Abstract
Heavy metal toxicity is one of the major abiotic stresses caused by physiological and biochemical changes. Plants have evolved various phytochemical defense mechanisms to cope with this abiotic stress conditions. Phenolic compounds are one of the stress responses and have multiple roles in respect to adaptation of plants to the environment. In the present study, we aimed to evaluate the differential accumulation of various phenolics with HPLC in the leaves of corn exposed to increasing heavy metal doses in the plant growth medium. The application of Cd, Cu, and Pb increased the total phenolics in all treatments compared to control groups. Chlorogenic acid and rutin were the main phenolic compounds in respect to quantifying. However, the contents of caffeic acid, ferulic acid, and vanillic acid were comparatively lower than chlorogenic acid and rutin in all samples. The content of chlorogenic acid significantly increased and rutin slightly increased in the treatment of the heavy metals. The levels of caffeic acid and ferulic acid significantly decreased in all exposures of heavy metals compared to control groups. The content of vanillic acid changed according to heavy metal types and doses in the leaves of corn, and the low doses of Pb and Cd increased the level of vanillic acid. We show that there is a positive correlation with the total phenolic content and chlorogenic acid when the corn is exposed to Pb. Moreover, there are negative correlations between total phenolic compound and caffeic acid, ferulic acid in the application of Cu and Cd.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Heavy metals are one of the main abiotic stress factors, and they have caused some physiological and biochemical changes in the plants. The excess of heavy metals affects the various parameters of plant metabolism such as photosynthesis, mineral distribution, and antioxidant defense system (Nazar et al. 2012). The contamination of soil with heavy metals increases as a result of agricultural activities such as uses of fertilizers, herbicides, and pesticides practices (Demirezen and Aksoy 2005). Environmental conditions have changed the plant growth and development, and plants have acquired adaptation mechanisms to survive their life (Lequeux et al. 2010). Heavy metals such as Cd and Pb are unnecessary, and others such as Cu, Fe, and Zn are required for plant life cycles. However, the high level of Cu shows a toxic effect and caused retention of plant development. They are considered as very significant contaminant and affect the electron transport system (ETS), chloroplast, thylakoid membrane, plastoquinone, and carotenoids (Sharma and Dubey 2005).
The primary response of plants exposed to heavy metals is the formation of reactive oxygen species (ROS), and it leads to physiological changes (Yadav 2010). ROS can be assisted as a cellular indicator of the stress. Plant damage occurs when the capacity of antioxidant is lower than the amount of ROS (Michalak 2006). Heavy metals can induce oxidative stress, but plants have developed various defense mechanisms such as an enzymatic and non-enzymatic system that allows scavenging of free radicals. Plant enzymatic defense systems include catalase, peroxidase, ascorbate peroxidase, superoxide dismutase, and glutathione reductase to remove the oxidant molecules. Also, plants have non-enzymatic antioxidant responses such as ascorbate, glutathione, flavonoids, phenolic compounds, tocopherol, and carotenoids (Schützendübel and Polle 2002; Gratao et al. 2005).
Phenolic compounds which are found to protect against stress in plants show antioxidant action with their high tendency to chelate metals, and they are plant secondary metabolites (Tomas-Barberan et al. 2001; Cervilla et al. 2012). Some phenolic compounds are highly widespread in the plants, while others are specific for certain plant family or found only in certain plant tissues (Cheynier 2012). These compounds have considerable physiological and morphological importance for plants. They play an important role in growth and reproduction, and exhibit physiological properties such as antioxidant and antimicrobial activities (Balasundram et al. 2006). They possess hydroxyl and carboxyl groups to bind heavy metals. Chelating ability of phenolic compounds may relate to the high nucleophilic character of the aromatic rings. Heavy metals have caused free radical formation; they decompose lipid hydroperoxide, and lipid alkoxyl radicals occur. Phenolic compounds inhibit lipid peroxidation by trapping the alkoxyl radicals (Michalak 2006). Exposure to heavy metals increases the production of phenolic compounds in plants. They are important antioxidant chelating metals and have been considered as electron-donating agents. The protective role of plant phenolic compounds could explain the modulation of their levels depending on stressing conditions in the environment (Kovacik et al. 2008; Marguez-Garcia et al. 2009).
