Abstract
Background
Lymphatic oral drug delivery systems are considered optimal for enhancing oral drug delivery. They offer the advantage of delivering drugs directly to the immune system while bypassing first-pass metabolism. Consequently, they are actively researched in the field of pharmaceuticals. Novel drug delivery systems are employed to administer drugs through the lymphatic system via the oral route.
Area covered
This review delves into the current understanding of drug delivery systems through the lymphatic pathway, with a focus on those utilizing long-chain triglycerides. We explore the mechanisms of delivery to the lymphatic pathway, characteristics of drugs used for this purpose, composition of lipid-based formulations, preparation methods, evaluation methods, and notable case studies.
Expert opinion
With the discovery of more lipophilic drug candidates, there is a growing interest in designing formulations to maximize lymphatic transport. Stimulating intestinal lymphatic transport holds the potential for benefits such as reducing first-pass metabolism and delivering high drug concentrations to the lymphatic system. To enhance the utility of intestinal lymph as an alternative means of transport to the systemic circulation, further research is essential. This research should address the underlying mechanisms of drug binding to lipoproteins in intestinal cells, as well as the effects of lipids and formulation excipients on this process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To optimize the therapeutic efficacy of a drug, it is crucial to carefully choose an administration route that aligns with its unique characteristics and to devise an optimal drug delivery system. While a variety of drug delivery systems have been explored and developed, ongoing efforts continue to create new technologies that align with the unique attributes of newly developed drugs. An examination of LogP values for approved drugs spanning the years 1990–2021 (categorized in 5-year intervals) reveals a consistent increase in both the mean and median drug LogP values over time. The primary factor contributing to this apparent escalation in the overall lipophilicity of drugs is a reduction in the proportion of highly polar molecules. Furthermore, lipids are recognized for their diverse roles in facilitating the absorption of medications from the gastrointestinal tract.
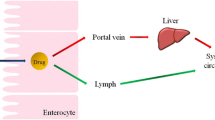
It is well-established that the postprandial absorption of medications is significantly influenced by the consumption of a lipid-rich diet, leading to substantial changes in the bioavailability of formulations. The uptake of drugs by the intestinal lymphatic system presents three potential therapeutic advantages. Firstly, these compounds circumvent first-pass liver metabolism because lymphatic vessels, in contrast to the portal vein, directly drain into the systemic circulation (Shackleford et al. 2003; Trevaskis et al. 2009). Secondly, successful targeting of drugs to the lymph, achievable through advanced lipid-based formulations, can enhance the bioavailability of drugs that are otherwise poorly absorbed (Zhang et al. 2016). Thirdly, in certain cases, drug absorption into the lymphatic system augments its therapeutic effects due to significantly elevated lymphatic concentrations. This is observed, for instance, in immunosuppressants and anti-HIV drugs (Takada et al. 1986; Han et al. 2014; Yoshida et al. 2016).
Recent advancements in mRNA technology and lipid nanoparticle-based delivery systems have facilitated the rapid development of mRNA COVID-19 vaccines. This progress has showcased the clinical potential of lipid nanoparticle-mRNA formulations, providing powerful tools for their continued advancement (Hou et al. 2021).
In this review, our objective is to enhance comprehension of lymphatic oral drug delivery systems, with a particular focus on those utilizing long-chain triglycerides. Consequently, we delve into the delivery mechanism to the lymphatic pathway, the characteristics of drugs employed for this purpose, the composition of lipid-based formulations, manufacturing techniques, evaluation methods, and notable case studies.
Lipid-based formulation
Oil solutions, emulsions, dispersions, micelles, self-emulsifying drug delivery systems (SEDDS), and self-microemulsifying drug delivery systems (SMEDDS) can be broadly categorized into four types according to the lipid-based formulation classification system proposed by Pouton (Table 1) (Pouton 2006). Clinical applications for each type are outlined in Table 2. Type I lipid formulations contain oils that require digestion to form mixed micelles. These formulations are typically biocompatible, and they are often the first choice if the active pharmaceutical ingredient (API) dose can be fully solubilized in the vehicle. Examples of drugs formulated as type I lipid formulations include vitamin D analogs and retinoids. Type II lipid formulations consist of a blend of lipids and water-insoluble surfactants (HLB < 12). They self-emulsify into crude oil-in-water emulsions, usually with the application of external energy. Type III lipid formulations include water-soluble surfactants (HLB > 12) and cosolvents. In the presence of an aqueous environment, these formulations spontaneously self-emulsify. Cases where the dispersion droplet size exceeds 200 nm are referred to as SEDDS, while those with a size below 200 nm are termed SMEDDS. In contrast, Type IV lipid formulations are based on water-soluble surfactants and co-solvents, with no inclusion of oil. These formulations enable rapid release and absorption of drugs when in contact with fine dispersions in an aqueous environment. However, this may lead to the precipitation of molecules in the gastrointestinal (GI) tract, as these systems are typically limited in their ability to sustain poorly soluble drugs in a solvent.
Excipients for lipid-based formulation
Suppliers provide a variety of lipid excipients, and their impact on the absorption process underscores the importance of comprehending the qualities of different excipients (Pouton and Porter 2008). The selection of excipients for lipid-based formulations is influenced by several factors, including miscibility, solvent capacity, self-dispersibility, and the potential to facilitate the self-dispersion of the formulation. Additionally, considerations encompass digestibility and the fate of the digested products, regulatory concerns (such as irritancy, toxicity, purity, and chemical stability), compatibility with capsules, melting point, and cost.
Lipid-based formulations commonly incorporate dietary oils containing medium- and long-chain triglycerides, along with solvents and surfactants (Christie 1987). These lipids exhibit amphiphilic properties, possessing both lipophilic (fatty acids) and hydrophilic components (Christie 1989). The melting point of these lipids increases with the length of the fatty acid chain but decreases with higher unsaturation, leading to increased susceptibility to oxidation (Jannin et al. 2008).
The Food and Drug Administration (FDA) currently lacks an independent evaluation process or mechanism for assessing the safety of individual excipients. Instead, in the case of premarket-approved drugs or biological products, the excipients undergo evaluation and receive approval as 'components' within the application. From a scientific standpoint, this regulatory approach is reasonable because excipients play a crucial role in the formulation and cannot be evaluated in isolation from the drug. This is particularly pertinent for lipid excipients due to their unique physicochemical properties and their potential for intricate interactions with other ingredients or physiological conditions that may arise in vivo (Chen 2008). The structure of several excipients of lipid-based formulation in Fig. 1.
