Abstract
An efficient micropropagation protocol has been developed through nodal explant by suppressing apical dominance for the enhanced production of multiple shoots by the physical method for the first time in Piper sarmentosum. Shoot regeneration was induced from the nodal explant on MS medium supplemented with varying concentrations and combination of different cytokinins (BA, KIN, and 2-ip) and auxins (IAA, IBA, and NAA). Of the different cytokinins used, higher number of the shoots (6.23 ± 0.13) and the higher shoot length (6.27 ± 0.17 cm) were observed on MS medium with 4.44 µM BA. But a remarkable increase in multiple shoots produced when suppressing the apical dominance by the physical method in the optimal concentration of 4.44 µM BA was observed, and this is the first report in tissue culture study. Rhizogenesis was observed on MS medium supplemented with IBA 4.9 µM and plantlets so obtained were hardened and the plant was established successfully on the natural environment with the survival rate of 89.68%. The present protocol suggests that by suppressing the apical dominance through the physical method without any expensive chemicals in the tissue culture system, shoot regeneration percentage can be increased, so we can adopt this method in tissue culture system for the production of a huge number of plants having strong apical dominance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The genus Piper, belonging to the family Piperaceae comprising about 2000 species, is distributed in tropical and subtropical regions toward the Asian and Southeast Asia regions [1]. In India, the genus Piper comprises 110 species [2, 3], and it is rather poorly represented in Andaman and Nicobar Islands with eight species and almost all the wild Piper species are very rare in distribution and confined to their occurrence in isolated pockets. Piper sarmentosum is a lesser known rare species reported from North Bay of the South Andaman and the Nicobar Islands in 2004 [4] occurring along sea level in isolated pockets. The tsunami in Andaman and Nicobar islands during the month of December in 2004 had severely affected the existence of this species. The natural occurrence of this species has not far been reported in Andaman and Nicobar Islands, elsewhere from the North Bay of the South Andaman Islands [5].
Piper sarmentosum is a herbaceous creeper with procumbent flowering and fruiting branches and is widely used in many Southeast Asian cuisines, especially in Malaysia and Thailand [1]. On account of its ethnopharmacology, since centuries ago, various parts of the taxon, viz. leaves, fruits, and roots, are widely used in Southeast Asian countries for the treatment of various kinds of ailments such as hypertension, diabetes, joint aches, muscle pain, cough, influenza, toothache, and rheumatism [6, 7]. Though the plant is grown in the natural condition through stem cuttings, it is not sufficient for successful vegetative propagation and gives only 10% success rate (the data were not published). So P. sarmentosum being a lesser known botanical entity of the Indian flora with a limited occurrence, while several useful attributes are little known in the Indian context, highlights its high requisite of ex situ conservation and protocol standardization for mass multiplication. So due to the limited occurrence and its rarity, P. sarmentosum has never been subjected to in vitro studies through the materials from the Indian Subcontinent and also these plants have a strong apical dominance.
Apical dominance is the control in the apical part of the shoot and the control may extend to orientation and outgrowth of the leaves, branches, flowers, roots, rhizomes, stolons, tubers, etc. [8]. The presence of auxin in the meristematic region suppresses the growth of axillary buds and also cytokinins which enhances the growth of axillary bud proliferation [9]. The major sources of apical dominance have been considered as the shoot apex and the young leaves [10]. Apical dominance is the major problem of this plant, so the breakage of apical dominance is necessary for the vigorous regrowth of this plant. The present investigation reports, for the first time, the in vitro propagation of P. sarmentosum carried out through suppressing the apical dominance by removing the apical part through the physical method for the production of a large number of multiple shoots.
Material and Methods
Source of Explant
The plants of P. sarmentosum were collected from Andaman and Nicobar Islands and maintained in the ex situ germplasm in Jawaharlal Nehru Tropical Botanic Garden and Research Institute, Palode (JNTBGRI, Kerala), and the saplings were also maintained in greenhouse, Department of Botany, University of Kerala, India. The plant was properly identified with the help of authentic literature and documented with their characteristic features, and a voucher specimen (KUBH 9793) was deposited in the Botany department herbarium, University of Kerala.
Surface Sterilization
Young shoot cuttings having 3–4 nodes were collected from the acclimatized greenhouse-grown plants, defoliated, and initially washed under running tap water for 10–20 min. They were then immersed in 10% Labolene (Galaxo India Ltd., Mumbai) (for 5–10 min), frequently shaking, and subsequently kept under running tap water for 20–30 min. The washed top cuttings were further rinsed with distilled water for 2–3 times and surface-sterilized with 0.1% HgCl2 for 5–6 min. duration. To remove every trace of sterilant, the explants were washed with sterile distilled water at least four to six times. The surface-sterilized shoot cuttings were then cut into appropriate size (1.5–2 cm) single nodal segments and were used as explant. The sterilized nodal explants were inoculated onto culture medium, and the whole operations were carried out under the aseptic condition under the laminar air flow hood (Klenzaids, India).
