Abstract
Purpose
We aimed to evaluate HIV-1 compartmentalization between the cerebrospinal fluid (CSF) and plasma and investigate as to which extent HIV-1 strains in CSF differ from those in blood and whether a correlation with either plasma viral load (pVL) or an altered blood–brain barrier (BBB) does exist.
Study design
We retrospectively evaluated paired CSF/blood samples collected from 86 HIV+ patients. HIV-RNA quantification, pol (PR/RT), and V3 sequencing were performed. HIV coreceptor tropism (CRT) was inferred (g2p, false-positive rate 10%, FPR). Data of standard CSF analysis were also reviewed; an altered CSF/plasma albumin ratio signified BBB damage. Neurological abnormalities (NA) were recorded.
Results
Overall, 32% of patients had a CSF/plasma HIV-RNA ratio > 1 (discordance); 3% of patients had detectable CSF HIV-RNA despite suppressed pVL (escape). Discordance was more frequent in ART-treated patients (p < 0.001) and in patients with NA (p = 0.016), but was independent of BBB damage (p = 0.65) and AIDS diagnosis (p = 0.96). Finally, CSF/plasma discordance was significantly more frequent (p < 0.0001) in patients with lower pVL values (< 10.000 copies/ml). Env divergence > 10% was found in 44% of sequences and was associated with ART (p = 0.008) and NA (p = 0.037). Overall, 24% of patients had a discordant CSF/blood CRT. A 100% nucleotide identity was observed in only 7.3% of pol sequences; notably, 10% of patients had resistance-associated mutations in CSF, but not in blood.
Conclusions
Our data confirm an independent replication and evolution of HIV within the CSF. A number of factors either hinder or contribute to the compartmentalization of HIV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Human Immunodeficiency Virus (HIV-1) penetrates the Central Nervous System (CNS) during the early stages of infection. In the CNS, including the cerebrospinal fluid (CSF), HIV stays in a very different environment where viral compartmentalization occurs due to an adaptation of HIV to the target cells. In fact, the low population of circulating CD4+ lymphocytes in CNS/CSF forces HIV to infect and replicate primarily in monocyte-derived cells [1], promoting a different viral evolution from plasma [1]. Moreover, the presence of blood–brain barrier (BBB) modifies the pharmacokinetics and pharmacodynamics of antiretroviral drugs in CNS with respect to blood causing a possible restart of HIV-1 replication [2] and, due to suboptimal drugs exposure, the emergence of drug resistance [2].
The sequestration of HIV in CNS/CSF implicates that in some patients CSF HIV-1 RNA can be detected at higher levels than in plasma [3, 4], and viral population is qualitatively divergent from variants in the peripheral district [5]. In fact, the presence of viruses with a different phenotype in paired CSF and peripheral blood mononuclear cells (PBMC) has been described [6], thereby proving that genetic characteristics can distinguish CNS/CSF isolates from blood viruses [7]. Finally, HIV establishes a viral reservoir within the CNS/CSF with the potential of replicating and reseeding the periphery [8].
Continuous HIV replication within the CNS/CSF (compartmentalization) has been associated with persistent inflammation and neuronal injury causing over time neurocognitive impairment or other persistent neurological disorders [5, 6, 8] that cannot be completely restored after the initiation of an effective antiretroviral treatment.
Compartmentalization of HIV-1 within the CNS/CSF has been repeatedly reported [9,10,11] even if on a limited number of patients; however, a controversial question is whether HIV-RNA in CSF derives from viral replication within the CNS or from blood. In fact, it has been suggested that for high level of plasma viral load (pVL) HIV in CSF originates from the blood compartment by diffusion across BBB [12, 13] and in this event a low diversity between CSF and plasma viruses should be observed.
The comprehension of what happens to HIV-1 when it penetrates and replicates into the CNS is fundamental for the management of HIV compartmentalization and its clinical implication.
Herein, we evaluated HIV-1 compartmentalization between CSF and plasma in a substantial group of HIV positive patients and investigated as to which extent HIV-1 strains in CSF differ from those in blood and whether a correlation with either pVL or an altered BBB does exist.
Study design
This is a retrospective study including data recorded from 86 HIV-1 positive subjects who underwent a lumbar puncture in our Institute between 2001 and 2015 due to either neurological symptoms or altered psychometric testing and/or abnormalities in Magnetic Resonance Imaging (MRI) of the brain. Paired blood and CSF samples were evaluated for HIV-1 viral load, bulk genotypic resistance tests, and viral coreceptor tropism. Standard CSF analysis was also reviewed and the CSF to serum albumin concentration quotient (QAlb) was used to estimate the BBB integrity; a QAlb > 7 signified BBB damage.