Phytochemicals are the individual chemicals from which plants are produced (Cseke et al. 2006). Phenolic compounds are characteristic of plants that have one or more hydroxyl groups bounded directly to an aromatic ring and range from simple phenolic molecules to highly polymerized compounds (Vermerris and Nicholson 2006). Antioxidant activities of phenolic compounds arise from their ability to scavenge free radicals, donate hydrogen atoms, and perform metal chelating activity (Balasundram et al. 2006). Phenolic acids are hydroxylated derivatives of benzoic and cinnamic acids. Caffeic acid is a metabolite of the phenylpropanoid pathway found in several plant species. Caffeic acid has changed the plant development, photosynthesis, and ROS generation. Caffeic acid has been accumulated in the cell wall-bound fraction of stressed plant (Bubna et al. 2011). Chlorogenic acids (CGAs) are the family of ester phytochemicals formed between cinnamic acid derivatives and quinic acids. CGAs are produced through the shikimate and phenylpropanoid pathways and have been identified in responses against both biotic and abiotic stressors (Ncube et al. 2014). Ferulic acid is the most abundant hydroxyl cinnamic acid in the plant, and shows antioxidant activity in response to free radicals via donating one hydrogen atom from its phenolic hydroxyl group. Ferulic acid exhibits wide variety of biological activities such as antioxidant, metal chelation, and modulation of enzyme activity. It is found in the plant cell wall components as covalent side chains and is a strong UV absorber (Kumar and Pruthi 2014). Rutin is one of the bioactive phenolic compounds, which can also be used as a natural coloring agent, and an oxidation inhibitor (Musallam et al. 2012). Vanillic acid is a derivate of benzoic acid, and exhibits free radical scavenging activity, reducing power, and inhibition of lipid peroxidation. Also, vanillic acid reduced lipid peroxidation and significantly restored enzymatic antioxidants (Calixto-Campos et al. 2015).
Studies on plant abiotic stress have been intensively researched on antioxidant enzymes and total phenolic compound by plant physiologists (Verma and Dubey 2003; Rellan-Alvarez et al. 2006; Gonçalves et al. 2007; Rastgoo and Alemzadeh 2011; Hassan and Mansoor 2014). Also, individual phenolic contents of various plants grown in normal environment have been studied by chemists (Rauha et al. 2000; Zheng et al. 2001; Cai et al. 2004; Wojdylo et al. 2007; Ramos-Escudero et al. 2012; Erenler et al. 2015). Although there are few studies on the relationship between heavy metal and phenolic compounds such as (Kovacık et al. 2009), no general conclusion about the heavy metal stress on phenolic compound levels. In the current study, we evaluated the effect of heavy metals on total phenolic contents and individual phenolic compounds of corn. The aim of the present work is primarily to reveal which phenolic compounds effect to the total phenolic changes. Also, we investigated influential phenolic compounds in corn exposed to the different doses of cadmium, copper, and lead.
Materials and methods
Plant material and growth conditions
Corn (Zea mays convar. saccharata var. rugosa) seeds were sown in multiple seedling vials filled with peat. After growing to sufficient size, the seedlings were cultivated in plastic boxes contained by 10 kg equal mix of peat and garden soil in unheated greenhouse conditions. The experiment was conducted as a randomized plot design with three replications. The characteristics of plant growth soil are pH 7:67, lime: 3.31 %, Mg: 5.9 (ppm), Ca: 57.6 (ppm), Fe: 11.5 (ppb), Zn: 119.7 (ppb), Cu: 30.75 (ppb), Pb: 12.3 (ppb), and Cd: 0.9 (ppb). The whole experiment was performed in greenhouse conditions with 16:8 photoperiods, at 25 ± 3 °C in the periods of May and June. After 2 weeks, CuSO4, Pb(NO3)2, and CdCl2 were added to the plant growth soil as a 10, 20, and 50 ppm doses. The heavy metal treatments were applied three times with 2 days interval. The leaves of corn were harvested 2 weeks after the heavy metal treatment and they were stored at −80 °C until the analysis.
Extraction of the phenolic compounds
Fresh corn leaves (2 g) were ground into liquid N2 with mortar and pestle, extracted with methanol–chloroform (4:1), and the homogenates were sonicated for 20 min at ambient temperature. The obtained extracts were centrifuged at 4500×g for 10 min at room temperature. The pellets were re-extracted under identical conditions. Supernatants were used for the total phenolic content and quantification of individual phenolic compounds.