Triglycerides
Triglyceride vegetable oils serve as predominant excipients in lipid-based drug delivery systems. This specific lipid class raises no safety concerns, as it undergoes complete metabolism and assimilation within the body (Gibson 2007). These vegetable oils are essentially glyceride esters, comprising a blend of unsaturated long-chain fatty acids commonly referred to as long-chain triglycerides. Oils derived from various plant sources exhibit distinct proportions of each fatty acid (Jannin et al. 2008). D-α-tocopheryl polyethylene glycol 1000 succinate (Vitamin E TPGS) is derived from vegetable tocopherols. It is water-soluble and serves as an absorption enhancer for poorly water-soluble drugs. Refined vegetable oils contain pure triglycerides (Collnot et al. 2006, Griffin and O'driscoll 2006; Collnot et al. 2007).
Mixed glycerides and polar oils
Mixed glycerides were produced through the partial hydrolysis of vegetable oils. The composition of these mixed glycerides was determined by the initial material, such as triglycerides, and the extent of hydrolysis. Medium-chain mixed glycerides exhibit resistance to oxidation, possess a high solvent capacity, and contribute to enhanced emulsification. These polar oily additives not only boost the solvent capacity of the formulation but also improve dispersibility (Porter et al. 2004). An illustrative example of such a polar oil is sorbitan trioleate. Oleic acid finds application in various commercial products (Strickley 2007).
Co-solvents
To enhance solubilization, various lipid-based products on the market incorporate water-soluble cosolvents (Porter et al. 2004; Strickley 2007). Commonly utilized materials in lipid-based formulations include polyethylene glycol 400, propylene glycol, ethanol, glycerol, and other cosolvents approved for experimental studies. Cosolvents serve multiple purposes in these formulations. Initially, ethanol was employed in early cyclosporine products to facilitate drug dissolution during manufacturing. Generally, cosolvents are added to boost the solvent capacity of drug formulations that readily dissolve. However, achieving a substantial increase in solvent capacity requires high concentrations of cosolvents, potentially leading to drug precipitation upon mixing with water. Cosolvents rapidly lose their solvent capacity upon dilution (Yalkowsky 1999).
Another function of cosolvents is to disperse systems containing high levels of water-soluble surfactants. Practical constraints on cosolvent concentrations arise due to potential immiscibility with oil components and the risk of incompatibility with capsule shells, particularly with low-molecular-weight cosolvents (Cole et al. 2008).
Water-insoluble surfactants
Non-ionic esters lacking polyethoxylation or polyglycerol modification are categorized as polar oils. In the context of oral lipid-based formulations, we designate a subset of excipients with intermediate Hydrophilic-Lipophilic Balance (HLB) values ranging from 8 to 12 as 'water-insoluble surfactants' (Wakerly et al. 1986). These substances strongly adsorb at oil–water interfaces. Although these materials are not sufficiently hydrophilic to dissolve in water or form micelles, they possess enough hydrophilicity to facilitate self-emulsification (Pouton 1997). The solubility of water-insoluble surfactant components in water is limited, contingent on their degree of ethoxylation, and generally remains quite low. Oleate esters, such as polyoxyethylene (20) sorbitan trioleate and polyoxyethylene (20) glyceryl trioleate, exemplify water-insoluble surfactants with HLB values ranging from 11 to 11.5. However, the blend of Tween-80 and Polyoxyethylene (80) sorbitan monooleate, with an average HLB value of 11, was not similar to that of polyoxyethylene (20) sorbitan trioleate. The former is composed of both water-soluble and water-insoluble molecules, while the latter is predominantly composed of water-insoluble molecules (Pouton 2000).
Water-soluble surfactants
Surfactants play a crucial role in the development of self-emulsifying drug delivery systems (Pouton 2006). Surfactants with an HLB value of approximately 12 or higher can form micellar solutions even in low water concentrations by surpassing their critical micellar concentration (Hauss 2007). These surfactants can be synthesized by blending polyethylene glycols with hydrolyzed vegetable oils. Alternatively, a common method involves the reaction of alcohols with ethylene oxide to produce alkyl ether ethoxylates, such as the well-known cetostearyl alcohol ethoxylate, also referred to as 'cetomacrogol.' Polysorbates, primarily ether ethoxylates, result from the reaction of sorbitan esters with ethylene oxide (Kolling 2004). Examples such as Cremophor RH40 and RH60, derived from hydrogenated vegetable oils, exemplify this category. Another notable surfactant is Cremophor EL, derived from non-hydrogenated castor oil. Cremophors enhance absorption by inhibiting efflux pumps; however, the specific mechanism of inhibition remains unclear. This phenomenon may arise from non-specific structural changes, where surfactant molecules permeate membranes, adhere to the efflux pump surface, or interact with their intracellular domains (Hugger et al. 2002; Shono et al. 2004; Grove et al. 2007).
Additives
In formulations, it is possible to incorporate lipid-soluble antioxidants, such as α-tocopherol, β-carotene, propyl gallate, butylated hydroxytoluene, or butylated hydroxyanisole. These antioxidants serve the purpose of safeguarding unsaturated fatty acid chains or drugs from oxidation (Gibson 2007).
Long-chain triglycerides oils
Triglycerides are classified based on their chain length as short (up to five carbons), medium (6–12 carbons), or long (more than 12 carbons). Artificial hydrogenation can be applied to reduce unsaturation and enhance resistance to oxidative deterioration. Triglycerides preferentially undergo lymphatic transport or enter the venous system, bypassing first-pass metabolism. The separation of glyceride components in natural product oils is employed to create additives that optimize desirable physical properties and facilitate drug absorption. This process also helps minimize issues such as vulnerability to oxidation (Greenberger et al. 1966). Examples of long-chain triglycerides include corn, olive, peanut, rapeseed, sesame, and soybean oils, which may contain hydrogenated soybean and vegetable oils. Long-chain triglycerides have been utilized in products such as Marino (Dronabinol®) and others (Table 3) (Savla et al. 2017).
Preparation method of lipid-based formulation
Oral delivery is the preferred method for drug administration. Both liquids and solids can be administered through this route. Physically and chemically stable liquids and semisolids can be converted into solid particles, such as powders or granules. These particles can then be filled into capsules or compressed into tablets using appropriate excipients.
Reverse phase evaporation
The formation of inverted micelles constitutes the fundamental principle underlying reverse-phase evaporation. In this method, lipids were dissolved in an organic solvent, facilitating the generation of inverted micelles. Subsequently, a predetermined quantity of aqueous phase (buffer) was introduced into the solution. The rearrangement of lipids at the water–oil interface resulted in the formation of a water-in-oil (W/O) microemulsion. To achieve a homogeneous dispersion, mechanical or sonication techniques were employed to emulsify W/O microemulsions. The elimination of the organic solvent occurred through continuous rotating evaporation under reduced pressure until a dense gel was obtained. The breakdown of inverted micelles and the subsequent production of lipid vesicles are both favored by slower organic solvent removal (Lombardo and Kiselev 2022). Notably, hepatitis B antigen-loaded solid lipid nanoparticles, modified with a TLR-4 agonist, were prepared using the reverse-phase evaporation method. This approach was chosen to ensure effective colonic uptake of the developed vaccine (Duong et al. 2023).