Culture Condition
The culture was initiated in MS medium [11] containing salts and vitamins supplemented with 3% (w/v) sucrose (SRL, India) and gelled with 0.8% (w/v) agar (SRL, India), and the pH of the medium was adjusted to 5.8 ± 0.2 with 0.1 N NaOH or 0.1 N HCl after addition of plant growth regulators. The medium was dispensed into the autoclaved culture tube (25 × 1.5 cm) and culture bottle (13 × 6 cm) and autoclaved at 121 °C for 18 min under 15 lbs. pressure in an autoclave (Nat steel, Mumbai). All the cultures were maintained in the culture room at 25 ± 2 °C under 16/8 h photoperiod with light intensity of 2500 lux (μmol−2 s−1) provided with white fluorescent tubes (Philips Ltd., Mumbai) and 55–60% relative humidity. All the cultures were subcultured on the fresh medium after 45 days.
Influence of Nodal Position
Influence of different positions of the node was also tested in MS medium supplemented with the optimal dose of cytokinin—4.44 µM BA. Different nodal positions (first to the fourth nodes) were selected from the field-grown plants to find out the nodes having the maximum percentage of response, number of multiple shoots and the maximum responding (position) nodes in the presence of 4.44 µM BA. The cultures were kept at 25 ± 2 °C with 16/8 h photoperiod, and observations were made after 45 days of inoculation. The maximum responding node was selected for the further experiment.
Culture Initiation and Shoot Multiplication
The surface-sterilized nodal segments (1.5–2 cm) were cultured on MS medium supplemented with varying concentration of different cytokinins individually, viz. 6-benzyl adenine (BA 2.22–22.2 µM), kinetin (KIN 2.32–23.2 µM), and 2-isopentenyl adenine (2-ip 2.46–24.6). Effect of the optimal cytokinin BA (4.44 µM) in combination with different concentrations of cytokinin and different range of auxins, viz. Indole-3-acetic acid, Indole-3-butyric acid and α-naphthalene acetic acid (IAA, IBA, and NAA) (0.5–6 µM), was also tested for shoot multiplication. MS medium without plant growth regulators was served as control.
Suppression of Apical Dominance by the Physical Method
To study the influence of suppression of apical dominance for the production of multiple shoots in MS medium supplemented with the optimal cytokinin, apical part of the microshoots developed from the nodal segments on MS medium supplemented with 4.44 µM BA in the 3-week old plant in the culture system were excised. The apical regions were removed by using the simple physical method such as sterilized forceps and blade without the addition of any expensive chemicals that suppress apical dominance. After 8 weeks of culture (the apical part removed plant in culture), the data on total number of shoots and shoot length (cm) were recorded.
Rooting
For in vitro rooting, the healthy shoots (5 cm) having about six leaves were excised and transferred to half strength MS medium supplemented with different individual concentrations (2.5–8.5 µM) of auxins (IAA, IBA, and NAA) used.
Hardening and Field Establishment
After 30 days of culture in the rooting medium, the cultures were taken from the incubation room and were kept at room temperature for one week. After one week, the rooted plants were deflasked and washed with tap water to remove the traces of agar and transferred to plastic pots (6.5 × 7.5 cm) filled with sand and garden soil (1:1) and plantlets were covered with a transparent polythene bag to maintain humidity. After a month, the plants were transferred to greenhouse condition and the polythene bags were removed. The plants were watered daily. Hardened plants were transferred to plastic pots (17 × 15 cm) containing garden soil/sand/cow dung (1:1:1) and finally to the field.
Experimental Design and Data Collection
For each treatment, 20 replicates were used and the experiments were repeated thrice. Observations of the culture were made at weekly intervals, and the data were recorded after 45 days of inoculation. Data were statistically analyzed by ANOVA using SPSS software (version 22.0).
Results and Discussion
Disinfections of Plant Material
The explants were surface-sterilized with 0.1% mercuric chloride for 4–6 min. The nodal explant showed maximum survival rate when treated with 0.1% HgCl2 for 5 min. The nodal explants remained green and also induced single shoot on basal MS medium.
