demonstrated that encephalomalacia had developed throughout the cortex of both the left cerebral hemisphere and a small portion of the anterior right frontal lobe, corresponding to the abnormalities on the initial DWI.
Discussion
The term AHT applies to all mechanisms of inflicted central nervous system injury. It is also referred to with such terms as “shaken baby syndrome,” “shaken infant syndrome,” “nonaccidental trauma,” and “inflicted traumatic brain injury,” to name a few, and there is a higher incidence in the first year of life [8, 13–16, 19]. Although the diagnosis of AHT is predominantly a clinical one, the role of the radiologist in evaluation is paramount, since a history of inflicted injury to the child is often absent from the clinical history, and the child might lack the external signs of craniofacial injury that would alert the clinician [8, 13–16, 19].
Although the diagnosis of AHT can be elusive and controversial, the triad of encephalopathy, subdural hematomas (particularly of differing ages), and retinal hemorrhages has traditionally been an accepted indicator of AHT; other potential neuroimaging findings include DAI, multiple or healed skull fractures, and diffuse cerebral swelling, even with only minimal signs of external trauma [20–25]. Although the presence of this triad is highly suspicious for AHT, it is not entirely conclusive, and forensic evidence might be necessary to ascertain whether the injuries are definitively related to AHT; however, “reasonable medical certainty” is the legal standard [26]. Some authors suggest that these three signs are not pathognomonic for AHT and could have traumatic and nontraumatic etiologies unrelated to inflicted injury, such as prematurity, increased intracranial pressure, and cerebral venous hypertension. For example, unilateral retinal hemorrhages have been described in witnessed accidental household trauma [27, 28]. Regarding SDHs, the CT and MRI characteristics can suggest or mitigate the likelihood of AHT; for example, homogeneous density SDHs on noncontrast CT are thought to be more common in accidental trauma, while mixed-density SDHs (as in patient 2 with unilateral HIE described above) are thought to be more common in AHT [29]. The SDH in patient 1 with unilateral HIE described above was too small to discern, but it appeared relatively homogeneous. In this study, in four of the six patients with suspected AHT and SDH or epidural hematoma, the hematomas were clearly mixed-density (Figs. 2 and 3), while in two the hematomas were too small (<5 mm) to clearly discern (Fig. 1). However, the temporal ages of the SDHs (on CT and MRI) in both patients with unilateral HIE were consistent with the insult timing and congruent with reports that routine MRI sequences can adequately characterize the SDH age based on the appearance of the sediment [18].
There has been debate regarding the exact etiology of the diffuse brain swelling and parenchymal insults in severe AHT. Although initially described as “shaken-baby syndrome,” shaking might not be the sole etiology of brain injury in AHT, particularly when DAI is not demonstrated [3, 8, 14–16, 30, 31]. Recent studies suggest that the most severe clinical and histologic manifestations of AHT are usually related to HIE, while DAI is less common than once thought; however, this topic remains controversial and might affect legal outcomes, as the term “shaken baby syndrome” might imply DAI [3, 8, 14–19, 32, 33]. However, there should be an evaluation for DAI by MRI, because CT is insensitive to small nonhemorrhagic lesions; on MRI, approximately 20–25% of lesions are hypointense (hemorrhagic) on T2*-W GRE, 60% bright on FLAIR images, and 15–20% are seen only with DW images [34]. In several studies, only a small minority of patients have had evidence of DAI on neuroimaging or neurohistology [14, 16, 33]. Our patients with unilateral HIE also lacked such MRI findings but suffered severe HIE-type insults and brain swelling. Interestingly, a recent article suggests that in young children (<36 months) presenting with acute head trauma, HIE more commonly occurs in AHT than in accidental head trauma [17]. Accordingly, we noted that in our patients of a similar age group and in those patients presenting with acute head trauma, HIE occurred in only 1 of the 43 patients who suffered what was considered clinically to be accidental trauma, while occurring in 3 of the 11 suspected to have suffered AHT. Interestingly, from our search of pediatric patients with HIE that were not listed as having suffered acute head trauma, we discovered an additional patient with suspected abuse who showed HIE (possibly related to forced submersion) without SDH, skull fracture, or scalp bruises, but had suffered extensive solid organ injury. This patient was likely not classified as acute head trauma because external craniocervical bruising was lacking, and in such a situation a head CT scan can be interpreted as normal in the initial phase of HIE if other signs of trauma are lacking while the diffuse brain swelling has not yet peaked (Fig. 4). The brain might even visually appear nearly normal in the acute phase on FLAIR or DW MR images if the insult is symmetric and diffuse and involves the cerebral cortex bilaterally and globally [11]. Hence, radiologists must be aware that young children with AHT might lack external signs of inflicted injury and can present with HIE causing diffuse brain swelling, paying careful attention to DW MR images in combination with ADC maps in this scenario.