Spray congealing
The process known as spray cooling involves spraying molten lipids into a cooling chamber, resulting in the formation of solid spherical particles upon contact with cool air. These particles can be administered in the form of capsules or tablets, with ultrasonic atomizers commonly used for this purpose. Rapid solidification depends on key factors such as the excipient melting point, formulation viscosity, and chamber air temperature (Albertini et al. 2008).
In a study conducted by Albertini, Beatrice, et al., various formulations were prepared through spray congealing. A lipid–hydrophilic matrix (e.g., Gelucire® 53/10) served as the carrier, with the addition of several mucoadhesive polymers. This approach led to a significant improvement in the solubility of low-solubility drugs, mucosal adhesion strength, and in vitro bioavailability (Albertini et al. 2009).
Spray drying
The presented method shares similarities with spray congealing, but it diverges in terms of the air temperature within the atomizing chamber. In this approach, the drug solution (comprising the drug dissolved in either an organic solvent or water) is sprayed into a chamber filled with hot air. Subsequently, the organic solvent or water evaporates, resulting in the formation of solid microparticles of the drug. Throughout this procedure, solid carriers, such as silicon dioxide, can be incorporated alongside lipid excipients.
According to Chauhan et al. (2005), the dissolution rate of the poorly water-soluble drug etoricoxib is significantly improved through the preparation of solid dispersions using lipid carriers (e.g., Gelucire™) via a spray drying technique.
Adsorption on solid a carrier
This method is both simple and economical, especially regarding equipment investment. In this approach, a liquid–lipid formulation is absorbed onto a solid carrier, such as silicon dioxide, calcium silicate, or magnesium aluminometasilicate. The liquid–lipid formulation is incorporated into the carrier through blending in a mixer. The choice of the carrier is crucial; it should possess a superior ability to absorb the liquid formulation and exhibit favorable flow properties after absorption. This adsorption technique has proven successful with gentamicin and erythropoietin, utilizing caprylocaproyl polyoxylglyceride formulations. These formulations retained their bioavailability-enhancing effects even after being adsorbed onto carriers (Ito et al. 2005; Venkatesan et al. 2005, 2006).
Melt granulation
The process known as melt granulation involves the transformation of a powder mix, containing a drug, into granules or pellets (Royce et al. 1996; Voinovich et al. 2001; Schaeffer 2004). In this method, a thermosoftening binder is mixed with the drug substance and other excipients under high shear to produce pellets or granules with the desired particle size (Schæfer, Taagegaard et al. 1993; Voinovich et al. 2001; Schaeffer 2004). Depending on the fineness of the powder, 15–25% of a lipid-based binder can be utilized. Key parameters to consider during the process include binder particle size, mixing time, impeller speed, and the viscosity of the binder upon melting (Seo and Schæfer 2001).
Research has shown that the dissolution rate of diazepam can be improved by formulating melt agglomerates containing solid dispersions of diazepam. For example, lactose monohydrate was melt-agglomerated with a meltable binder such as polyethylene glycol 3000 or Gelucire® 50/13 in a high shear mixer (Seo et al. 2003). Melt pelletization has been employed not only to enhance but also to retard the release rate of incorporated drugs (Schæfer and Mathiesen 1996; Thies and Kleinebudde 1999; Voinovich et al. 2000; Mehuys et al. 2004a, b).
Melt extrusion/extrusion spheronization
Extrusion is the process that transforms a raw material with plastic properties into a product of uniform shape and density by compelling it through a die under controlled temperature, product flow, and pressure conditions (Breitenbach 2002, Verreck and Brewster 2004). A "system-in-cylinder" molding technique was employed to create a dual-purpose formulation involving propranolol hydrochloride, aiming to enhance bioavailability and ensure controlled release. The core matrix, composed of a blend of Gelucire® 44/14 and hypromellose, was shaped within an ethylcellulose pipe to safeguard it from hydrodynamic and mechanical stress. Results demonstrated a controlled release of propranolol hydrochloride over 24 h, exhibiting a fourfold increase in oral bioavailability compared to a commercial formulation when tested in dogs (Mehuys et al. 2004a, b; Mehuys et al. 2004a, b; Mehuys et al. 2005).
Supercritical fluid-based method
The presented formulation technique utilizes lipids to coat drug particles, creating solid dispersions. The process involves initially dissolving the drug and lipid-based additives in an organic solvent and subsequently in a supercritical fluid, such as carbon dioxide, achieved through elevating temperature and pressure. Coating is accomplished by controlled reduction in pressure and temperature, causing the solubility of the coating material to decrease and deposit onto drug particles, forming a coating layer (Dos Santos et al. 2003, Thies et al. 2003). Critical considerations for this technique include: (i) solubility of formulation components in the supercritical fluid, (ii) integrity/stability of the active substance under process conditions, and (iii) energy or environmental concerns related to solvent evaporation, if applicable. Various lipid-based and lipid-related additives were explored, including glyceryl trimyristate (e.g., Dynasan™ 114) and stearoyl polyoxylglycerides (e.g., Gelucire® 50/02) for controlled-release applications (Sethia and Squillante 2002; Dos Santos et al. 2003; Thies et al. 2003; Sethia and Squillante 2004).
Recent advancements include the effective enhancement of carbamazepine bioavailability using Vitamin E TPGS and Gelucire® 44/14. Solid dispersions of carbamazepine were prepared employing either the traditional solvent evaporation technique or the supercritical carbon dioxide method. Both methods and additives increased intrinsic dissolution rates of carbamazepine. Although all formulations improved drug bioavailability, the degree of enhancement varied based on the additive and method used. Vitamin E TPGS performed better with the supercritical fluid method, while Gelucire® 44/14 exhibited superior results with the classic solvent evaporation method.
Lymphatic pathway of lipid-based formulation
In lipid-based delivery systems, vehicles undergo transformation into secondary lyotropic vesicular and micellar structures, exhibiting enhanced mucus-penetrating ability upon lipolysis by constituent lipid-acting lipases in the gastrointestinal tract (Zhang et al. 2021). Post-lipolysis in the intestinal lumen, primarily 2-monoglycerides, fatty acids, and monoglycerides are released and subsequently absorbed by enterocytes. This absorption is facilitated through a mechanism mediated by fatty acid-binding membrane proteins and fatty acid-related transporters residing on the apical membrane surfaces (Berthelsen et al. 2019). Chylomicrons (CMs), lipid spheroids formed in enterocytes in response to lipid ingestion and digestion, play a pivotal role in transporting lipids, especially highly lipophilic triglycerides (TG). The common structure of the CM comprises TG and cholesterol esters in the core, surrounded by phospholipids, cholesterol, and wrapping proteins on the surface (Beilstein et al. 2016).