Influence of Position of Nodal Explants on Shoot Multiplication
The nodal explant position has played a major role in the multiple shoot production of in vitro regeneration [12]. The different positions of nodal explants (first–fourth nodes) were dissected and inoculated in medium containing 4.44 µM BA, and growth was observed to check which node gave more effect for shoot initiation and multiplication. In the present study of the different nodal position tested, the maximum number of multiple shoots was produced in second nodes in 4.44 µM BA. The maximum number of multiple shoots in the second node was also recorded in Guava cultivars [13].
Effect of Various PGRs on Shoot Multiplication
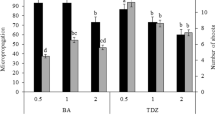
Mass propagation of plant using nodal segment culture has proved to be the most suitable and reliable method of in vitro regeneration [14]. Surface-sterilized nodal explant (second node) was cultured on MS media supplemented with various concentration and combinations of cytokinins and auxins. The response of nodal explant cultured on MS medium supplemented with various PGRs for shoot multiplication over a period of 45 days is presented in Table 1. The surface-sterilized nodal explants were inoculated on MS medium supplemented with the varying individual concentration and combinations of different cytokinins like BA, KIN and 2-ip. Of the different cytokinins used, the higher number of shoots (6.23 ± 0.13) and higher shoot length (6.27 ± 0.17 cm) were observed on MS medium with 4.44 µM BA after 45 days of culture (Table 1). Compared to BA, KIN and 2-ip and combination of optimal BA with KIN and 2-ip gave reduced number of shoots/node (Table 1) and also the optimal BA in combination with different concentrations of auxins like NAA, IAA, and IBA showed no shoot regeneration, but callus and root formation was noticed after 3 weeks. The intensity of callusing was increased when the concentration of auxin increases.
In the present investigation, 4.44 µM BA showed better regeneration response as compared to all other tested cytokinins like KIN and 2-ip and their combinations. BA induced more shoots which was also reported in various explants of cultivated piper species [15]. However, Anand and Rao [16] observed that BA and KIN combination was more effective than BAP alone in Piper barberi, a critically endangered and endemic piper species of Western Ghats of India, which is contradictory to the present study. The superiority of BA over other cytokinins in shoot bud regeneration has been well documented in Syzygium alternifolium [17], Pterocarpus marsupium [18], and Gynochthodes umbellata [19].
The combination of BA with different auxins like IAA, IBA, and NAA in different concentrations did not produce multiple shoots; instead, basal callusing was observed. This is in agreement with the finding of piper species where BA in combination with IAA produced a lesser number of shoots [20] and the contradictory study reported in P. nigrum where BA in combination with IAA gave better response for shoot multiplication [21].
Breakage of Apical Dominance
The experiments carried out to suppress the apical dominance, especially by tissue removal, demonstrated that the apical portion of the shoot possesses the source of control for the growth of lateral buds. If the shoot is decapitated, the lateral bud grows effectively. Removal of apical part of the plant was carried out in MS medium supplemented with the optimal cytokinin—4.44 µM BA—and produced a significantly higher number of shoots than medium supplemented with BA alone. In brief, the excision of the apical part of the microshoot of the nodal explants resulted in a threefold increase in shoot number. An average of 19.34 ± 0.26 number of shoots with 5.38 ± 0.18 cm length was produced (Fig. 1c) within 45 days of culture as compared to 4.44 µM BA without any breakage of apical dominance, but the average length of shoot marginally reduced.
In vitro propagation of Piper sarmentosum: a explant inoculated on MS medium, b shoot with basal callusing in 1 mg/l BAP + 2-ip 3 mg/l, c multiple shoot formation by suppressing apical dominance, d rooting of microshoots in 1 mg/l IBA, e in vitro raised plantlet transplanted to pot with sand and garden soil (1:1) after 2 week and f established plants on the natural environment after 2 months
The enhanced multiple shoot production recorded in the present study explains the apical dominance of the plant and that might be the reason for the production of less number of shoots. Tanaka et al. [9] and Chabikwa et al. [22] reported that auxins are the major reason for apical dominance suppressing the growth of axillary buds [9, 22]. From the present study, nodal segments cultured under in vitro at optimal BA (4.44 µM) showed less number of shoots, due to the apical dominance. So the removal of apical part showed a significant effect on the suppression of apical dominance and produced threefold increase of shoots. The previous study showed that the apical dominance under in vitro system can be suppressed by the addition of expensive chemicals as reported by several workers. Incorporation of auxin transport inhibitors (quercetin) under in vitro showed marked enhancement in the number of shoots in Oldenlandia umbellata [23]. Similar results were reported in Alnus glutinosa where incorporation of 1-naphthyl phthalamic acid (NPA) and TIBA in the medium improved shoot number [24]. However, in the present investigation, this is the first report based on the physical method under in vitro culture system to suppress the apical dominance. The present study revealed the major fact that breakage of apical dominance that can be done through physical method is more convenient and adaptable than the chemical method (Table 2).