Plasma and CSF HIV-RNA levels were quantified by Nucleic Acid Sequence–based Amplification kit (Nuclisens, version 2.1 [Bio-Merieux]) with a detection limit of 25 copies/mL both in plasma and CSF. The CSF/plasma ratio of HIV-RNA was calculated; a CSF/plasma ratio > 1 (indicating levels of HIV-RNA in CSF higher than those in plasma) was arbitrarily labeled as a “discordance” between CSF and plasma. CSF “escape” was defined as detectable CSF HIV RNA in the setting of suppressed plasma viral load.
Protease and reverse transcriptase genes were sequenced on paired CSF and plasma samples using the commercially available Viroseq HIV Genotyping Kit (Applied Biosystems, Foster City, CA, U.S.A). For patients with undetectable pVL, pol sequences were obtained from proviral DNA with the use of a “home-made” protocol [14]. The patterns of resistance-associated mutations in paired blood/CSF samples were analysed.
Genotypic determination of HIV-1 co-receptor usage was also performed by population sequencing of the V3-coding region of HIV-1 env as previously described [15]. According to current European guidelines [16] R5 and non-R5 (either X4 and dual tropic) coreceptor tropism was inferred using the Geno2pheno algorithm (https://coreceptor.geno2pheno.org/) with a false-positive rate (FPR) of 10%. A FPR value below 10% is predictive for an X4 virus whereas a value above 10% reflects a R5 virus.
Nucleotide divergence of pol and env sequences was evaluated by aligning paired sequences using EMBOSS Needle software (http://www.ebi.ac.uk/Tools/psa/emboss_needle/nucleotide.html).
Descriptive statistics [means, medians, SD, interquartile range (IQR), percentages, and frequencies] were performed. Statistical significance was estimated according to univariate test as appropriate; a p value < 0.05 was considered significant.
Results
Patient characteristics
Patients were mostly males (81.4%) with a median age of 42.5 years (IQR 37.5–48) and the majority of them (55.8%) had acquired HIV infection through sharing syringes or needles during drug use. When lumbar puncture was performed, median CD4+ cell count was 89 cells/µl (IQR, 34–262), and 67 (78%) patients were diagnosed with AIDS. AIDS diagnosis was due to opportunistic infections of CNS in 34.8% of all patients, and an additional 33.7% of patients had altered psychometric testing with or without MRI abnormalities. Overall, 62.8% of patients were naïve to antiretroviral therapy; 26.7% were receiving an ART regimen and 10.5% ART experienced patients had suspended their treatment because of a scarce adherence or because they were prevented from taking the medicines for any clinical reason.
Viral load
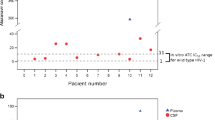
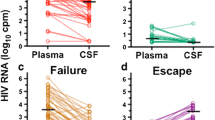
HIV-RNA on paired CSF/plasma samples was available for 75 patients. Median CSF and plasma VL were 3.53 log10 cp/ml (IQR 2.73–4.72 log10 cp/ml) and 4.92 log10 cp/ml (IQR 3.86–5.47 log10 cp/ml), respectively. CSF and plasma VL were independent of AIDS diagnosis (p = 0.43 and p = 0.77, respectively), but they were associated with cART taking (p = 0.013 and p < 0.001).
A CSF/plasma discordance was found in 24/75 (32%) patients, two of whom (3%) had detectable CSF HIV-RNA despite a suppressed pVL (escape). CSF/plasma discordance (or escape) was independent of AIDS diagnosis (p = 0.96) but it significantly correlated with a history of neurological disability and/or MRI abnormalities (p = 0.016) which were attributable to opportunistic infections in 9/24 (36%) subjects, and in the remaining patients to HIV itself. Discordance (or escape) was also more frequently observed in patients on ART (p = 0.0002) (Table 1). Finally, based on pVL strata (pVL < 10,000; 10,000 < pVL < 100,000; pVL > 100,000 copies/ml), CSF/plasma discordance was significantly more frequent (p < 0.0001) in patients with lower pVL values (15/20 patients, 75%) than in those with higher pVL levels (8/22 patients, 36.3% and 1/33, 3%, respectively).
