Abstract
Heavy metals such as Fe, Mn, Cu, Ni, Co, Cd, Zn, Hg and As have long been gathering in soils as a result of variety of anthropogenic activities particularly after the setup of industrial sector. Plants exposed to high levels of these heavy metals undergo number of changes in their physiology and metabolism. The most typical and visible manifestation of heavy metal toxicity includes reduced plant growth, leaf chlorosis, leaf necrosis, turgor loss, instant drop in seed germination rate and a defunct photosynthetic apparatus, which is frequently associated with progressive senescence processes or ensuing plant death. In plants, heavy metal stress also increases the generation of free radicals and many other detrimental species. To defend themselves against heavy metal stress, plants employ a various avoidant or tolerant mechanisms. One such mechanism that provides plants with a defensive strategy to deal with severe heavy metal toxicity is the synthesis of phenolics, which are secondary natural metabolites originating biogenetically from either the shikimate/phenylpropanoid pathway or acetate/malonate pathway. The present review lays focus on the structure, synthesis, accumulation and role of phenolics in ameliorating the detrimental effects of heavy metals in plants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
From roughly 300,000 recorded species of higher plant species, around 200,000 chemical entities have been extracted and identified (Fiehn 2002). Metabolites such as sugars, fatty acids, amino acids and nucleic acids that are required by all plants for growth and development are known as primary metabolites (Wu and Chappell 2008). Plants, apart from these primary metabolites, produce structurally and chemically countless and varied array of compounds known as secondary metabolites. These compounds occur in specialized and distinctive cells and are not vital for photosynthesis and respiration, but are believed to play role in plant persistence in specific environment (Boudet 2007; Harborne and Green 1980). There are vast numbers of secondary metabolites in plants which have the potential of responding to biotic and abiotic stresses. The pathways responsible for their synthesis have frequently been recruited from vital primary metabolism pathways following initial gene duplication, which resulted in the new functions and diversified roles of these duplicated genes, and have become fundamental part of the plant developmental programme. The onset of developmental stage is often accompanied by accumulation of secondary metabolites. This accumulation pattern of secondary metabolites is strictly controlled by gene expression both spatially and temporarily. The transportation of the metabolic intermediates further provides an extra level of regulation. Ontogeny and circadian clock-controlled gene expression also play vital role in synthesizing these metabolites. Phenolics are one of the most prevalent categories of secondary metabolites generated by plant kingdom members (Boudet 2007; Harborne and Green 1980). Phenolic compounds have piqued the interest of scientists due to their biological activity, which has been shown in in vitro and in vivo studies to be advantageous to human health (Vazquez-Olivo et al. 2020). When it comes to plant phenolics, the term “phenol” is used for the compound that has one or more hydroxyl substituents attached to the phenyl ring. The term “polyphenol” would accordingly be defined as any natural product that contains minimum of two phenyl rings with one or more hydroxyl groups, including their functional derivatives (e.g. esters and glycosides). However, this definition would include several compounds which have terpenoid origin, e.g. gossypol, oestrone, etc., thus making the definition unsatisfactory (Harborne 1989). According to Quideau et al. (2011), phenolics should only be used for secondary metabolites that are generated biogenetically by the shikimic acid/phenylpropanoid pathway, which directly makes available phenylpropanoids (Fig. 15.1), or the acetate/malonate pathway, which is also referred to as the polyketide pathway and can yield simple phenols, or both of the pathways. Both of these routes synthesize a huge collection of monomeric as well as polymeric phenol structures that play a variety of roles in plants, ranging from structural components of cell walls to involvement in plant development and survival under diverse abiotic and biotic stress situations. The term polyphenols is used with regard to structure containing more than one phenolic ring. Therefore, the plant phenolics are an extremely diverse assemblage which already contains tens of thousands of members with varied structures, and the number is continuously increasing (Quideau et al. 2011).