Following a series of intracellular processes, pre-CMs mature into CMs that are secreted into the mesenteric lymph and subsequently transferred via the lymphatics into the systemic circulation (Zhang et al. 2021) The typical intestinal tract is abundantly supplied with both blood and lymph due to the close proximity of the lamina propria surrounding the enterocytes to blood and lymph vessels. After absorption and transition across enterocytes, two potential scenarios may unfold. In one scenario, drugs may prefer entry into blood capillaries, while in the other, entry into lymph capillaries is favored. The former pathway, wherein absorbed drugs are transported into the portal blood, is the most common mechanism due to the higher rate of fluid flow in the portal blood compared to that in the intestinal lymph (500-fold higher for portal blood) (Trevaskis et al. 2008). Large or high molecular weight drugs face limitations in diffusing across blood capillaries and thus utilize more permeable lymphatic capillaries for absorption. Lipoproteins play a crucial role upon entry into the enterocyte, binding to macromolecules to facilitate the entry process and, more importantly, aiding in movement across the enterocyte. The physical size of lipoproteins varies, restricting their diffusion across vascular endothelium. Consequently, lipoprotein size limitations in diffusion, combined with the unobstructed diffusion across lymphatic capillaries, result in the preferential access of lipoproteins and the drug to the lymphatics (Yáñez et al. 2011).
Drug-related factors for delivery to the lymphatic system
The physicochemical properties of drugs play a critical role in determining the efficacy of oral delivery to the lymphatic system, as they impact the loading or concentration of drugs per chylomicron. These properties, which are summarized in Table 4, include the partition coefficient and TG solubility, with the latter being particularly vital for lymphatic delivery. Charman and Stella (1986) proposed that effective drug candidates should have a LogP > 5 and TG solubility > 50 mg/mL. In their study, they compared the lymphatic transport of dichlorodiphenyltrichloroethane (LogP 6.19) with hexachlorobenzene (LogP 6.53). Despite similar LogP values, 33.5% and 2.3% of the administered dosages of dichlorodiphenyltrichloroethane and hexachlorobenzene, respectively, reached the lymphatic system, primarily due to a 13-fold difference in TG solubility between the two compounds (Charman and Stella 1986; Myers and Stella 1992). However, it is noteworthy that a high partition coefficient and TG solubility is not sufficient for lymphatic drug delivery. For instance, in one trial, only 3% of the administered panclomedine, with a LogP of 5.48 and TG solubility of 175 mg/mL, reached the intestinal lymph. Similarly, CI-976, a lipophilic lipid regulator with a LogP of 5.83 and TG solubility > 100 mg/mL, exhibited very poor transport, with < 1% of the dose reaching the intestinal lymph (Myers and Stella 1992).
We observed weak correlations between intestinal lymphatic bioavailability and logP (r2 = 0.50, P = 0.0326), solubility in long-chain triglycerides (r2 = 0.55, P = 0.0341), and molecular weight (r2 = 0.12, P = 0.3828). Similarly, the correlations between the uptake of lipophilic compounds by the isolated CM and LogP (r2 = 0.48, P = 0.0182), solubility in long-chain triglycerides (r2 = 0.55, P = 0.0147), and molecular weight (r2 = 0.12, P = 0.2974) were found to be weak (Gershkovich and Hoffman 2005).
The logarithm of octanol solubility (log [Oct]) exhibited a linear relationship with the predicted LogP, unrelated to poor water solubility. A Log[Oct] value > 2.5 might serve as a more reliable predictor of suitability in lipid-based drug delivery systems than LogP alone (Savla et al. 2017).
The partition coefficient and solubility in TG are crucial factors influencing the lymphatic transport of a drug. However, further research is required to definitively identify all the key factors contributing to the successful lymphatic transport of drugs (Kim et al. 2013).
Models for evaluation of lymphatic transport
Numerous models have been created to evaluate lymphatic transport. In vitro models seek to replicate the digestive processes and functions of either M cells or CMs. In contrast, in vivo models primarily focus on gathering data and estimating drug transport through the lymphatic system via lymphatic cannulation. The digestion model utilized to assess lipid-based drug delivery systems is detailed in Table 5.
In vitro model: in vitro digestion models
When simulating the intricate physiological and physicochemical events occurring in the human GI tract after the oral administration of a lipid-based drug delivery system, it is essential to ensure that each stage of the digestion process closely mirrors realistic pH and enzymatic conditions, transit times, and mixing. Moreover, consideration must be given to factors such as the resident microbiota, immune system, feedback mechanisms, and specific hormonal controls to accurately replicate human digestion (Guerra et al. 2012). Additionally, to achieve an accurate prediction of the in vivo behavior of lipid-based drug delivery systems, it may be necessary to mimic the uptake mechanism in the small intestine, lymphatic transport, and hepatic metabolism. Unfortunately, the simulation of these complex multistage processes is technically challenging, time-consuming, and costly. Therefore, working under the assumption that a simplified digestion model can effectively capture the relevant effects of human GI digestion on the oral performance of lipid-based drug delivery systems, a series of simplified in vitro digestion models were developed.
The most commonly utilized in vitro digestion models for evaluating lipid-based drug delivery systems focus on simulating enzymatic digestion. These models typically replicate the intestinal (or both gastric and intestinal) phase of digestion using physiologically relevant media designed to mimic GI fluids, including digestive enzymes, at relevant activities. All experiments were conducted at 37 °C, often maintaining a fixed pH. Notably, within the field of food sciences, in vitro models commonly incorporate digestion in the oral cavity (Lucas-González et al. 2018).
While most in vitro digestion models for lipid-based drug delivery systems primarily concentrate on enzymatic digestion processes, there are alternative approaches. Simple dispersion tests, which involve no added enzymes, can be regarded as basic in vitro models that only simulate the preprocessing of lipid-based drug delivery systems during gastric mechanical digestion in vivo. Furthermore, the dynamic gastric model and TNO gastrointestinal model 1, originally designed for food research, prove suitable for studying both enzymatic digestion and the impact of gastric emptying rate and GI hydrodynamics on lipid-based drug delivery systems (Griffin et al. 2014).