Regarding outcomes, the literature suggests that differences in outcomes of AHT are related to the mechanism of injury, while the most severe outcomes usually involve HIE. SDH, while common in AHT, is often not the immediate cause of mortality as the brain swelling is often disproportionate to the size of the SDH [7, 12, 16]. Accordingly, Kemp et al. [16] found that in patients with AHT presenting with apnea who died, the majority (85%) of those with neuroimaging had HIE, while the SDHs were usually too small to have exerted a significant swelling or mass effect. In this regard, DWI is imperative in the work-up of acute or subacute HIE, as diffuse, symmetric, bilateral cortical insults seen on DWI have a poor outcome; permanent effects range from quadriparesis to persistent vegetative state and even brain death [3, 4, 7–11]. In both of our patients, the initial MRI at our hospital demonstrated diffuse but unilateral white matter restricted diffusion, most consistent with the subacute phase of HIE, given the presence on FLAIR imaging of hyperintensity in the cortex in a gyriform pattern. Acute (<3 days) insults usually have cortical abnormalities on DWI and can even appear normal on FLAIR imaging [11, 35]. This temporal pattern of acute cortical restriction on DWI, followed by the white matter, is thought to be related to the cortex having a higher metabolic need and hence being more susceptible acutely, followed by Wallerian degeneration in the white matter in incomplete global ischemia [36, 37]. Rarely, AHT presents with unilateral HIE, as in our two patients. Patients with this unilateral variant might have improved survival, albeit with severe residual neurologic deficits, compared to those with the more common bilateral insults [3, 4, 9–11, 38, 39]. Therefore, DWI was essential for diagnosis and prognosis of HIE in our patients. Recognition of such subacute, or late, DWI patterns of HIE-type insults by the radiologist is paramount, because early MR imaging might be absent, and this appearance portends a severe outcome.
The authors wish to emphasize that HIE in young children is not in itself a determinant of AHT, as correlation with the clinical, imaging, and legal scenario is of paramount importance. Accordingly, a search of our registry yielded five young children with HIE who were listed as not having suffered acute head trauma (Fig. 4). Overall, HIE (in nontraumatic cases) is most commonly related to cardiopulmonary arrest, even in young children, but also can be related to drowning, anemia, electric shock, hypoglycemia, drug overdose, and environmental toxins, to name only a few of myriad potential causes [1]. However, unilateral HIE was not noted in any of the patients with HIE in this study who were not suspected of having AHT, which raises the possibility that unilateral HIE is another potential sign of AHT in the appropriate clinical and legal setting. Hence, further research is necessary to correlate this appearance of unilateral HIE on DW MRI with a biomechanical cause.
Determining the biomechanical cause of HIE and brain swelling in AHT has been elusive. Stretching of the cervical cord/brainstem, chest squeezing, strangling, and concussion have all been suggested to impair respiration and lead to HIE [12–15, 40–42]. However, each of those mechanisms, with the exception of strangling, would be expected to cause bilateral insults. Our two patients with unilateral HIE lacked spinal cord/brainstem and arterial injury on MRI. Accordingly, an earlier study of patients with AHT presenting with HIE demonstrated histologic findings of cord injury only in the minority [14]. This raises the possibility of upper spinal cord injury without radiographic abnormality (SCIWORA) causing the HIE. However, Pang [43] has shown that all patients with SCIWORA with normal cord signal on MRI have complete neurologic recovery, making this syndrome an unlikely cause of HIE in our patients. We excluded aberrant cervicocranial vasculature as a cause by MRA, in accordance with previous reports that the large majority of patients with AHT and HIE have normal MRA findings [3]. In addition, we believe that MRI/MRA was adequate to evaluate for internal carotid artery dissection using axial, thin-section T1-W and T2-W sequences with fat saturation, as the sensitivity of this combination has been reported as near that of catheter angiography [44]. One plausible mechanism causing unilateral HIE is transient narrowing from vascular compression, which could theoretically result from neck hyperflexion/extension involved in shaking or strangling, or from hyperextension/flexion caused by cranial impact [45]. Hence, we propose that transient compression caused diminished, nonocclusive blood flow results in unilateral HIE, as there was no evidence of brainstem or cord injury or arterial dissection. Interestingly, temporary occlusion of one common carotid artery has produced similar unilateral HIE-type insults on DWI in animal models [46].