The biosynthetic pathway of phenolic compounds. A diagram depicting the biosynthesis of phenolic compounds in plants via the pentose phosphate, shikimate and phenylpropanoid pathways. (Source: Redrawn from Lattanzio, V., 2013. Phenolic compounds: introduction. In: Ramawat, K.G., Mérillon, J.M. (Eds.), Natural Products, Springer-Verlag: Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-22144-6_57 and Lin et al. (2016))
Higher plants are known for synthesizing tens of thousands of various phenolic compounds. In their leaves, vascular plants contain amides, esters and glycosides of hydroxycinnamic acids; glycosylated flavonoids, particularly flavonols; and proanthocyanidins and their relatives. Chlorogenic acid is one of the few examples of soluble phenolics which is widely distributed across plant families. However, the presence of other phenolics is limited to particular genera or families, thereby making them important biomarker for taxonomic analysis. The examples of phenolic-containing polymers include suberin, lignin and sporopollenin of pollen grains (Boudet 2007). Phenolics are not so common in bacteria, fungi and algae. However, bryophytes regularly synthesize vast array of phenolic compounds that include polyphenols like flavonoids (flavones and flavonols) and proanthocyanidins and their relatives (Swain 1975). It is widely believed that land-adapted plants appeared in the mid-Palaeozoic era, i.e. between about 480 and 360 million years ago. These plants consist of embryophytes (liverworts, hornworts, mosses) and tracheophytes which have arisen from charophycean algae. To cope with the incessantly changing environmental challenges over the evolutionary time, plants synthesized phenolic compounds to address the specific needs. Thus phenolic-producing plants were selected throughout the process of evolution in diverse plant lineages. It provided “phenolic UV light screens” to higher members of Charophyceae which first successfully adapted to the land after emerging from the aquatic environment (Boudet 2007). Apart from this, phenylpropanoid pathway that produces lignin comprises common set of metabolic processes in higher plants, which also showed its presence 400 million years ago with the appearance of stiff vascular land plants. These secondary metabolites have evolved over time to provide the specific adaptations to plants which became one of the reasons for their diversity that we observe today (Boudet 2007). Plant’s highly structured interactions with their biotic and abiotic surroundings have been a primary driving force behind the formation of these specialized natural metabolites. In this regard, phenolic buildup in plant tissues is seen as a frequent adaptive response of plants to adversarial environmental settings, hence boosting evolutionary fitness. The plant phenolics are thought to play an important defensive role in plants when environmental challenges like strong light, freezing temperatures, pathogen and herbivore attacks, nutritional shortage and heavy metal stress cause a surge in the formation of free radicals and many other oxidative species. Plants respond to biotic and abiotic stresses by increasing their capacity to reduce reactive oxygen species, according to emerging studies. Secondary metabolism gene expression is frequently stimulated by biotic and abiotic stress by the incorporation of signalling chemicals such as salicylic acid, jasmonic acid and its derivatives (Winkel-Shirley 2002).
15.2 Structure and Classification of Phenolics
Plant phenolics consist of several thousand chemically known members whose structures range from monomeric to dimeric and polymeric phenolics. Harborne and Simmonds (1964) categorized them according to the number of carbons in the molecule as given in Table 15.1.
The phenolics with lower molecular weight are ubiquitous in higher plants. Certain phenolics among them are restricted to some species only, while others are common in higher plants. Proanthocyanidins, which are also referred to as condensed tannins, are the higher molecular weight polyphenols commonly present in woody plants but generally lacking in nonwoody plants. The hydrolysable tannins are found in only 15 of the 40 orders of dicotyledons, making them less common than proanthocyanidins (Ishimaru et al. 1987; Quideau 2009). According to Palanisamy et al. (2020), tannins attach to proteins and other natural substances to precipitate them. The leaves of Vaccinium species contain a simple phenol arbutin (hydroquinone b-D-glucoside, I). It is so uncommon that it has been recommended as a signal for pear juice adulteration of other liquids (Kutchan and Dixon 2005).
Phenolic acids are commonly found in complex structures like lignins and hydrolysable tannins as well as bound with carbohydrates or organic acids (Tomas-Barberan and Clifford 2000). A C6–C2 compound picein (II), occurring in the needles of spruce trees (Picea abies), is also found in many other plant species. Coumarins, C6–C3 derivatives, are benzo-a-pyrones (lactones) produced through the interaction between the o-hydroxy and carboxyl groups and subsequent cyclization. This group is found in several eudicotyledon families either free or in conjunction with sugars heterosides and glycosides (Petersen et al. 1999).
Naphthoquinones, such as plumbagin (VI), are a kind of quinone pigments. There are several higher plant families such as Avicenniaceae, Bignoniaceae, etc. that contain this class of phenolics. They are produced through many pathways such as the polyketide pathway, the shikimate/succinyl-CoA combination pathway and the shikimate/mevalonate pathway (Babula et al. 2009). Xanthones, another class, are present in only a few higher plant families; they have thus high taxonomic importance. Mangiferin (VII) is an unusual natural xanthone in that it has significantly larger natural occurrence than the other members of same class. It was discovered in the leaves of Mangifera indica L. for the first time (Bennett and Lee 1989).
Stilbene family is extensively prevalent across the plant kingdom, while certain members of this group are peculiar to specific plant families only. Similarly, different glycosylated and acyl flavonoids and their bound forms comprise a wide range of natural compounds, with over 10,000 distinct structures having been discovered (Veitch and Grayer 2008). They are present in a variety of plant tissues and have chemical structures that are based on a C6–C3–C6 skeleton. The two main families of complicated C6–C3–C6 plant phenolics include bi- and triflavonoids, as well as proanthocyanidins. Because these compounds are the result of oxidative coupling of diverse flavonoid structures, they all have a carbonyl group at C–4 or its equivalent in every component unit.