Determination of the total phenolic contents
Total phenolic contents were determined using Folin–Ciocalteu reagent (Singleton and Slinkard 1977). Briefly, 100 µL of extracts was dissolved into 4.5 mL distilled water. One milliliter of Folin–Ciocalteu reagent was pipetted to the mixture and kept at 25 °C for 3 min; 300 µL of 2 % Na2CO3 was added to the mixture. After 2 h, the absorbance of samples was measured at 760 nm. The results were expressed as milligrams of gallic acid equivalents (GAEs) per gram fresh weight using a calibration curve obtained from gallic acid as standard.
Analysis of the phenolic compounds by HPLC
Individual phenolics of leaf extracts were analyzed by high performance liquid chromatography (HPLC) with direct injection of 20 µL of filtrated (0.22 μm) methanol–chloroform extracted sample. Analysis of phenolic compounds was carried out using Shimadzu HPLC LC 20AT pump and DAD-M20A detector. A gradient of solvent A (deionized water) and solvent B (water:formic acid, 90:5) were applied to reversed-phase Prontosil C18-EPS 3 μm Reversed-Phase HPLC Columns (4.6 × 150 mm) as follow: 0–8 min, 10 % A and 90 % B; 8–29 min, 15 % A and 85 % B; 29–40 min, 30 % A and 70 % B; 40–50 min, 55 % A and 45 % B; 60 min, 0 % A and B washing and equilibration of the column. The flow rate was 1 mL min−1, the column temperature was set at 40 °C. Detection was performed by scanning from 190 to 800 nm and read at 280 nm. Identification of individual phenolic compounds was performed by comparing their retention times and spectra with those of original phenolic standards. The standards were obtained from Merck (cinnamic acid and chlorogenic acid), and Sigma-Aldrich (cerulic acid, caffeic acid, vanillic acid, and rutin). The results were expressed as milligram per kilogram of fresh weight.
Statistical analysis
Statistical analysis was performed with one-way ANOVA followed by Duncan multiple tests. The numerical data of phenolics are presented as mean ± standard deviation. Significant differences in relation to the control groups were indicated at p < 0.05.
Results
The effect of heavy metals on total phenolic contents
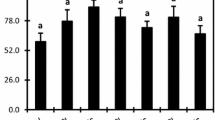
The application of Cd, Cu, and Pb to the corn growth medium changed the total phenolic contents, and their levels are shown in Fig. 1. Heavy metals increased the content of total phenolics in all treatments compared to control groups except for 20 of Cd, and it is not changed significantly. The most greatest increase of the total phenolic is seen in the Pb (10 ppm), Cd (50 ppm), and Cu (20 ppm) treatments at significant levels, respectively (p < 0.05). However, other applied doses slightly increased the total phenolic contents in the leaves of corn.
The content of individual phenolics in leaves of corn
The contents of caffeic acid, ferulic acid, chlorogenic acid, rutin, and vanillic acid are determined in the leaves of corn exposed to Cd, Cu, and Pb. The treatment of heavy metals changed the level of individual phenolics. Chlorogenic acid and rutin were the main phenolics in respect to quantifying. However, the contents of caffeic acid, ferulic acid, and vanillic acid were comparatively lower than chlorogenic acid and rutin in all leaves.
The effect of heavy metals on chlorogenic acid and rutin are shown in Fig. 2. The content of chlorogenic acid significantly increased in all application and doses of heavy metals except for 10 ppm of Cu. The level of rutin slightly increased in the treatment of Cd, Pb, and Cu except 50 ppm doses of copper. The levels of caffeic acid and ferulic acid significantly decreased in all exposures of heavy metals compared to control groups (p < 0.05). The quantity of vanillic acid in leaves of corn cultivated in heavy metal polluted pots changed according to heavy metal types and doses. The treatment of Cd and Pb significantly increased the content of vanillic acid at low doses (10 and 20 ppm). However, the application of them at high doses (50 ppm) decreased the content of vanillic acid. Also, Cu only increased the level of vanillic acid at 20 ppm doses (p < 0.05). The results of caffeic acid, ferulic acid, and vanillic acid mentioned above are shown in Fig. 3.
The correlation values among the phenolic compounds
The correlation analysis among the phenolic compounds of corn leaves in the growth medium containing heavy metals was performed with bivariate (Pearson’s) correlation. We show that there is a positive correlation with the total phenolics and chlorogenic acid when the corn is exposed to Pb, especially (p < 0.01). Also, there are negative correlations between total phenolic content and caffeic acid, ferulic acid in the leaves of corn exposed to all heavy metal applications except Cd for ferulic acid. The correlations of all samples are shown in Table 1.