Causes of HIE other than diffuse brain swelling and ischemic insults were considered in both patients with unilateral HIE, including venous thrombosis, vasospasm, and swelling or mass effect from the SDH or surgery (patient 2). Regarding venous thrombosis, patient 2 had a normal MR venogram. In patient 1, venous sinus thrombosis was a consideration, but there was no evidence of parenchymal hematoma or parasagittal hemorrhages, or lack of venous dural sinus patency on thin-section cranial and cervical imaging. As for vasospasm, which can sometimes occur after traumatic brain injury, this usually occurs after subarachnoid hemorrhage (only minimally present only in patient 2), and not solely from SDH [47]. On MRA, there was no evidence of spasm in either patient, and additionally spasm-related ischemia with restricted diffusion would appear wedge-shaped, akin to the appearance of thromboembolic insults, and not gyriform. Regarding the possibility that the extensive infarcts in both patients arose from the mass effect of the SDHs on the vasculature, the SDH in patient 1 was too small, while the SDH in patient 2 was larger, but the mass effect from an SDH is unlikely to unilaterally involve both the anterior and middle cerebral artery distributions. Although the MRI was performed after surgical decompression in patient 2, we do not believe that this confounded the diagnosis of HIE, or that the surgery caused HIE, because loss of gray–white differentiation was already present prior to surgery (Fig. 2). The repeat MRI demonstrated diffuse white matter restricted diffusion, with cortical hyperintensity on FLAIR images, indicating an HIE-type insult [35]. Hence, acute SDHs might cause a mass effect and infarction, but these infarcts do not typically cause a global and simultaneously unilateral insult; these infarcts are usually in a wedge-shaped distribution conforming to one cerebral arterial supply. The sequential imaging in both patients was also consistent with HIE, with cortical necrosis leading to atrophy.
Other disorders can occasionally mimic AHT, such as meningitis and coagulopathies [48]. In both patients with unilateral HIE, meningoencephalitis is unlikely to have caused these imaging appearances, as both were afebrile. Meningitis is usually bilateral or multifocal, and not confined to global involvement of one hemisphere [49]. Also, to our knowledge, meningoencephalitis can cause focal restricted diffusion of affected areas but does not typically cause a global but unilateral appearance of restricted diffusion, as seen in our two patients; the DWI abnormalities in both were largely unilateral and confluent [49, 50]. Although both of our patients with unilateral HIE presented with SDHs, coagulopathies are very unlikely to have caused this appearance, as the diffuse cortical infarctions on DWI would be more likely seen in a thrombogenic state (leading to vascular compromise) than in a coagulopathic state. Accordingly, no coagulopathies were revealed in the clinical and laboratory evaluations of either child.
It is important to consider other potential causes of diffuse, largely unilateral restricted diffusion on MRI. The DWI findings of status epilepticus could potentially overlap with HIE; however, this condition would be obvious from the history and usually involves restricted diffusion within the hippocampus, amygdala, and pulvinar regions (not present in our patients with unilateral HIE); also, to our knowledge this does not typically progress temporally to diffuse white matter restricted diffusion, while the DWI abnormalities in status epilepticus can be reversible [51, 52]. In regard to potential underlying metabolic abnormalities, these are also unlikely, because metabolic insults usually result in a bilateral, symmetric appearance of restricted diffusion, whether in the cortex or basal ganglia [53].]
Conclusions
Unilateral HIE in a young child may be a sign of AHT when it occurs in conjunction with SDH and retinal hemorrhages. However, clinical correlation and forensic investigation for a history of abuse is necessary in such cases, in which the patient can lack skull fractures, DAI, and external cranial signs of trauma. The proposed mechanism is transient, unilateral vascular compression, as dedicated craniocervical MRA and cervical MRI might be unremarkable.