Lignans and neolignans are plant phenolics generated by the oxidative dimerization of two phenylpropanoid units found in a broad array of plant species. Lignans are phenylpropanoid dimers with C3 side chains that are primarily C–C connected via their C3 side chains (tail-to-tail such as pinoresinol). Eusiderin and other neolignans are phenylpropanoid dimers linked head-to-tail (Moss 2000). Lignin, the key structural polymer of xylem and the second most plentiful organic material in plants after cellulose, is present as an important component of all vascular plant’s cell walls. Lignin has a well-known protective effect against phytopathogenic fungi. It possesses antifungal properties, prevents fungal attack by physically stopping their entry and inhibits the spread of their poison (Rashad et al. 2020). The coniferyl alcohol and small quantity of p-coumaryl alcohol are the primary sources of lignins in gymnosperms, whereas coniferyl and sinapyl alcohols are found in about equal amounts in angiosperm lignins.
Plant tannins are high molecular weight phenolic compounds that may form complexes with carbohydrates and proteins. Tannins found in higher plants are metabolites of two types: hydrolysable tannins and proanthocyanidins, which are commonly called as condensed tannins. Proanthocyanidins occur in vast amounts in woody plants but are uncommon in nonwoody plants and usually confined to some specific tissues such as seed coat of Arabidopsis thaliana L. or alfalfa. Only 15 of the 40 orders of eudicots contain hydrolysable tannins (Ishimaru et al. 1987), making them less common than proanthocyanidins. A third family of tannins, phlorotannins, was recently discovered in a variety of algal species. The phlorotannins are composed of phloroglucinol subunits connected together either by C–C bonds or C–O–C (aryl-ether) bonds. Acids, bases and, in certain situations, hydrolytic enzymes break down hydrolysable tannins into sugars (often D-glucose) or similar polyols and phenolic acid (tannase). In the case of gallotannins, which are polygalloyl esters, this is gallic acid. Ellagitannins are hexahydroxydiphenoyl carbohydrate esters or cyclitols in a restricted sense, but compounds originating from subsequent oxidative reactions are also included in a broader sense. When the hexahydroxydiphenoyl group is broken down, the parent acid lactonizes quickly to produce the dilactone ellagic acid (IX). Under highly acidic circumstances, proanthocyanidins which are flavan-3-ol oligomers and polymers undergo cleavage of C–C interflavanic linkages to produce anthocyanidins (or C–C and C–O–C in A-type proanthocyanidins). Proanthocyanidins can form polymers with up to 50 units (Quideau 2009). Ishimaru et al. in 1987 reported that flavano-ellagitannins are found in some plants; however, they are far less prevalent (e.g. acutissimin A and B (X–XI), which were initially identified from Quercus acutissima Carruthers). Lastly, melanins are high molecular weight dark brown- or black-coloured pigments that are generated from phenolic compounds by the oxidative polymerization. Generally, they are ortho-dihydroxyphenol conjugated polymers (Bell and Wheeler 1986).
15.3 Accumulation of Phenolics in Plants
The buildup of phenolics occurs when plants face the challenges of biotic or abiotic stress such as that of low temperatures, pathogen infection, heavy metals, etc. Several articles have looked at the impact of nonfreezing temperature stress on phenolic metabolism. These researches have revealed that cold stress increases phenolic metabolism and affects its behaviour. However, there is a certain threshold temperature below which a rise in phenolic metabolism occurs, and this temperature is critical at which chilling injury may occur. It was found that cold-induced stress increases the level of phenylalanine ammonia-lyase activity (PAL, EC 4.3.1.5) and other enzymes involved in the phenolic biosynthesis pathway. These low-temperature responses in phenolic metabolism (increased enzyme activity and phenolic compound levels) also affect the shelf life of stored fruit and vegetables by providing an adequate substrate for browning reactions (Lattanzio et al. 1994, 2001). Plant development is dependent on the availability of recycled nutrients, whereas the external nutrient provides only a modest percentage of the overall need. Climate, substrate (litter) quality and decomposer species all have an influence on nutrient mineralization carried out by soil microbes. Polyphenols have been found as soil process regulators, with the potential to affect the total amount and form of nutrients accessible to plants and/or microbes. Polyphenols go into the soil mostly as leachates from above- and below-ground plant parts and/or above- and below-ground plant litter. Phenolic compounds, for example, can have a direct influence on the activity and composition of decomposers, influencing rate of decomposition and nutrient cycling. Furthermore, plants rely on their root’s capacity to interact with bacteria. The opposite is also correct; numerous bacteria and fungi rely on relationships with plants, which are frequently controlled by root exudates. Root exudate is teeming with isoflavonoids and flavonoids, triggering certain genes responsible for nodulation and that may be involved in vesicular-arbuscular mycorrhiza colonization. The roots of the host plant initiate nodule formation by releasing flavonoids into the rhizosphere. Because each rhizobia species reacts differently to these compounds, the uniqueness of the symbiotic association is hence defined by the exudate. Flavonoids attract bacteria and activate rhizobia Nod (nodulation) gene expression, resulting in the formation and release of strain-specific Nod factors (NF). The oligosaccharide backbone of NF is composed of N-acetyl-D-glucosamine units connected to a nonreducing sugar by a fatty acyl group. The various NF substituents linked to the oligosaccharide backbone are regarded to be a key factor of host-symbiotic specialization. In most situations, the occurrence of suitable NF is adequate to initiate nodule development (Mathesius 2008). Anthocyanins