Env sequences and coreceptor tropism
Paired CSF/plasma env sequences were successfully obtained from 82/86 (95.3%) patients. 88% of these paired V3 sequences had some degree of discrepancy. Median nucleotide divergence for paired V3 (105 bp long) sequences was 8.1% (IQR 1.9–14.0). Env nucleotide divergence was > 10% in 44% of sequences; it was unrelated to AIDS diagnosis (p = 0.85), but it was more likely in patients exposed to ART than in naïves (naïve, 31.3%; current ART, 69.5%; previous ART, 50%; p = 0.008) and in patients with neurological abnormalities (p = 0.037) (Table 1).
Regarding coreceptor tropism, a R5 strain was found in 58 (70.7%) and 56 (68.2%) CSF and blood samples, respectively. A discordant CSF/blood tropism was observed in 20 (24%) patients (11, R5CSF/non-R5blood; 9, non-R5CSF/R5blood); moreover, even when CRT in the two compartments coincided, a FPR difference > 10% between CSF and blood sequences was observed in 52% of patients.
Pol sequences and resistance mutations
A total of 41 paired pol sequences was obtained, and a complete (100%) identity was observed in only 7.3% of sequences; nevertheless, median nucleotide divergence was low (1.5%; IQR 0.7–2.1). A total of 13/41 (32.5%) patients had signature mutations either in CSF, in plasma or both (Table 2) and six of these patients had discordant results in CSF compared to plasma; in particular, 10% of patients had resistance-associated mutations in CSF which were absent in blood. Finally, at signature positions, substitutions in blood were more likely to be mixture of wild-type and mutated aminoacids.
Correlation with BBB damage
A BBB damage (Qalb>7) was observed in 56% of patients; median CSF HIV-RNA levels were 3.73 log10 (IQR 2.81–4.77 log10) and 3.22 log10 (IQR 2.5–4.22 log10) copies/ml, respectively, in patients with or without an altered BBB (p = 0.19).
Discordance (or escape) was also unrelated to the BBB damage (p = 0.65); moreover, even without reaching statistical significance, patients with normal BBB had a higher median nucleotide V3 [8.55 (2.8–14.1) vs 5.7 (1.0–14.0), p = 0.47] and pol [1.9 (IQR 0.8–2.4) vs 0.9 (0.3–1.7) p = 0.10] sequence divergence than patients with an altered BBB.
Discussion
Cerebrospinal fluid viral discordance wherein CSF HIV-RNA is detectable and plasma RNA is suppressed (escape) or CSF HIV-RNA levels are higher than in plasma supports the potential of the CNS as a distinct compartment [17] whence HIV-1 viral particles may be released independently of blood. In this study, HIV discordance between CSF and plasma HIV-RNA was found in one-third of patients. This rate of discordance was higher than in other reports [13] possibly because of a different patient selection and discordance definition [5]; when CSF viral discordance was defined as a CSF HIV RNA load > 0.5 log10 copies/mL greater than pVL, a 20% discordance was observed in our series (data not shown) which was comparable to other case studies [4, 13]. Moreover, similar to previous observations [10, 18], HIV-RNA discordance mainly applied to patients with neurological signs (p = 0.019) most likely due to HIV itself and was significantly associated with ART use (p = 0.0002). An explanation for this latter finding can be offered by the particular structure of the BBB which is characterized by tight junctions and the lack of intercellular pores thereby preventing most molecules from entering the CNS. Therefore, beyond antiretroviral drug characteristics (molecular weight, protein binding, lipophilicity, etc), the presence of BBB plays an important role in reducing drug penetration. The HIV-1 exposure in CNS to suboptimal drug concentration is not without consequences, since it allows local replication of HIV-1 [2] and differential viral evolution.
CCR5-positive mononuclear cells, macrophages, and microglia in the central nervous system of HIV-positive subjects represent an important target for virus replication [19]; accordingly, in the present study, 70.7% of viral strains from CSF were classified as R5 variants. Although a divergent coreceptor tropism between HIV-1 variants in CSF and blood was observed in 24% of patients, a false-positive rate difference > 10% between CSF and blood HIV strains pertained to half of our cases; this result along with discordant patterns of signature mutations in the pol gene detected in 15% of cases suggests a different evolution of HIV in the two compartments. Ten percent of our patients had important mutations in CSF but not in plasma; moreover, wherein amino acid changes referred to positions shared by CSF and plasma sequences, mutants were seen in CSF, whereas mixtures of mutant and wild-type sequences were found in plasma. This result underlines the role of CNS as a distinct compartment where multiple influences can promote specific HIV-1 strains to replicate independently from periphery.