Discussion
Phenolic compounds are commonly found in plants, and they have been noticed to have multiple biological effects as antioxidant activity (Wojdylo et al. 2007). Environmental stresses such as drought, salt, and heavy metal have been demonstrated to increase the ROS production. ROS homeostasis in plant cells is achieved by antioxidant compounds and enzymes. Antioxidant molecules including ascorbic acid, glutathione, and phenolic compounds inhibit the oxidation and have crucial roles in stress responses (Racchi 2013). In higher plants, phenolic compounds are considered as secondary metabolites, and they have been recognized as serving roles in the responses to environmental stimulus. They participate in several physiological processes associated with plant growth and development (Tattini et al. 2004; Garica-Sanchez et al. 2012).
In the present study, the application of heavy metals has globally increased the total phenolic compounds in the leaves of corn compared to control groups. It was previously shown that Cd increased the total phenolics in the Lepidium sativum applied CdCl2 at low doses (0.5 mg L−1) but it decreased at high doses. The application of Se (selenium) increased the total phenolic of cress (Elguera et al. 2013). The total concentration of phenols significantly increased under the treatment with 2 mM of boron in the leaves of tomato (Cervilla et al. 2012). Marguez-Garcia et al. (2012) reported that the treatment of CdSO4 did not generally show a significant change with regard to total phenolic in the leaves of Erica andevalensis. Also, it was stated that water deficit increased the total phenolic of olive cultivars (Olea europaea) in the long term (Petridis et al. 2012). Our results show that the total phenolic compounds of corn significantly increased compared to controls (p < 0.05), but these increases have changed heavy metal types and their concentration.
The possible indication of the presence of abiotic stress in the plant is the alteration of the composition of phenolic compounds (Cervilla et al. 2012). In this study, we investigated some individual phenolic compounds; it can be observed that phenolic compound levels changed according to the variety of compounds. The chlorogenic acid level significantly increased in the leaves of corn exposed to each heavy metal applications. The content of rutin slightly increased in the application of heavy metal excluding the high doses of copper in the leaves (p < 0.05). Chlorogenic acid is an important antioxidative molecule and its accumulation was induced by the heavy metals and this may indicate, as naturally expected, that accumulation of phenolic compounds such as chlorogenic acid and rutin is related to the amount of heavy metal in the plant organs (Kovacık et al. 2009a). However, the content of caffeic and ferulic acid significantly decreased with the application of heavy metals, but the level of vanillic acid changed to heavy metal types and doses in the leaves of corn. It is thought that the decrease in phenolic metabolites may result from a decline in the activity of key enzymes related to the biosynthesis of these phenolic compounds (Chung et al. 2006).
Phenolic acids as secondary metabolites have several functions; they play an important role in the adaptation of plants to the environment and in overcoming stress conditions. Elguera et al. (2013) indicated that CdCI2 decreased the content of free phenolics such as chlorogenic acid, ferulic acid, caffeic acid, and vanillic acid in the leaves of L. sativum, while the contents of chlorogenic acid and caffeic acid increased in the leaves exposed to sodium selenite. The application of NiCI2 increased the total phenolic content, chlorogenic acid, and caffeic acid while it decreased ferulic acid, and the level of vanillic acid changed depending on the applied doses in the leaf rosettes of Matricaria chamomilla (Kovacık et al. 2009b). The treatment of CdSO4 increased the level of rutin in the leaves of E. andevalensis (Marguez-Garcia et al. 2012). The content of chlorogenic acid slightly increased with increasing CuCI2 concentration; however, the level of vanillic acid remained constant in the leaf rosette of M. chamomilla (Kovacık et al. 2008). Salt stress stimulated the increase of ferulic acid and vanillic acid, and it decreased the chlorogenic acid and caffeic acid in the leaf rosettes of M. chamomilla. Changes in the phenolic acids should be affected by the amount of metal accumulated in the plant tissue. Enhanced accumulation of phenolic compounds is a universal response to abiotic stress such as heavy metals. These changes may be related to the synthesis of barriers and phenols against stressors (Kovacık et al. 2009c). The contents of various secondary plant metabolites are strongly dependent on the cultivating conditions and have impact on the metabolic pathways responsible for the accumulation of the related natural products (Ramakrishna and Ravishankar 2011).
In the present study, we show that the application of heavy metal generally increased the total phenolics of the leaves of the corn. This increase can be a result of the rises of chlorogenic acid and rutin which already the main phenolics in point of quantitatively, and they may affect the changes in the total phenolic content. However, the contents of caffeic acid, ferulic acid, and vanillic acid decreased with the treatment of heavy metals. They may be responsible for the small changes in the content of total phenolics in our results. We demonstrated some phenolic compounds of corn, and the present study can provide further experimental evidence on the impact of heavy metal stress, but additional experiments can be performed in the variation of phenolic compounds for clarification, in future.