Rooting
Rooting of in vitro shoot is an essential step for successful completion of any micropropagation program. Auxin-supplemented basal medium is generally referred to as rooting media, and their role in root development is well documented [25]. For in vitro rooting, the shoots having 5 cm length were harvested and transferred to rooting media. For rooting, half strength MS media supplemented with different auxins (IAA, IBA, and NAA) with different individual concentrations (2.5–8.5 µM) were used. The rooting percentage, number of roots/shoots, and length of roots were recorded after 30 days of culture (Table 3). Of the different auxins used, the highest number of roots (29.34 ± 0.81) with an average length of 3.69 ± 0.20 cm was recorded on ½ MS medium with 4.9 µM IBA within 30 days of culture (Fig. 1d). Increased concentration of IBA showed callusing from the base of microshoots. The root produced from the medium supplemented by IBA was thick and strong as compared to IAA. Of the different individual concentrations of IAA used, 8.5 µM produced 12.09 ± 0.16 roots and an average root length with callusing at base and NAA showed no root induction in all the concentrations tried and produced the only callus at the cut end of the microshoots. The in vitro raised shoots were transferred to half strength MS medium supplemented with different concentration of auxins, IBA 4.9 µM was most effective for root induction. IBA has been used successfully to obtain the higher rooting efficiency as reported by various workers [26,27,28,29,30,31]. The excellent rooting was found in P. nigrum in the presence of IBA [21].
Acclimatization and Hardening
After 30 days, the rooted plants were deflasked, washed, and treated with 1% Indofil M-45 and planted in pots containing sand and garden soil (1:1) and transferred to greenhouse condition (Fig. 1e). The rooted plantlets were successfully transferred to pots and then to the field. The plant was established in the field with 89.68% of survivability in the natural environment (Fig. 1f). The established plants do not show any morphological abnormalities as compared to that of the mother plant. Hence, this standardized protocol would be beneficial to rapid in vitro propagation and conservation of this rare edible and medicinally valuable plant.
Conclusion
The present study reveals that a remarkable number of shoots (19.34 shoots/node) can be produced from P. sarmentosum using MS media formulation by suppressing the apical dominance by simply removing the apical part of the plant using forceps and blade in culture condition. Through this method, there was a threefold increase in shoot number than the optimal BAP in normal condition.
Future Prospects
This is the most reliable and cost-effective protocol for enhanced shoot multiplication of the species because there is no addition of any expensive chemicals for the suppression of apical dominance in the medium. The developed protocol is simple, cost-effective, and reproducible, and this method can be used for mass propagation and conservation of Piper sarmentosum.
References
Rukachaisirikul T, Siriwattanakit P, Sukcharoenphol K, Wongvein C, Ruttanaweang P, Wongwattanavuch P, Suksamrarn A (2004) Chemical constituents and bioactivity of Piper sarmentosum. J Ethnopharmacol 93(2–3):173–176
Hooker J (1886) The flora of British India, vol 5. Reeve & Co., London
Purseglove JW, Brown E, Green C, Robbins S (1981) Spices, vol 2. Longman Group Ltd., Harlow
Karthigeyan K, Sumathi R, Jayanthi J, Diwakar P, Lakra G (2004) Piper sarmentosum Roxb.—an addition to the flora of Andaman Islands. Curr Sci 87(2):140–141
Mathew S, Chitra C, Anilkumar C (2016) Propagation and ex situ conservation of Endocomia macrocoma subsp. prainii (Myristicaceae) from the Andaman Islands in the Bay of Bengal. J Biodivers Endanger Species 4(167):2
Perry LM, Metzger J (1980) Medicinal plants of east and southeast Asia: attributed properties and uses. MIT press, Cambridge
Hussain K, Ismail Z, Sadikun A, Ibrahim P (2008) Analysis of proteins, polysaccharides, glycosaponins contents of Piper sarmentosum Roxb. and anti-TB evaluation for bio-enhancing/interaction effects of leaf extracts with Isoniazid (INH). Nat Prod Radiance 7(5):402–408
Hillman JR (1984) Apical dominance. In: Wilkins MB (ed) Advanced plant physiology. Pitman, London, pp 127–148
Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H (2006) Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J 45(6):1028–1036