References
Commichau C (2003) Hypoxic-ischemic encephalopathy. In: Noseworthy J (ed) Neurological therapeutics: principles and practice (1st edn) Martin Dunitz, New York, pp 470–480
Hossmann KA, Hoehn-Berlage M (1995) Diffusion and perfusion MR imaging of cerebral ischemia. Cerebrovasc Brain Metab Rev 7:187–217
Biousse V, Suh DY, Newman NJ et al (2002) Diffusion-weighted magnetic resonance imaging in shaken baby syndrome. Am J Ophthalmol 133:249–255
Parizel PM, Ceulemans B, Laridon A et al (2003) Cortical hypoxic-ischemic brain damage in shaken-baby (shaken impact) syndrome: value of diffusion-weighted MRI. Pediatr Radiol 33:868–871
Grant PE, Yu D (2006) Acute injury to the immature brain with hypoxia with or without hypoperfusion. Radiol Clin North Am 44:63–77
James HE (1999) Pediatric head injury: what is unique and different. Acta Neurochir Suppl 73:85–88
Haviland J, Russell RI (1997) Outcome after severe non-accidental head injury. Arch Dis Child 77:504–507
Suh DY, Davis PC, Hopkins KL et al (2001) Nonaccidental pediatric head injury: diffusion-weighted imaging findings. Neurosurgery 49:309–318
Kawahara H, Takeda Y, Tanaka A et al (2000) Does diffusion-weighted magnetic resonance imaging enable detection of early ischemic change following transient cerebral ischemia? J Neurol Sci 181:73–81
Wijdicks EF, Campeau NG, Miller GM (2001) MR imaging in comatose survivors of cardiac resuscitation. AJNR 22:1561–1565
McKinney AM, Teksam M, Felice R et al (2004) Diffusion-weighted imaging in the setting of diffuse cortical laminar necrosis and hypoxic-ischemic encephalopathy. AJNR 25:1659–1665
Shannon P, Becker L (2001) Mechanisms of brain injury in infantile child abuse. Lancet 358:686–687
Shannon P, Smith CR, Deck J et al (1998) Axonal injury and the neuropathology of shaken baby syndrome. Acta Neuropathol 95:625–631
Geddes JF, Hackshaw AK, Vowles GH et al (2001) Neuropathology of inflicted head injury in children. I. Patterns of brain damage. Brain 124:1290–1298
Geddes JF, Vowles GH, Hackshaw AK et al (2001) Neuropathology of inflicted head injury in children. II. Microscopic brain injury in infants. Brain 124:1299–1306
Kemp AM, Stoodley N, Cobley C et al (2003) Apnoea and brain swelling in non-accidental head injury. Arch Dis Child 88:472–476
Ichord RN, Naim M, Pollock AN et al (2007) Hypoxic-ischemic injury complicates inflicted and accidental traumatic brain injury in young children: the role of diffusion-weighted imaging. J Neurotrauma 24:106–118
Vinchon M, Noule N, Tchofo PJ et al (2004) Imaging of head injuries in infants: temporal correlates and forensic implications for the diagnosis of child abuse. J Neurosurg 101:44–52
Keenan HT, Runyan DK, Marshall SW et al (2003) A population-based study of inflicted traumatic brain injury in young children. JAMA 290:621–626
Richards PG, Bertocci GE, Bonshek RE et al (2006) Shaken baby syndrome. Arch Dis Child 91:205–206
Petitti N, Williams DW 3rd (1998) CT and MR imaging of nonaccidental pediatric head trauma. Acad Radiol 5:215–223
Wilkinson WS, Han DP, Rappley MD et al (1989) Retinal hemorrhage predicts neurologic injury in the shaken baby syndrome. Arch Ophthalmol 107:1472–1474
Zimmerman J, Bilaniuk LT, Bruce D et al (1978) Interhemispheric acute subdural hematoma: a computed tomographic manifestation of child abuse by shaking. Neuroradiology 16:39–40
Nimkin K, Kleinman PK (2001) Imaging of child abuse. Radiol Clin North Am 39:843–864
Jaspan T, Griffiths PD, McConachie NS et al (2003) Neuroimaging for non-accidental head injury in childhood: a proposed protocol. Clin Radiol 58:44–53
Mackey M (2006) After the Court of Appeal: R v Harris and others [2005] EWCA crim 1980. Arch Dis Child 91:873–874
Geddes JF, Tasker RC, Hackshaw AK et al (2003) Dural haemorrhage in non-traumatic infant deaths: does it explain the bleeding in “shaken baby syndrome”? Neuropathol Appl Neurobiol 29:14–22
Christian CW, Taylor AA, Hertle RW et al (1999) Retinal hemorrhages caused by accidental household trauma. J Pediatr 135:125–127