In vitro model: chylomicron association model
The virtual association of drug molecules with chylomicrons in enterocytes governs the efficiency of lymphatic drug transport. The in vitro chylomicron association model holds significant promise in providing crucial insights for predicting in vivo mesenteric transport. Dichlorodiphenyltrichloroethane, exhibiting a higher affinity for chylomicrons, demonstrated markedly increased lymphatic transport compared to diazepam, which exhibits limited binding efficiency (Fig. 2) (Gershkovich and Hoffman 2007). A linear correlation between chylomicron-binding capability and lymphatic transport was established using nine fat-soluble components, including halofantrine, probucol, and vitamins, among others (Gershkovich and Hoffman 2005). This correlation was further validated using a physicochemical in silico model.
While natural chylomicrons may offer superior predictive capabilities, biomimetic chylomicrons are preferred due to their lower intergroup variations, reduced sacrifice of animals, and the elimination of labor-intensive procedures involved in natural chylomicron separation (Lu et al. 2015). In addition to the fundamental physicochemical properties of drugs, various factors, such as the addition of surfactants, may play a crucial role in altering drug solubility (Kim et al. 2015). Therefore, the contribution of excipients should also be taken into consideration.
In vitro model: combined digestion-permeation model
Most of the digestion-permeation models designed employ the conventional pH–stat lipolysis model for simulating digestion. In this process, the digested contents are subsequently transferred to a distinct compartment for the permeation step (Table 5).
In vivo model: lymphatic cannulation model
The absorption of particles or drug-associated CMs occurs through lacteals from the apical side coverage at the mesenteric lymphatics. To effectively collect and measure total lymphatic drug transport, mesenteric lymphatic cannulation emerges as a favorable approach. A model employing mesenteric lymphatic cannulation was established and utilized for evaluating lymphatic transport (Holm et al. 2003). Details of the surgical procedures can be found in the literature (Chaudhary et al. 2014).
Large animals such as pigs and dogs are preferred over smaller counterparts like rats and mice due to their physiological and anatomical similarities, especially in aspects like biliary secretion, when compared to humans (Edwards et al. 2001). Nevertheless, rat models remain the most popular. Notwithstanding the simplicity of sample collection and quantification, lymphatic cannulation is an invasive technique with inherent limitations (Trevaskis et al. 2015). The procedure involves intricate surgery and may potentially affect lymph flow and vessel pressure gradients, posing challenges for consecutive sampling after multiple treatments. Due to the influence of various factors, the success rate of lymphatic cannulation is generally low (Patel and Sawant 2019).
In vivo model: lymph blocking model
To alleviate the challenges associated with cannulation-related operational stress, lymph-blocking models have been established using blocking reagents. Cycloheximide, a protein synthesis inhibitor, selectively suppresses CM secretion without affecting other absorption pathways. Despite the irreversible nature of the blockage, no apparent adverse effects have been reported (Dahan and Hoffman 2005). The robust correlation observed between the CM blocking model and the lymphatic cannulation model validates its reliability in predicting lymphatic absorption (Lind et al. 2008). However, the specificity of cycloheximide in blocking CMs remains uncertain. Research suggests that cycloheximide may also inhibit M cells, thereby compromising the accuracy of estimating the CM pathway (Sun et al. 2011).
In vitro–in vivo correlation (IVIVC)
The enhancement of lipid-based formulation development and commercialization potential can be significantly achieved through in vitro-in vivo correlations. Establishing a model that connects in vitro and in vivo data not only shortens the drug development timeline but also improves product quality. Various in vitro techniques, including assessing solubility, dissolution, and lipolysis of lipid excipients, along with intestinal membrane techniques such as isolated animal tissue and cell culture models, can be employed to evaluate lipid-based formulations (Seeballuck et al. 2003). The pH–stat lipolysis model, which mainly simulates enzymatic digestion, is the most frequently used model in the evaluation of lipid-based formulations (Fig. 3). While these techniques provide insights into specific formulation aspects, it is crucial to recognize their limitations in understanding the in vivo interactions and performance of such systems. Similar to enterocytes, Caco-2 cells produce and release chylomicrons upon exposure to lipids. Additional research is needed to identify the most suitable in vivo model for evaluating lipid-based formulations. Dahan and Hoffman (2007) conducted a study on the effect of different lipid-based formulations of lipophilic drugs on in vitro solubilization and ex vivo intestinal permeability processes. They predicted the in vivo oral bioavailability of two model drugs, griseofulvin and dexamethasone, by designing in vitro lipolysis and ex vivo intestinal permeability models. Both drugs exhibited a good correlation between their in vitro and in vivo data regarding lipolysis and absorption. However, the actual in vivo data did not align with the ex vivo permeation studies. Griseofulvin, with limited solubility (5 µg/mL), is influenced by the presence of lipid excipients, bile salts, phospholipids, and lipolytic products in the digestive media. Therefore, the careful selection of the most appropriate in vitro and in vivo models is paramount for establishing reliable in vitro–in vivo correlations in lipid-based formulations.
Conclusion
This overview summarizes research developments aimed at gaining a better understanding of the unique characteristics of the lymphatic system. Given the effective sale of numerous medications in lipid-based formulations, lipid-based drug delivery systems exhibit a wide range of potential applications for enhancing solubility and bioavailability.
In recent times, there has been a growing interest in understanding the mechanisms through which drugs enter the lymph, the formulation strategies that can either maximize or minimize lymphatic transport, and the potential impacts of lymphatic transport on drug processing within enterocytes and the liver. The potential advantages of stimulating intestinal lymphatic transport include a reduction in first-pass metabolism and the efficient transfer of high medication doses to the lymphatic system.
Due to their roles in both transport and immunomodulation, lymphatic vessels have become a focus in therapeutic design. Following noninvasive oral or intradermal/subcutaneous distribution, lymphatic vessels can effectively facilitate immunomodulatory treatments, thereby enhancing the efficacy of therapies requiring access to lymph nodes for optimal effectiveness. Although there has been some progress in understanding how to target lymphatic transport, the precise methods to target specific immune cell populations after their transportation to the lymph nodes remain not fully comprehended.
Lipid-based formulations will also continue to change the face of oral peptide and protein medication delivery, capitalizing on current advancements in the field and exploring potential avenues for future development.