are kind of flavonoids that provide many flowers and fruits their red and blue/purple hues. These chemical substances act as visual signals to entice pollinators and various animals for seed dispersal. They are housed in vacuole of specialized cells. Anthocyanins are also responsible for the stunning exhibitions of varied red to reddish-orange hue in deciduous tree leaves. Leaf colour dynamics are not just a byproduct of leaf senescence; various concepts have evolved in the last decade to elucidate the evolutionary process of autumn colours which are primarily caused by carotenoids (yellow-orange pigments) and anthocyanins (red-purple). Carotenoids exist in the leaves all year, but they are concealed by the green of chlorophyll in mature leaves; in the fall, they become visible owing to the disappearance of chlorophyll. Anthocyanins, on the other hand, are created in abundance during the autumn season just before the leaves are shed. Hence, red is produced throughout fall and isn’t merely a byproduct of leaf withering. Why do leaves that are ready to fall become red? The usefulness of the fall hues is still debated. According to photoprotection hypothesis, red may shield the foliage from the detrimental effects of low temperature, permitting for more effective nutrient resorption, particularly nitrogen. According to coevolution theory, red does serve as a threatening signal to animals, mainly to feeding insects such as aphid. It has also been said that cold, dry and bright sunlight stresses are alleviated by red pigments by the process referred to as light attenuation that lowers the absorption of green sunlight. Recent research on fall and juvenile leaves, on the other hand, suggests that the red pigments prevent leaf injury by making them less appetizing or apparent to animals deficient of red visual receptor, or by indicating poor leaf quality. In their natural habitat, plants are subjected to a plethora of pests and diseases. In response to such creature attacks, the plant may develop tolerance or resistance mechanisms which allow it to thrive. Plants generate diverse phenolic metabolites that perform both functions, i.e. repelling and attracting various organisms in the plant’s surroundings. Fraenkel in 1959 identified phenolics as “trigger” molecules that boost or restrict nutrient intake by animal herbivores; plant phenolics have been recognized as significant in chemoecology, particularly in herbivore eating behaviour (Heil 2008). They operate as repellents, inhibitors, natural animal toxicants and insecticides against attacking species (Cornell and Hawkins 2003; Bhattacharya et al. 2010). Preformed antibiotic compounds found in healthy plants are thought to act as intrinsic chemical barriers against herbivorous and fungal adversaries, protecting plants from a wide spectrum of pests and diseases. In contrast, stimulated defensive chemicals are synthesized as plant’s reaction to biotic stress both at the location of attack and far away from this site. The latter is signalled by salicylates and jasmonates which soon upon infection are synthesized and prevent further spread and subsequent attacks. A network of interrelated signal transduction pathways governs induced resistance, with phenolic acids functioning as critical signalling molecules (Smith et al. 2009; Runyon et al. 2010). Plant survival in an environment rich in potentially dangerous microorganisms is dependent on effective microbe sensing and quick defensive responses. Plant immunity is dependent on each cell’s capacity to identify pathogens. An initial stage of microbe detection is carried out by membrane proteins known as pattern recognition receptors (PRRs) that detect the chemical fingerprints of a broad class of bacteria known as pathogen-associated molecular patterns (PAMPs). As soon as plant PRRs identify prospective pathogens via conserved PAMPs, phenolics are produced, resulting in PAMP-triggered immunity (Nicaise et al. 2009; Zipfel 2008). When a plant recognizes a pathogen, it activates its endogenous multicomponent defensive mechanism. Because a large number of defence-related genes must be activated for plants to respond to pathogen assault, the multicomponent defence response triggered following pathogen infection involves a major investment of cellular resources, including extensive genetic reprogramming. Many defence-related genes form metabolites referred to as phytoalexins, which have antimicrobial property and are synthesized as a result of the interaction between the host’s and a fungal parasite’s metabolic systems. Phytoalexins formed in the different species of family Leguminosae are isoflavonoids, with pterocarpans such as medicarpin and glyceollin II (XXIII) being the most common isoflavonoid subclass, while phytoalexins from Vitaceae appear to form a rather restricted group of molecules that belong to the stilbene family (3,5,40-trihydroxystilbene). The bulk of plants from which such chemicals have been found are dicotyledonous (Lattanzio et al. 2006; Vermerris and Nicholson 2006). Plants and insects have a complicated ecological connection that includes both physical and chemical interactions. Plant variables, insect factors and certain insect-plant factors, such as hypersensitivity response and plant resistance to insect-borne illnesses, all have an impact on this interaction. Secondary metabolites in sufficient quantity to produce an unpleasant physiological impact are plant elements that make a host unappealing. Plant phenolics are now widely recognized to carry out protection of plants against insects. Tannins may also have an impact on insect development in three different ways: they possess an astringent taste that reduces palatability and in turn consumption; they mix with proteins to create complexes with low digestibility; and they function as enzyme inactivators. Recent research on tannin oxidation in insects by Raymond Barbehenn and coworkers shows that tannin activity cannot be described so easily, since tannin oxidation should also be considered as a plant defence mechanism (Constabel and Barbehenn 2008; Barbehenn et al. 2010).