Thimann KV (1977) Hormone action in the whole life of plants. University of Massachusetts Press, Amherst
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Mishra M, Pathak R (2001) Effect of nodal position and season on in vitro shoot proliferation in aonla. J Appl Hortic 3(2):103–104
Shekafandeh A, Khosh-Khui M (2008) Effects of bud position and culture medium on shoot proliferation from nodal culture of two mature guava cultivars. Asian J Plant Sci 7(2):177–182
George EF, Sherrington PD (1984) Plant propagation by tissue culture. Exegetics Ltd, Eversley
Bhat S, Chandel K, Malik S (1995) Plant regeneration from various expiants of cultivated Piper species. Plant Cell Rep 14(6):398–402
Anand A, Rao CS (2000) A rapid in vitro propagation protocol for Piper barberi Gamble, a critically endangered plant. Vitro Cell Dev Biol Plant 36(1):61–64
Khan PSV, Prakash E, Rao K (1997) In vitro micropropagation of an endemic fruit tree Syzygium alternifolium (Wight) walp. Plant Cell Rep 16(5):325–328
Chand S, Singh AK (2004) In vitro shoot regeneration from cotyledonary node explants of a multipurpose leguminous tree, Pterocarpus marsupium roxb. Vitro Cell Dev Biol Plant 40(5):464–466
Anjusha S, Gangaprasad A (2016) In vitro propagation and anthraquinone quantification in Gynochthodes umbellata (L.) Razafim. & B. Bremer (Rubiaceae)—A dye yielding plant. Ind Crops Prod 81:83–90
Rubluo A, Barroso ML (1992) In vitro morphogenetic responses and cytokinin-auxin interaction for callus production in pepper. Anales del Instituto de Biología Serie Botánica 63(2):195–201
Khan S, Banu TA, Islam M, Habib A, Ferdousi A, Das N, Akter S (2017) In vitro regeneration of Piper nigrum L. Bangladesh J Bot 46(2):789–793
Chabikwa TG, Brewer PB, Beveridge CA (2019) Initial bud outgrowth occurs independent of auxin flow from out of buds. Plant Physiol 179(1):55–65
Krishnan SS, Siril E (2017) Enhanced in vitro shoot regeneration in Oldenlandia umbellata L. by using quercetin: a naturally occurring auxin-transport Inhibitor. Proc Nat Acad Sci India Sect B Biol Sci 87(3):899–904
Lall S, Mandegaran Z, Roberts A (2005) Shoot multiplication in cultures of mature Alnus glutinosa. Plant Cell, Tissue Organ Cult 83(3):347–350
Kackar N, Solanki K, Singh M, Vyas S (1991) Micropropagation of Prosopis cineraria. Indian J Exp Biol 29(1):65–67
El Bouzdoudi B, Saïdi R, El Ansari ZN, L’bachir El Kbiach M, Martin P, Badoc A, Lamarti A (2017) Micropropagation of Carob (Ceratonia silique L.) through Adventitious Buds of Immature Embryonic Cotyledons. Am J Plant Sci 8(9):2180
Muthukrishnan S, Benjamin JF, Muthukumar M, Rao M (2012) In vitro propagation of Ceropegia thwaitesii Hook-an endemic species of Western Ghats of Tamil Nadu, India. Afr J Biotechnol 11(59):12277–12285
Ahmad N, Fazal H, Abbasi BH, Rashid M, Mahmood T, Fatima N (2010) Efficient regeneration and antioxidant potential in regenerated tissues of Piper nigrum L. Plant Cell, Tissue Organ Cult 102(1):129–134
Kartsonas E, Papafotiou M (2007) Mother plant age and seasonal influence on in vitro propagation of Quercus euboica Pap., an endemic, rare and endangered oak species of Greece. Plant Cell, Tissue Organ Cult 90(1):111–116
Chandrasekara A, Yapabandara Y, Weerasinghe P (2011) In-vitro propagation of locally selected black pepper (Piper Nigrum L.). Research Symposium, University of Srilanka, 435
Shiji P, Siril E (2018) An improved micropropagation and ex vitro rooting of a commercially important crop Henna (Lawsonia inermis L.). Physiol Mol Biol Plants 24(6):1273–1284
Acknowledgements
The authors are thankful to the Head, Department of Botany, University of Kerala, India, for providing the facility for doing this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest to publish this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Significance Statement
An efficient micropropagation protocol has been developed by suppressing apical dominance for the enhanced production of multiple shoots for the first time through Piper sarmentosum a rare lesser known wild edible species from the Andaman Island.
Rights and permissions
About this article
Cite this article
Stephin, S., Gangaprasad, A., Mathew, S.P. et al. Enhanced In Vitro Shoot Multiplication of Piper sarmentosum by Suppression of Apical Dominance. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 90, 87–94 (2020). https://doi.org/10.1007/s40011-019-01086-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-019-01086-w