With the discovery of more lipophilic drug candidates, there is a growing interest in formulation design strategies aimed at optimizing lymphatic transport. Stimulating intestinal lymphatic transport offers potential benefits, including the reduction of first-pass metabolism and the delivery of high drug concentrations into the lymphatic system. While numerous studies have contributed to a deeper understanding of the role of lipid precursors in lymphatic drug transport and the development of diverse formulations, our comprehension of drug delivery via the lymphatic route at the cellular level remains limited. Consequently, to enhance the utility of intestinal lymph as an alternative mode of transport to the systemic circulation, further research is imperative. This research should focus on elucidating the underlying mechanisms of lipoprotein and drug binding in intestinal cells, as well as the impact of lipids and formulation excipients on this intricate process.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Albertini B, Passerini N, Pattarino F, Rodriguez L (2008) New spray congealing atomizer for the microencapsulation of highly concentrated solid and liquid substances. Eur J Pharm Biopharm 69(1):348–357
Albertini B, Passerini N, Di Sabatino M, Vitali B, Brigidi P, Rodriguez L (2009) Polymer–lipid based mucoadhesive microspheres prepared by spray-congealing for the vaginal delivery of econazole nitrate. Eur J Pharm Sci 36(4–5):591–601
Alskär LC et al (2019) Effect of lipids on absorption of carvedilol in dogs: is coadministration of lipids as efficient as a lipid-based formulation? J Control Release 304:90–100
Beilstein F, Carrière V, Leturque A, Demignot S (2016) Characteristics and functions of lipid droplets and associated proteins in enterocytes. Exp Cell Res 340(2):172–179
Berthelsen R, Klitgaard M, Rades T, Müllertz A (2019) In vitro digestion models to evaluate lipid based drug delivery systems; present status and current trends. Adv Drug Deliv Rev 142:35–49
Bibi HA, Holm R, Bauer-Brandl A (2017) Simultaneous lipolysis/permeation in vitro model, for the estimation of bioavailability of lipid based drug delivery systems. Eur J Pharm Biopharm 117:300–307
Breitenbach J (2002) Melt extrusion: from process to drug delivery technology. Eur J Pharm Biopharm 54(2):107–117
Caliph SM, Charman WN, Porter CJ (2000) Effect of short-, medium-, and long-chain fatty acid-based vehicles on the absolute oral bioavailability and intestinal lymphatic transport of halofantrine and assessment of mass balance in lymph-cannulated and non-cannulated rats. J Pharm Sci 89(8):1073–1084
Charman W, Stella V (1986) Estimating the maximal potential for intestinal lymphatic transport of lipophilic drug molecules. Int J Pharm 34(1–2):175–178
Chaudhary S, Garg T, Murthy R, Rath G, Goyal AK (2014) Recent approaches of lipid-based delivery system for lymphatic targeting via oral route. J Drug Target 22(10):871–882
Chauhan B, Shimpi S, Paradkar A (2005) Preparation and characterization of etoricoxib solid dispersions using lipid carriers by spray drying technique. AAPS PharmSciTech 6:E405–E409
Chen M-L (2008) Lipid excipients and delivery systems for pharmaceutical development: a regulatory perspective. Adv Drug Deliv Rev 60(6):768–777
Christie WW (1987) High-performance liquid chromatography and lipids. A practical guide 183–208
Christie WW (1989) Gas chromatography and lipids: a practical guide (No Title)
Cole ET, Cadé D, Benameur H (2008) Challenges and opportunities in the encapsulation of liquid and semi-solid formulations into capsules for oral administration. Adv Drug Deliv Rev 60(6):747–756
Collnot E-M, Baldes C, Wempe MF, Hyatt J, Navarro L, Edgar KJ, Schaefer UF, Lehr C-M (2006) Influence of vitamin E TPGS poly (ethylene glycol) chain length on apical efflux transporters in Caco-2 cell monolayers. J Control Release 111(1–2):35–40
Collnot E-M, Baldes C, Wempe MF, Kappl R, Hüttermann J, Hyatt JA, Edgar KJ, Schaefer UF, Lehr C-M (2007) Mechanism of inhibition of P-glycoprotein mediated efflux by vitamin E TPGS: influence on ATPase activity and membrane fluidity. Mol Pharm 4(3):465–474
Crum MF et al (2016) A new in vitro lipid digestion–in vivo absorption model to evaluate the mechanisms of drug absorption from lipid-based formulations. Pharm Res 33:970–982
Dahan A, Hoffman A (2005) Evaluation of a chylomicron flow blocking approach to investigate the intestinal lymphatic transport of lipophilic drugs. Eur J Pharm Sci 24(4):381–388
Dahan A, Hoffman A (2007) The effect of different lipid based formulations on the oral absorption of lipophilic drugs: the ability of in vitro lipolysis and consecutive ex vivo intestinal permeability data to predict in vivo bioavailability in rats. Eur J Pharm Biopharm 67(1):96–105
Dos Santos IR, Thies C, Richard J, Le Meurlay D, Gajan V, VandeVelde V, Benoit J-P (2003) A supercritical fluid-based coating technology. 2: solubility considerations. J Microencapsul 20(1):97–109
Duong V-A, Nguyen T-T-L, Maeng H-J (2023) Recent advances in intranasal liposomes for drug, gene, and vaccine delivery. Pharmaceutics 15(1):207
Edwards GA, Porter CJ, Caliph SM, Khoo S-M, Charman WN (2001) Animal models for the study of intestinal lymphatic drug transport. Adv Drug Deliv Rev 50(1–2):45–60
El Tayar N et al (1993) Solvent-dependent conformation and hydrogen-bonding capacity of cyclosporin A: evidence from partition coefficients and molecular dynamics simulations. J Med Chem 36(24):3757–3764
Gershkovich P, Hoffman A (2005) Uptake of lipophilic drugs by plasma derived isolated chylomicrons: linear correlation with intestinal lymphatic bioavailability. Eur J Pharm Sci 26(5):394–404
Gershkovich P, Hoffman A (2007) Effect of a high-fat meal on absorption and disposition of lipophilic compounds: the importance of degree of association with triglyceride-rich lipoproteins. Eur J Pharm Sci 32(1):24–32
Gibson L (2007) Lipid-based excipients for oral drug delivery. Drugs Pharm Sci 170:33
Greenberger NJ, Rodgers JB, Isselbacher KJ (1966) Absorption of medium and long chain triglycerides: factors influencing their hydrolysis and transport. J Clin Investig 45(2):217–227