15.4 Effects of Heavy Metals on Plants
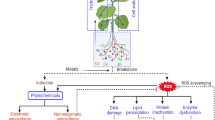
Heavy metals are defined as elements with specific weights more than or equal to 5 g/cm3. Several of them (Co, Fe, Mn, Mo, Ni, Zn, Cu) are key micronutrients involved in redox reactions, electron transfers and other critical metabolic functions. Non-essential metals (Pb, Cd, Cr, Hg and so on) may be toxic to plants. Human activities such as industrial waste, fertilizer application, smelting and disposal of sewage have been instrumental for accumulation of Fe, Mn, Cu, Ni, Co, Cd, Zn, Hg and arsenic in soils due to the human activities (Wagay et al. 2020; Aydinalp and Marinova 2009). As a result of these operations, metals are leached into groundwater or collected on the soil surface (Aydinalp and Marinova 2009; Gupta et al. 2012; Hakeem et al. 2015; Basheer 2018). All heavy metals are non-biodegradable, which means they can’t be removed from the environment naturally. Some are immobile, meaning they cannot be moved from where they have accumulated, while others are mobile, meaning they are often absorbed by plant roots through diffusion, endocytosis or metal transporters (Ali and Jain 2004). However, several of these metals, such as zinc, copper and nickel, are important micronutrients that must be consumed in small amounts as cofactors for specific enzymes. Plants growing in an environment containing hazardous quantities of heavy metals undergo a variety of changes in their physiology and metabolism (Dubey and Naik 2011; Villiers et al. 2011). However, because different heavy metals contain different locations of action inside the plant, the overall toxic effect varies. Their most commonly found visual effect is decreased growth in plants preceded by leaf chlorosis, necrosis, turgor loss, reduction in seed germination and a damaged photosynthetic apparatus and is frequently associated with ongoing senescence processes or eventual plant death (Sharma and Dubey 2007). All of these impacts are connected to heavy metal-induced ultrastructural, biochemical and molecular alterations in plant tissues and cells. Because of the evil ecological consequences, heavy metal pollution of agricultural soil has emerged as a major environmental problem. Such hazardous substances are classified as soil pollutants due to their widespread prevalence and significant negative effects on plants grown in such soils (Gamalero et al. 2009).
15.4.1 Zinc Effects on Plants
Zinc (Zn) is a heavy metal that can remain intact in soil for long periods and can alter several metabolic processes in plants. Zn and Cd phytotoxicity has been investigated in a range of plant species, including Phaseolus vulgaris, Brassica juncea and tobacco, such as a decrease in growth and development and metabolism and oxidative damage (Cakmak and Marschner 1993; Tkalec et al. 2014). Cd and Zn both have been shown influencing enzyme’s catalytic performance in Phaseolus vulgaris and pea plants (Van Assche et al. 1988). Zn concentrations in polluted soils typically exceed nutritional requirements, which can lead to phytotoxicity. Warne et al. (2008) demonstrated that Zn concentrations in polluted soils are between 150 and 300 mg/kg. High Zn levels in soil impede several plant metabolic systems, delaying development and causing senescence. Zinc toxicity in plants stopped root and shoot development (Malik et al. 2011). Zinc toxicity also produces chlorosis in the newly formed leaves, which can spread to the older leaves following continuous exposure to high Zn levels in the soil (Ebbs and Kochian 1997). Because hydrated Zn2+ and Fe2+ ions have comparable radii, chlorosis may be caused in part by an induced iron (Fe) deficit (Marschner et al. 1986). Increased amounts of Zn can also cause deficits in manganese (Mn) and copper (Cu) in above-ground plant parts. These deficits have been linked to a slowed transport of these micronutrients from below- to above-ground plant parts. This impediment stems from the fact that the concentrations of Fe and Mn in plants growing on Zn-rich medium are higher in the root than in the shoot. Another common symptom of Zn poisoning is the formation of a purplish-red hue in leaves, which is caused by a lack of phosphorus (P) (Ebbs and Kochian 1997).