References
Balasundram N, Sundram K, Samman S (2006) Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem 99:191–203
Bubna GA, Lima RB, Zanardo DYL, Santos WD, Ferrarese MLL, Ferrarese-Filho O (2011) Exogenous caffeic acid inhibits the growth and enhances the lignification of the roots of soybean (Glycine max). J Plant Physiol 168:1627–1633
Cai Y, Lua Q, Sun M, Corke H (2004) Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci 74:2157–2184
Calixto-Campos C, Carvalho TT, Hohmann MSN, Pinho-Ribeiro FA, Fattori V, Manchope MF, Zarpelon AC, Baracat MM, Georgetti SR, Casagrande R, Verri WA (2015) Vanillic acid inhibits inflammatory pain by inhibiting neutrophil recruitment, oxidative stress, cytokine production, and NFκB activation in mice. J Nat Prod 78:1799–1808
Cervilla LM, Blasco B, Rios JJ, Rosales MA, Sanchez-Rodriguez E, Rubio-Wilhelmi MM, Romero L, Ruiz JM (2012) Parameters symptomatic for boron toxicity in leaves of tomato plants. J Bot. doi:10.1155/2012/726206
Cheynier V (2012) Phenolic compounds: from plants to foods. Phytochem Rev 11:153–177
Chung IM, Kim JJ, Lim JD, Yu CY, Kim SH, Hahn SJ (2006) Comparison of resveratrol, SOD activity, phenolic compounds and free amino acids in Rehmannia glutinosa under temperature and water stress. Environ Exp Bot 56:44–53
Cseke LJ, Kirakosyan A, Kaufman PB, Warber SL, Duke JA, Brielmann HL (2006) Natural products from plants. Taylor and Francis, New York
Demirezen D, Aksoy A (2006) Heavy metal levels in vegetables in Turkey are within safe limits for Cu, Zn, Ni and exceeded for Cd and Pb. J Food Chem 29:252–265
Elguera JCT, Barrientos EY, Wrobel K, Wrobel K (2013) Effect of cadmium (Cd(II)), selenium (Se(IV)) and their mixtures on phenolic compounds and antioxidant capacity in Lepidium sativum. Acta Physiol Plant 35:431–441
Erenler R, Telci I, Ulutas M, Demirtas I, Gul F, Elmastas M, Kayır O (2015) Chemical constituents, quantıtatıve analysıs and antioxidant activities of echinacea purpurea (L.) moench and Echinacea pallıda (Nutt.) Nutt. J Food Biochem 39:622–630
Garcia-Sanchez M, Garrido I, Casimiro IJ, Casero PJ, Espinosa F, Garcia-Romera I, Aranda E (2012) Defence response of tomato seedlings to oxidative stress induced by phenolic compounds from dry olive mill residue. Chemosphere 89:708–716
Gonçalves JF, Becker AG, Cargnelutti D, Tabaldi L, Pereira LB, Battisti V, Spanevello RM, Morsch VM, Nicoloso FT, Schetinger MRC (2007) Cadmium toxicity causes oxidative stress and induces response of the antioxidant system in cucumber seedlings. Braz J Plant Physiol 19(3):223–232
Gratao PL, Polle A, Lea PL, Azevedo RA (2005) Making the life of heavy metal-stressed plants a little easier. Funct Plant Biol 32:481–494
Hassan M, Mansoor S (2014) Oxidative stress and defense mechanism in mung bean seedlings after lead and cadmium treatments. Turk J Agric For 38:55–61
Kovacik J, Gruz J, Backor M, Tomko J, Strnad M, Repcak M (2008) Phenolic compounds composition and physiological attributes of Matricaria chamomilla grown in copper excess. Environ Exp Bot 62:145–152
Kovacik J, Klejdus B, Hedbayny J, Stork F, Backor M (2009) Comparison of cadmium and copper effect on phenolic metabolism, mineral nutrients and stress-related parameters in Matricaria chamomilla plants. Plant Soil 320:231–242
Kovacık J, Klejdus B, Backor M (2009a) Phenolic metabolism of Matricaria chamomilla plants exposed to nickel. J Plant Physiol 166:1460–1464
Kovacık J, Klejdus B, Hedbavny J, Backor M (2009b) Salicylic acid alleviates NaCl-induced changes in the metabolism of Matricaria chamomilla plants. Ecotoxicology 18:544–554
Kumar N, Pruthi V (2014) Potential applications of ferulic acid from natural sources. Biotechnol Rep 4:86–93
Lequeux H, Hermans C, Lutss S, Verbruggen N (2010) Response to copper excess in Arabidopsis thaliana: impact on the root system architecture, hormone distribution, lignin accumulation and mineral profile. Plant Physiol Biochem 48:673–682