Griffin BT, O’driscoll CM (2006) A comparison of intestinal lymphatic transport and systemic bioavailability of saquinavir from three lipid-based formulations in the anaesthetised rat model. J Pharm Pharmacol 58(7):917–925
Griffin BT, Kuentz M, Vertzoni M, Kostewicz ES, Fei Y, Faisal W, Stillhart C, O’Driscoll CM, Reppas C, Dressman JB (2014) Comparison of in vitro tests at various levels of complexity for the prediction of in vivo performance of lipid-based formulations: case studies with fenofibrate. Eur J Pharm Biopharm 86(3):427–437
Grove M, Müllertz A, Pedersen GP, Nielsen JL (2007) Bioavailability of seocalcitol: III. Administration of lipid-based formulations to minipigs in the fasted and fed state. Eur J Pharm Sci 31(1):8–15
Guerra A, Etienne-Mesmin L, Livrelli V, Denis S, Blanquet-Diot S, Alric M (2012) Relevance and challenges in modeling human gastric and small intestinal digestion. Trends Biotechnol 30(11):591–600
Han S, Quach T, Hu L, Wahab A, Charman WN, Stella VJ, Trevaskis NL, Simpson JS, Porter CJ (2014) Targeted delivery of a model immunomodulator to the lymphatic system: comparison of alkyl ester versus triglyceride mimetic lipid prodrug strategies. J Control Release 177:1–10
Hauss DJ (2007) Oral lipid-based formulations: enhancing the bioavailability of poorly water-soluble drugs. CRC Press
Holm R, Porter CJ, Edwards GA, Müllertz A, Kristensen HG, Charman WN (2003) Examination of oral absorption and lymphatic transport of halofantrine in a triple-cannulated canine model after administration in self-microemulsifying drug delivery systems (SMEDDS) containing structured triglycerides. Eur J Pharm Sci 20(1):91–97
Hou X, Zaks T, Langer R, Dong Y (2021) Lipid nanoparticles for mRNA delivery. Nat Rev Mater 6(12):1078–1094
Hugger ED, Novak BL, Burton PS, Audus KL, Borchardt RT (2002) A comparison of commonly used polyethoxylated pharmaceutical excipients on their ability to inhibit P-glycoprotein activity in vitro. J Pharm Sci 91(9):1991–2002
Ito Y, Kusawake T, Ishida M, Tawa R, Shibata N, Takada K (2005) Oral solid gentamicin preparation using emulsifier and adsorbent. J Control Release 105(1–2):23–31
Jannin V, Musakhanian J, Marchaud D (2008) Approaches for the development of solid and semi-solid lipid-based formulations. Adv Drug Deliv Rev 60(6):734–746
Kaukonen AM et al (2004) Drug solubilization behavior during in vitro digestion of simple triglyceride lipid solution formulations. Pharm Res 21:245–253
Keemink J, Bergström CA (2018) Caco-2 cell conditions enabling studies of drug absorption from digestible lipid-based formulations. Pharm Res 35:1–11
Keemink J, Mårtensson E, Bergström CA (2019) Lipolysis-permeation setup for simultaneous study of digestion and absorption in vitro. Mol Pharm 16(3):921–930
Kim H, Kim Y, Lee J (2013) Liposomal formulations for enhanced lymphatic drug delivery. Asian J Pharm Sci 8(2):96–103
Kim H, Seong I, Ro J, Hwang S-H, Yun G, Lee J (2015) Enhanced association of probucol with chylomicron by pharmaceutical excipients: an in vitro study. Drug Dev Ind Pharm 41(7):1073–1079
Kolling WM (2004) Handbook of pharmaceutical excipients. Am J Pharm Educ 68(15):BF1
Lind ML, Jacobsen J, Holm R, Müllertz A (2008) Intestinal lymphatic transport of halofantrine in rats assessed using a chylomicron flow blocking approach: the influence of polysorbate 60 and 80. Eur J Pharm Sci 35(3):211–218
Lombardo D, Kiselev MA (2022) Methods of liposomes preparation: formation and control factors of versatile nanocarriers for biomedical and nanomedicine application. Pharmaceutics 14(3):543
Lu Y, Qiu Y, Qi J, Feng M, Ju D, Wu W (2015) Biomimetic reassembled chylomicrons as novel association model for the prediction of lymphatic transportation of highly lipophilic drugs via the oral route. Int J Pharm 483(1–2):69–76
Lucas-González R, Viuda-Martos M, Pérez-Alvarez JA, Fernández-López J (2018) In vitro digestion models suitable for foods: opportunities for new fields of application and challenges. Food Res Int 107:423–436
Mehuys E, Vervaet C, Gielen I, Van Bree H, Remon JP (2004a) In vitro and in vivo evaluation of a matrix-in-cylinder system for sustained drug delivery. J Control Release 96(2):261–271
Mehuys E, Vervaet C, Remon JP (2004b) Hot-melt extruded ethylcellulose cylinders containing a HPMC–Gelucire® core for sustained drug delivery. J Control Release 94(2–3):273–280
Mehuys E, Remon JP, Korst A, Van Bortel L, Mols R, Augustijns P, Porter C, Vervaet C (2005) Human bioavailability of propranolol from a matrix-in-cylinder system with a HPMC-Gelucire® core. J Control Release 107(3):523–536
Myers R, Stella V (1992) Factors affecting the lymphatic transport of penclomedine (NSC-338720), a lipophilic cytotoxic drug: comparison to DDT and hexachlorobenzene. Int J Pharm 80(1–3):51–62
Niethammer A et al (2001) Synthesis and preclinical characterization of a paclitaxel prodrug with improved antitumor activity and water solubility. Bioconjug Chem 12(3):414–420
Patel MH, Sawant KK (2019) Self microemulsifying drug delivery system of lurasidone hydrochloride for enhanced oral bioavailability by lymphatic targeting: in vitro, Caco-2 cell line and in vivo evaluation. Eur J Pharm Sci 138:105027
Porter CJ, Kaukonen AM, Boyd BJ, Edwards GA, Charman WN (2004) Susceptibility to lipase-mediated digestion reduces the oral bioavailability of danazol after administration as a medium-chain lipid-based microemulsion formulation. Pharm Res 21:1405–1412
Pouton CW (1997) Formulation of self-emulsifying drug delivery systems. Adv Drug Deliv Rev 25(1):47–58
Pouton CW (2000) Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and ‘self-microemulsifying’drug delivery systems. Eur J Pharm Sci 11:S93–S98
Pouton CW (2006) Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci 29(3–4):278–287
Pouton CW, Porter CJ (2008) Formulation of lipid-based delivery systems for oral administration: materials, methods and strategies. Adv Drug Deliv Rev 60(6):625–637
Royce A, Suryawanshi J, Shah U, Vishnupad K (1996) Alternative granulation technique: melt granulation. Drug Dev Ind Pharm 22(9–10):917–924
Savla R, Browne J, Plassat V, Wasan KM, Wasan EK (2017) Review and analysis of FDA approved drugs using lipid-based formulations. Drug Dev Ind Pharm 43(11):1743–1758
Schaeffer T (2004) Pelletisation with meltable binders in sustained release formulations with lipid based excipient. Bull Tech Gattefosse 97:113–124