15.4.2 Cadmium Effects on Plants
According to Salt et al. (1995), cadmium (Cd) in agricultural soil has a regulatory limit of 100 mg/kg soil. Plants growing in soil with elevated levels of Cd exhibit evident damage signs such as chlorosis, growth inhibition, root tip browning and eventually death (Mohanpuria et al. 2007; Guo et al. 2008). Cd-induced inhibition of root Fe(III) reductase resulted in Fe(II) shortage, which severely hampered photosynthesis (Alcantara et al. 1994). It has been demonstrated that Cd interferes with the absorption, relocation and use of numerous elements (such as Ca, Mg, P, K) and transportation of water by plants in general (Das et al. 1997). Hernandez et al. (1996) showed that Cd also inhibited nitrate absorption and its transportation from below- to above-ground parts of plants by decreasing nitrate reductase activity in the plant shoots. Plants of Silene cucubalus also showed significant suppression of nitrate reductase activity (Mathys 1975). Balestrasse et al. 2003 showed that nitrogen fixation and primary ammonia assimilation in soybean plant nodules reduced under Cd treatments. Metal poisoning can reduce plasma membrane permeability, resulting in decrease in water content. Furthermore, Cd has been shown to interfere with the water balance as was studied by Costa and Morel (1994). Cadmium treatments have been found to impair the activity of ATPase in wheat and sunflower root plasma membrane fractions (Fodor et al. 1995). Cadmium alters membrane functioning by causing lipid peroxidation and disrupts chloroplast metabolism by limiting chlorophyll production and decreasing the activity of enzymes involved in CO2 fixation (Fodor et al. 1995).
15.4.3 Copper Effects on Plants
Copper (Cu) is a micronutrient for plants that aids in CO2 uptake along with ATP generation. Cu is required for the formation of several proteins, such as plastocyanin in the photosynthetic system and cytochrome oxidase in the respiratory electron transport chain (Demirevska-Kepova et al. 2004). However, increased industrial and mining activities have contributed to a surge in Cu concentration in ecosystems. Additionally, Cu gets integrated into soils due to several human activities such as mining and smelting of Cu-containing ores. Mining activities produce a significant amount of waste rocks and tailings, which end up remaining on the surface. Cu in excess is cytotoxic, causes stress and kills plants. This slows plant growth and produces chlorosis in the leaves. Plants are harmed by oxidative stress and ROS as a result of excessive Cu exposure (Stadtman and Oliver 1991). Oxidative stress disrupts physiological functions and also causes damage to macromolecules. Copper toxicity impeded the growth of Alyssum montanum (Ouzounidou 1994), cucumber (Moreno-Caselles et al. 2000) and Brassica juncea (Moreno-Caselles et al. 2000). Copper and cadmium together had affected the germination of seeds, length of seedling and the lateral root number in Solanum melongena (Neelima and Reddy 2002).
15.4.4 Mercury Effects on Plants
Hg is an unusual metal since it exists in several forms such as HgS, Hg2+, Hg and methyl-Hg. However, in soil Hg2+ predominates. Hg discharged into soil primarily stays in the solid phase due to adherence with sulphides, clay particles and organic materials. An elevated concentration of Hg2+ poses threat to plant by causing an apparent damage as well as physiological concerns. For instance, Hg2+ attaches to water channel proteins, causing closure of guard cells and hampering the water flow in plants (Zhang and Tyerman 1999). Furthermore, high levels of Hg2+ impede mitochondrial activity and induce oxidative stress by producing ROS. As a result, both lipids of cell membranes and plant cellular processes are disturbed (Cargnelutti et al. 2006).