Tung GA, Kumar M, Richardson RC et al (2006) Comparison of accidental and nonaccidental traumatic head injury in children on noncontrast computed tomography. Pediatrics 118:626–633
Caffey J (1972) Brain damage from whiplash-shaking of infants. Am J Dis Child 124:161–169
Duhaime AC, Gennarelli TA, Thibault LE et al (1987) The shaken baby syndrome: a clinical, pathological, and biomechanical study. J Neurosurg 66:409–415
Johnson DL, Boal D, Baule R (1995) Role of apnea in nonaccidental head injury. Pediatr Neurosurg 23:305–310
Ewing-Cobbs L, Prasad M, Kramer L (2000) Acute neuroradiological findings in young children with inflicted or non inflicted traumatic brain injury. Childs Nerv Syst 16:25–34
Huisman TA, Sorensen AG, Hergan K et al (2003) Diffusion-weighted imaging for the evaluation of diffuse axonal injury in closed head injury. J Comput Assist Tomogr 27:5–11
Arbelaez A, Castillo M, Mukherji SK (1999) Diffusion-weighted MR imaging of global cerebral anoxia. AJNR 20:999–1007
Chalela JA, Wolf RL, Maldjian JA et al (2001) MRI identification of early white matter injury in anoxic-ischemic encephalopathy. Neurology 56:481–485
Pantoni L, Garcia JH, Gutierrez JA (1999) Cerebral white matter is highly vulnerable to ischemia. Stroke 27:1641–1647
Field AS, Hasan K, Jellison BJ et al (2003) Diffusion tensor imaging in an infant with traumatic brain swelling. AJNR 24:1461–1464
Osborn AG, Blazer S, Salzman KL et al (2004) Diagnostic imaging: brain (1st edn), Amirsys, Salt Lake City, pp 38–41, 46–48
Geddes JF (2003) Comment. Apnoea and brain swelling in non-accidental head injury. Arch Dis Child 88:476
Alexander R, Sato Y, Smith W et al (1990) Incidence of impact trauma with cranial injuries ascribed to shaking. Am J Dis Child 144:724–726
Hadley MN, Sonntag VKH, Rekate HL et al (1989) The infant whiplash-shake injury syndrome: a clinical and pathological study. Neurosurgery 24:536–540
Pang D (2004) Spinal cord injury without radiographic abnormality in children, 2 decades later. Neurosurgery 55:1325–1342
Oelerich M, Stogbauer F, Kurlemann G et al (1999) Craniocervical artery dissection: MR imaging and MR angiographic findings. Eur Radiol 9:1385–1391
Goldsmith W, Plunkett J (2004) A biomechanical analysis of the causes of traumatic brain injury in infants and children. Am J Forensic Med Pathol 25:89–100
Dijkhuizen RM, Knollema S, van der Worp HB et al (1998) Dynamics of cerebral tissue injury and perfusion after temporary hypoxia-ischemia in the rat: evidence for region-specific sensitivity and delayed damage. Stroke 29:695–704
Oertel M, Boscardin WJ, Obrist WD et al (2005) Posttraumatic vasospasm: the epidemiology, severity, and time course of an underestimated phenomenon: a prospective study performed in 299 patients. J Neurosurg 103:812–824
Care M (2006) Neuroradiology. In: Frasier L, Rauth-Farley K, Alexander R et al (eds) Abusive head trauma in infants and children: a medical, legal, and forensic reference. GW Medical Publishing, St. Louis
Teixeira J, Zimmerman RA, Haselgrove JC et al (2001) Diffusion imaging in pediatric central nervous system infections. Neuroradiology 43:1031–1039
Kim JA, Chung JI, Yoon PH et al (2001) Transient MR signal changes in patients with generalized tonicoclonic seizure or status epilepticus: periictal diffusion-weighted imaging. AJNR 22:1149–1160
Wall CJ, Kendall EJ, Obenaus A (2000) Rapid alterations in diffusion-weighted images with anatomic correlates in a rodent model of status epilepticus. AJNR 21:1841–1852
Takahashi S, Higano S, Ishii K et al (1993) Hypoxic brain damage: cortical laminar necrosis and delayed changes in white matter at sequential MR imaging. Radiology 189:449–456
Sener RN (2004) Diffusion magnetic resonance imaging patterns in metabolic and toxic brain disorders. Acta Radiol 45:561–570
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McKinney, A.M., Thompson, L.R., Truwit, C.L. et al. Unilateral hypoxic-ischemic injury in young children from abusive head trauma, lacking craniocervical vascular dissection or cord injury. Pediatr Radiol 38, 164–174 (2008). https://doi.org/10.1007/s00247-007-0673-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-007-0673-0