Marguez-Garcia B, Fernandez-Angeles M, Cordoba F (2009) Phenolics composition in Erica sp. differentially exposed to metal pollution in the Iberian Southwestern Pyritic Belt. Bioresour Technol 100(1):446–451
Marguez-Garcia B, Fernandez-Recamales MA, Cordoba F (2012) Effects of cadmium on phenolic composition and antioxidant activities of Erica andevalensis. J Bot. doi:10.1155/2012/936950
Michalak A (2006) Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol J Environ Stud 15:523–530
Musallam I, Duwayri M, Shibli R, Alali F (2012) Investigation of rutin content indifferent plant parts of wild cape (Capparis spinosa L.) populations from Jordan. Res J Med Plant 6(1):27–36
Nazar R, Iqbal N, Masood A, Khan MIR, Syeed S, Khan NA (2012) Cadmium toxicity in plants and role of mineral nutrients in its alleviation. Am J Plant Sci 3:1476–1489
Ncube EN, Mhlongo M, Piater LA, Steenkamp PA, Dubery IA, Madala NE (2014) Analyses of chlorogenic acids and related cinnamic acid derivatives from Nicotiana tabacum tissues with the aid of UPLC-QTOF-MS/MS based on the in-source collision-induced dissociation method. Chem Cent J 8:66
Petridis A, Therios I, Samouris G, Koundoursa S (2012) Effect of water deficit on leaf phenolic composition, gas exchange, oxidative damage and antioxidant activity of four Greek olive (Olea europaea L.) cultivars. Plant Physiol Biochem 60:1–11
Racchi ML (2013) Antioxidant defenses in plants with attention to Prunus and Citrus spp. Antioxidants 2:340–369
Ramakrishna AR, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 6(11):1720–1731
Ramos-Escudero F, Munoz AM, Alvarado-Ortiz C, Alvarado A, Yanez JA (2012) Purple corn (Zea mays L.) phenolic compounds profile and its assessment as an agent against oxidative stress in isolated mouse organs. J Med Food 15:206–215
Rastgoo L, Alemzadeh A (2011) Biochemical responses of Gouan (Aeluropus littoralis) to heavy metals stress. Aust J Crop Sci 5(4):375–383
Rauha JP, Remes S, Heinonen M, Hopia A, Kahkönen M, Kujala T, Pihlaja K, Vuorela H, Vuorela P (2000) Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int J Food Microbiol 56:3–22
Rellan-Alvarez R, Ortega-Villasante C, Alvarez-Fernandez A (2006) Stress responses of Zea mays to cadmium and mercury. Plant Soil 279:41–50
Schützendübel A, Polle A (2002) Plant responses to abiotic stress: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53:1351–1365
Sharma P, Dubey RS (2004) Lead toxicity in plants. Braz Plant Physiol 17:35–52
Singleton VL, Slinkard K (1977) Total phenol analysis: automation and comparison with manual methods. Am J Enol Vitic 28:49–55
Tattini M, Galardi C, Pinelli P, Massai R, Remorini D, Agati G (2004) Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol 163:547–561
Tomas-Barberan FA, Espin JC (2001) Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J Sci Food Agric 81:853–876
Verma S, Dubey RS (2003) Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci 164:645–655
Vermerris W, Nicholson R (2006) Phenolic compound biochemistry. Springer, Dordrecht
Wojdylo A, Oszmianski J, Czemerys R (2007) Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem 105:940–949
Yadav SK (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot 76:167–179
Zheng W, Wang SY (2001) Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem 49:5165–5170
Acknowledgments
The authors are most grateful to the Plant Research Laboratory (BALAB) of Chemistry Department at the Gaziosmanpasa University, which provided us with the HPLC unit and had been accommodating us during our experimentation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kısa, D., Elmastaş, M., Öztürk, L. et al. Responses of the phenolic compounds of Zea mays under heavy metal stress. Appl Biol Chem 59, 813–820 (2016). https://doi.org/10.1007/s13765-016-0229-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13765-016-0229-9