15.4.5 Chromium Effects on Plants
Plants are poisoned by chromium (Cr) compounds, which have a deleterious influence on their growth and development. Because seed germination is altered by Cr, a seed’s ability to sprout in Cr-containing medium indicates its resistance to this heavy metal (Peralta et al. 2001). Twenty-five per cent decrease was observed in seed sprouting of the weed Echinochloa colona when the medium contained 20 ppm Cr (Rout et al. 2000). In Phaseolus vulgaris germination is up to 48% by hexavalent Cr (500 ppm) levels in soil (Parr and Taylor Jr 1982). Similarly, concentration of 40 ppm Cr(VI) in contaminated medium decreased lucerne seeds’ (Medicago sativa cv. Malone) germination and growth by 23% (Peralta et al. 2001). In another study, Zeid (2001) reported sugarcane bud germination was lowered by 32–57% at 20 and 80 ppm Cr, respectively. Seed germination reduced under Cr stress was because of Cr′s inhibitory effect on amylase function and on the transfer of carbohydrates to the embryo axis. Protease activity rises with Cr treatment that might explain why Cr-treated seeds germinate less well (Zeid 2001). Reduced root development in plants and crops is a well-established adverse effect of heavy metals. In Salix viminalis, Prasad et al. (2001) reported that the order of negative impacts of heavy metals on its new root primordia is Cd, Cr and Pb with Cr having a greater effect on root length than the other heavy metals tested. Cr stress is a significant component influencing photosynthetic CO2 fixation, electron transport, photophosphorylation and enzyme activity (Clijsters and Van Assche 1985). Although the effect of Cr on the process of photosynthesis in higher plants is well established, according to studies it is unclear whether the Cr-induced photosynthesis limitation is due to instability of chloroplast ultrastructure, obstruction of electron transport chain or the action of Cr on activities of Calvin cycle enzymes (Desmet et al. 1975; Van Assche and Clijsters 1983). In peas, Bishnoi et al. (1993) found that Cr(VI) had a stronger influence on the activity of PS I than on PS II in isolated chloroplasts but both were damaged in fully grown plants. In plants, chromium stress causes three kinds of physiological changes as (1) changes in the synthesis of pigments involved in photosynthesis, (2) elevation in the synthesis of metabolites (e.g. glutathione and ascorbic acid) as a direct and immediate response to Cr stress that might harm the plant and (3) metabolic pool changes to channel the synthesis of new metabolites such as phytochelatins and histidine that provide tolerance to Cr stress (Schmfger 2001). Two enzymes such as superoxide dismutase (SOD) and antioxidant catalase are induced as the key metal detoxifying enzymes in plants. It was reported that the activity of these enzymes peaked at lower levels of heavy metal whereas, at higher levels, SOD activity remained unchanged while catalase activity declined (Nematshahi et al. 2012).
15.4.6 Lead Effects on Plants
Lead (Pb) is one of the most common and widely spread toxic heavy metals in soil. It is detrimental to plant structure, growth and physiological activities. Seed germination in Spartina alterniflora and Pinus halepensis has been demonstrated to be reduced by lead. Germination may be inhibited as a result of lead interfering with key enzymes. Lead also hindered plant elongation, in addition to leaf growth in Allium and barley (Grunhage et al. 1985). According to Goldbold and Hutterman (1986), the extent to which root growth is hindered is determined by the medium’s lead content, ionic composition and pH. In Sesamum indicum, root development has been inhibited in a concentration-dependent manner. Many plant species exhibit aberrant morphology when subjected to high levels of lead in the soil. Apart from lignification of cortical parenchyma, lead also induces unequal radial thickening in pea roots, cell walls of endodermis. Lead at concentrations of 100–200 ppm was applied to potted sugar beet plants, causing chlorosis and growth loss (Paivoke 1983; Hewitt 1953). Lead levels as low as 0.005 ppm resulted in a substantial decrease in lettuce and carrot root development. Pb2+ inhibitory effects on growth and biomass production might be attributed to impacts on metabolic plant processes (Sharma and Dubey 2005). A lead-induced oxidation of IAA (indole-3-acetic acid) is the principal reason of cell growth inhibition. Lead has also been shown to impede photosynthesis by lowering carboxylating enzyme activity (Stiborova et al. 1987). Excess Pb also inhibits enzyme performance, causes water imbalance, affects membrane permeability and interferes with mineral feeding (Sharma and Dubey 2005). According to Reddy et al. (2005), Pb hinders enzyme function by interacting with their sulfhydryl groups at the cellular level. High Pb levels also increase ROS generation in plants, promoting oxidative stress.
15.4.7 Arsenic Effects on Plants
Arsenate (As) is a phosphate (P) analogue that interacts with the same kind of import transporters in the plasma membrane of plant roots (Meharg and Macnair 1992). Arsenate tolerance has been observed in a wide range of plant species, for instance, in grasses; tolerance is produced by the inhibition of a high-affinity P/As absorption route. This inhibition decreases As input to an extent where the plant can detoxify it, most likely through constitutive processes (Meharg and Macnair 1992; Meharg 1994). Within plant cells, As is also converted into less toxic As species (Meharg 1994). Arsenate is converted to arsenite, dimethylarsinic acid (DMA) and monomethyl arsenic acid in phytoplankton and macroalgae (MMA). These methylated forms of As are subsequently broken down into organophospholipids and arsenosugars (Phillips 1990).