In CSF, HIV-1 strains could reflect either a continuous replenishment from systemic replication [3, 20] or an incomplete suppression of viral replication due to an inefficient penetration of cART through the BBB. In any case, the source and causes of this persistent viral replication in CNS are still controversial and not completely understood [21]. Recent studies suggested that viral escape/compartmentalization in CSF greatly correlates with the peripheral viral replication. Mukerji et al. [22] found that pVL during the 6 months preceding the lumbar puncture predicts viral escape in ART-experienced subject; in particular, an inverse relationship would exist between CSF discordance and pVL [23]. Accordingly, in the present study CSF/plasma discordance was significantly more frequent (p < 0.0001) in patients with lower pVL values; moreover, it was also unrelated to the BBB damage (p = 0.65), thereby disproving a passive diffusion across the BBB.
In this study, neurological symptoms or MRI abnormalities, mostly directly caused by HIV-1 itself, were significantly associated with discordance/escape and env nucleotide divergence. This finding is consistent with previous studies which also claimed for this correlation [10, 24, 25]. HIV-1 replication in CNS causes an important elevation of proinflammatory cytokines, that is even higher in patients with discordance/escape [21], thereby giving a possible explanation of neurologic signs and symptoms in patients with persistent CNS viral replication.
Although our study evaluating paired CSF/plasma samples from 86 HIV+ subjects is one of the largest case-series on this topic, we acknowledge some limitations. Because of the retrospective nature of the study, there could be information or misclassification biases related to clinical history of patients. Moreover, we could not evaluate the actual clinical impact of our findings on history of patients, due to the absence of follow-up of neurological symptoms and/or a second lumbar puncture.
In conclusion, the analysis of viral sequences in patients enrolled in this study suggests that an independent evolution (compartmentalization) of HIV in CSF could occur. Moreover, our data indicate that both viral escape/discordance and sequence divergence could be associated with neurological signs and symptoms. Therefore, we believe in the necessity of the assessment of CNS compartment in HIV-1 infected subject, even in those with peripheral viral suppression.
References
Bednar MM, Sturdevant CB, Tompkins LA, et al. Compartmentalization, viral evolution, and viral latency of HIV in the CNS. Curr HIV/AIDS Rep. 2015;12:262–71. https://doi.org/10.1007/s11904-015-0265-9.
Aylin Yilmaz RW, Price. Magnus Gisslen. Antiretroviral drug treatment of CNS HIV-1 infection. J Antimicrob Chemother. 2012;67:299–311. https://doi.org/10.1093/jac/dkr492 (Epub 2011 Dec 8).
Eden A, Fuchs D, Hagberg L, et al. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis 202:1819–1825. https://doi.org/10.1086/657342 (Epub 2010 Nov 4).
Rawson T, Muir D, Mackie NE, Garvey, et al. Factors associated with cerebrospinal fluid HIV RNA in HIV infected subjects undergoing lumbar puncture examination in a clinical setting. J Infect 65:239–245. https://doi.org/10.1016/j.jinf.2012.04.007. (Epub 2012 Apr 17).
Churchill M, Nath A. Where does HIV hide? A focus on the central nervous system. Curr Opin HIV Aids. 2013;8:165–9. https://doi.org/10.1097/COH.0b013e32835fc601.
Di Stefano M, Monno L, Fiore JR, et al. Neurological disorders during HIV-1 infection correlate with viral load in cerebrospinal fluid but not with virus phenotype. AIDS 1998;12:737–43. https://doi.org/10.1097/COH.0b013e32835fc601.
Pillai SK, Pond SL, Liu Y, et al. Genetic attributes of cerebrospinal fluid-derived HIV-1 env. Brain. 2006;129:1872–83. https://doi.org/10.1093/brain/awl136.
Hellmuth J, Valcour V, Spudich S. CNS reservoirs for HIV: implications for eradication. J Vir Erad. 2015;1:67–71.
Schnell G, Price RW, Swanstrom R, et al. Compartmentalization and clonal amplification of HIV-1 variants in the cerebrospinal fluid during primary infection. J Virol. 2010;84:2395–407. https://doi.org/10.1128/JVI.01863-09 (Epub 2009 Dec 16).