Schæfer T, Mathiesen C (1996) Melt pelletization in a high shear mixer. VIII. Effects of binder viscosity. Int J Pharm 139(1–2):125–138
Seeballuck F, Ashford MB, O’Driscoll CM (2003) The effects of Pluronic® block copolymers and Cremophor® EL on intestinal lipoprotein processing and the potential link with P-glycoprotein in Caco-2 cells. Pharm Res 20:1085–1092
Seo A, Schæfer T (2001) Melt agglomeration with polyethylene glycol beads at a low impeller speed in a high shear mixer. Eur J Pharm Biopharm 52(3):315–325
Seo A, Holm P, Kristensen HG, Schæfer T (2003) The preparation of agglomerates containing solid dispersions of diazepam by melt agglomeration in a high shear mixer. Int J Pharm 259(1–2):161–171
Sethia S, Squillante E (2002) Physicochemical characterization of solid dispersions of carbamazepine formulated by supercritical carbon dioxide and conventional solvent evaporation method. J Pharm Sci 91(9):1948–1957
Sethia S, Squillante E (2004) Solid dispersion of carbamazepine in PVP K30 by conventional solvent evaporation and supercritical methods. Int J Pharm 272(1–2):1–10
Shackleford DM, Faassen WF, Houwing N, Lass H, Edwards GA, Porter CJ, Charman WN (2003) Contribution of lymphatically transported testosterone undecanoate to the systemic exposure of testosterone after oral administration of two andriol formulations in conscious lymph duct-cannulated dogs. J Pharmacol Exp Ther 306(3):925–933
Shono Y, Nishihara H, Matsuda Y, Furukawa S, Okada N, Fujita T, Yamamoto A (2004) Modulation of intestinal P-glycoprotein function by cremophor EL and other surfactants by an in vitro diffusion chamber method using the isolated rat intestinal membranes. J Pharm Sci 93(4):877–885
Strickley RG (2007) Currently marketed oral lipid-based dosage forms: drug products and excipients. Drugs Pharm Sci 170:1
Sun M, Zhai X, Xue K, Hu L, Yang X, Li G, Si L (2011) Intestinal absorption and intestinal lymphatic transport of sirolimus from self-microemulsifying drug delivery systems assessed using the single-pass intestinal perfusion (SPIP) technique and a chylomicron flow blocking approach: linear correlation with oral bioavailabilities in rats. Eur J Pharm Sci 43(3):132–140
Takada K, Yoshimura H, Yoshikawa H, Muranishi S, Yasumura T, Oka T (1986) Enhanced selective lymphatic delivery of cyclosporin A by solubilizers and intensified immunosuppressive activity against mice skin allograft. Pharm Res 3:48–51
Thies R, Kleinebudde P (1999) Melt pelletisation of a hygroscopic drug in a high shear mixer: part 1. Influence of process variables. Int J Pharm 188(2):131–143
Thies C, Dos Santos IR, Richard J, VandeVelde V, Rolland H, Benoit J-P (2003) A supercritical fluid-based coating technology 1: process considerations. J Microencapsul 20(1):87–96
Trevaskis NL, Charman WN, Porter CJ (2008) Lipid-based delivery systems and intestinal lymphatic drug transport: a mechanistic update. Adv Drug Deliv Rev 60(6):702–716
Trevaskis NL, Shackleford DM, Charman WN, Edwards GA, Gardin A, Appel-Dingemanse S, Kretz O, Galli B, Porter CJ (2009) Intestinal lymphatic transport enhances the post-prandial oral bioavailability of a novel cannabinoid receptor agonist via avoidance of first-pass metabolism. Pharm Res 26:1486–1495
Trevaskis NL, Hu L, Caliph SM, Han S, Porter CJ (2015) The mesenteric lymph duct cannulated rat model: application to the assessment of intestinal lymphatic drug transport. J Visual Exp 97:e52389
Venkatesan N, Yoshimitsu J, Ito Y, Shibata N, Takada K (2005) Liquid filled nanoparticles as a drug delivery tool for protein therapeutics. Biomaterials 26(34):7154–7163
Venkatesan N, Yoshimitsu J, Ohashi Y, Ito Y, Sugioka N, Shibata N, Takada K (2006) Pharmacokinetic and pharmacodynamic studies following oral administration of erythropoietin mucoadhesive tablets to beagle dogs. Int J Pharm 310(1–2):46–52
Verreck G, Brewster ME (2004) Melt extrusion-based dosage forms: excipients and processing conditions for pharmaceutical formulations. Bull Tech Gattefossé 97:85–95
Voinovich D, Moneghini M, Perissutti B, Filipovic-Grcic J, Grabnar I (2000) Preparation in high-shear mixer of sustained-release pellets by melt pelletisation. Int J Pharm 203(1–2):235–244
Voinovich D, Moneghini M, Perissutti B, Franceschinis E (2001) Melt pelletization in high shear mixer using a hydrophobic melt binder: influence of some apparatus and process variables. Eur J Pharm Biopharm 52(3):305–313
Wakerly MG, Pouton CW, Meakin BJ, Morton FS (1986). Self-emulsification of vegetable oil-nonionic surfactant mixtures: a proposed mechanism of action. ACS Publications
Yalkowsky SH (1999) Solubility and solubilization in aqueous media, Oxford University Press, New York
Yáñez JA, Wang SW, Knemeyer IW, Wirth MA, Alton KB (2011) Intestinal lymphatic transport for drug delivery. Adv Drug Deliv Rev 63(10–11):923–942
Yoshida T, Nakanishi K, Yoshioka T, Tsutsui Y, Maeda A, Kondo H, Sako K (2016) Oral tacrolimus oil formulations for enhanced lymphatic delivery and efficient inhibition of T-cell’s interleukin-2 production. Eur J Pharm Biopharm 100:58–65
Zhang B, Xue A, Zhang C, Yu J, Chen W, Sun D (2016) Bile salt liposomes for enhanced lymphatic transport and oral bioavailability of paclitaxel. Die Pharmazie-an International Journal of Pharmaceutical Sciences 71(6):320–326
Zhang Z, Lu Y, Qi J, Wu W (2021) An update on oral drug delivery via intestinal lymphatic transport. Acta Pharmaceutica Sinica B 11(8):2449–2468
Acknowledgements
This study was supported by Won-Kwang University in 2023. We would like to thank Editage (www.editage.co.kr) for the English language editing.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors (So-Jeong Joeng, Woo-Yul Song, Chun-Woong Park, and Dong-Wook Kim) declare that they have no conflict of interest.
Research involving human and animal rights
This article does not contain any studies with human and animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jeong, SJ., Song, WY., Park, CW. et al. Recent approaches to investigate drug delivery systems through the lymphatic pathway using oral lipid-based formulations. J. Pharm. Investig. 54, 131–144 (2024). https://doi.org/10.1007/s40005-023-00656-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-023-00656-5