Canestri A, Lescure F, Jaureguiberry S, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis. 2010;50:773–8.
Soulié C, Tubiana R, Simon A, et al. Presence of HIV-1 R5 viruses in cerebrospinal fluid even in patients harboring R5 × 4/X4 viruses in plasma. J Acquir Immune Defic Syndr. 2009;51:60–4.
Soulié C, Fourati S, Lambert-Niclot S, et al. HIV genetic diversity between plasma and cerebrospinal fluid in patients with HIV encephalitis. AIDS. 2010;24:2412–4. https://doi.org/10.1016/S1473-3099(13)70269-X.
Soulie C, Grudé M, Descamps D, et al. Antiretroviral-treated HIV-1 patients can harbour resistant viruses in CSF despite an undetectable viral load in plasma. J Antimicrob Chemother. 2017;72:2351–2354. https://doi.org/10.1093/jac/dkx128.
Monno L, Punzi G, Scarabaggio T, et al. Mutational patterns of paired blood and rectal biopsies in HIVinfected patients on HAART. J Med Virol. 2003;70:1–9.
Monno L, Brindicci G, Saracino A, et al. V3 sequences and paired HIV isolates from 52 non-subtype B HIV type-1 infected patients. AIDS Res Hum Retroviruses. 2010;26:365–72. https://doi.org/10.1089/aid.2009.0224.
Vandekerckhove LP, Wensing AM, Kaiser R, et al. European guidelines on the clinical management of HIV-1 tropism testing. Lancet Infect Dis. 2011 May;11:394–407. https://doi.org/10.1016/S1473-3099(10)70319-4.
Fois AF, Brew BJ. The Potential of the CNS as a Reservoir for HIV-1 Infection: Implications for HIV eradication. Curr HIV/AIDS Rep. 2015;12:299–303. https://doi.org/10.1007/s11904-015-0257-9.
Peluso MJ, Ferretti F, Peterson J, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS. 2012;26:1765–74. https://doi.org/10.1097/QAD.0b013e328355e6b2.
He J, Chen Y, Farzan M, et al. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–9.
Fletcher CV, Staskus K, Wietgrefe SW, et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci USA. 2014;111:2307–12. https://doi.org/10.1073/pnas.1318249111. (Epub 2014 Jan 27).
Nightingale S, Geretti AM, Beloukas A, et al. Discordant CSF/plasma HIV-1 RNA in patients with unexplained low-level viraemia. J Neurovirol. 2016;22:852–60. https://doi.org/10.1007/s13365-016-0448-1.
Mukerji SS, Misra V, Lorenz D, et al. temporal patterns and drug resistance in CSF viral escape among ART-experienced HIV-1 infected adults. J Acquir Immune Defic Syndr (1999). 2017;75:246–55. https://doi.org/10.1097/QAI.0000000000001362. 2017 (.
Di Carlofelice M, Everit A, Muir D, et al. Cerebrospinal fluid HIV RNA in persons living with HIV. HIV Med. 2018. https://doi.org/10.1111/hiv.12594.
Kugathasan R, Collier DA, Haddow LJ, et al. Diffuse white matter signal abnormalities on magnetic resonance imaging are associated with human immunodeficiency virus type 1 Viral escape in the central nervous system among patients with neurological symptoms. Clin Infect Dis. 2017;64:1059–65. https://doi.org/10.1093/cid/cix035.
Dravid AN, Natrajan K, Kulkarni MM, et al. Discordant CSF/plasma HIV-1 RNA in individuals on virologically suppressive antiretroviral therapy in Western India. Medicine 2018;97:e9969. https://doi.org/10.1097/MD.0000000000009969.
Acknowledgements
The authors would like to thank all patients who participated in the study. The authors also wish to thank Mrs Paulene Butts for her assistance in manuscript preparation.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No authors have any conflict of interest to declare.
Ethical approval
The research did not require approval from the ethics committee according to the Italian law since it was performed as an observational retrospective study in the context of normal clinical routines.
Rights and permissions
About this article
Cite this article
Bavaro, D.F., Calamo, A., Lepore, L. et al. Cerebrospinal fluid compartmentalization of HIV-1 and correlation with plasma viral load and blood–brain barrier damage. Infection 47, 441–446 (2019). https://doi.org/10.1007/s15010-019-01268-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-019-01268-8