15.4.8 Cobalt Effects on Plants
The three forms of cobalt (Co) in which it occurs in soil are cobaltite [CoAsS], erythrite [Co3(AsO4)2] and smaltite [CoAs2]. A trace quantity of Co can be absorbed by plants from the soil. Cobalt absorption and dispersion in plants differ by species and are influenced by a variety of factors (Kukier et al. 2004; Bakkaus et al. 2005). The phytotoxic consequences of increased Co levels are less well understood. A recent phytotoxicity study on Co in barley (Hordeum vulgare L.), oilseed rape (Brassica napus L.) and tomato (Lycopersicon esculentum L.) revealed an adverse impact on above-ground plant growth and biomass. Furthermore, high Co concentrations in cauliflower leaves reduced the activities of Fe, chlorophyll, protein and catalase and also impaired the transport of P, S, Mn, Zn and Cu from cauliflower roots to shoots. Compared to excess Cu or Cr, Co significantly diminished both water potential and transpiration rate. Chatterjee and Chatterjee (2000) discovered that exposing cauliflower leaves to excess Co enhanced their diffusive resistance and relative water content.
15.4.9 Nickel Effects on Plants
Nickel (Ni) is classified as a transition metallic element found in natural soils except in ultramafic and serpentinic soils. However, Ni2+ concentrations are rising in certain areas as a result of human activities such as mining, smelter emissions, coal and oil combustion, sewage, phosphate fertilizers and pesticides (Gimeno-García et al. 1996). According to Izosimova (2005), Ni2+ concentrations in contaminated soil can be 20–30 times (200–26,000 mg/kg) higher than in normal soil (10–1000 mg/kg). The elevated levels of Ni2+ in soil induce metabolic changes and toxicity indications such as chlorosis and necrosis in various plant species including rice (Pandey and Sharma 2002; Das et al. 1997). Vegetation growing in elevated Ni2+ soil had impaired nutritional balance, which led in a disruption of cell membrane activities. As a result, Ni2+ influences the plasma membrane’s lipid content and H-ATPase activity in Cucumis sativus shoots. Gonnelli et al. (2001) found that Ni2+-sensitive plants had higher MDA concentrations as compared to Ni2+-tolerant plants. These changes impair membrane function and ion balance in the cytoplasm, particularly for K2+, the most mobile ion crossing the plant cell membranes. Because of the increased Ni2+ absorption, the water content of dicot and monocot plant species has been found to decrease. This decrease in water absorption is used to track the evolution of Ni2+ toxicity in plants (Pandey and Sharma 2002; Gajewska et al. 2006).
15.4.10 Manganese Effects on Plants
Excessive manganese (Mn) accumulation in foliage slows photosynthetic carbon fixation. Mn is easily transported from below-ground to above-ground plant parts by transpiration; but, once it enters the leaves, it is difficult to remobilize to other plant organs via phloem (Loneragan 1988). The most common symptoms of Mn toxicity are necrotic black spots on leaves, petioles and stems (Wu 1994). The spotting first appears on the lower leaves and then advances to the upper leaves of foliage (Horiguchi 1988). The number and size of the speckles may increase with time, causing necrotic lesions, leaf browning and death. Cucumis sativus has shown general leaf bronzing and internode shortening. Apart from chlorosis and browning of tissues, “crinkle leaf” appears in the newest leaf, stem and petiole tissues subjected to Mn toxicity (Wu 1994; Bachman and Miller 1995). Mn-toxic roots are often brown and fragile (Le Bot et al. 1990; Foy et al. 1995). Excess Mn has been shown to limit chlorophyll d production by interfering with a Fe-related process (Clairmont et al. 1986). Mn toxicity in certain species commences with chlorosis of mature leaves and advances to newly formed ones. If the toxicity is mild, then these symptoms develop at the leaf edges and spread to the interveinal regions; but if it is severe, the condition progresses as leaf marginal and interveinal necrosis (Bachman and Miller 1995).
15.4.11 Iron Effects on Plants
Iron, a necessary metal for all plants, is involved in a variety of critical biological processes ranging from photosynthesis to chloroplast development and chlorophyll production. According to Marschner (1995), iron is an important part of many biological redox systems involving haem proteins as well as iron-sulphur proteins. Most soils contain high iron content, but the appearance of iron toxicity symptoms in leaf tissues occurs only when the soil is flooded, owing to the microbial reduction of insoluble Fe3+ to insoluble Fe2+ (Becker and Asch 2005). Therefore, iron poisoning in plants is caused by excessive Fe2+ absorption by roots and transfer to aerial parts via transpiration flow. Excess Fe2+ increases free radical generation, which irreversibly destroys cellular structure and affects membranes, DNA and proteins (De Dorlodot et al. 2005). In tobacco, canola, soybean and Hydrilla verticillata, iron toxicity is associated with decreased photosynthesis and yield, as well as increased oxidative stress and ascorbate peroxidase activity (Sinha et al. 1997).
15.5 Role of Phenolics
15.5.1 Physiological Roles of Phenolics in Plants
Phenolic compounds are widely distributed and perform critical functions in plant metabolism as well as in other biological processes (Boudet 2007). There are several growth- and development-related physiological process such as seed germination, cell division and the production of photosynthetic pigments which are influenced by phenolics (Tanase et al. 2019). There are a variety of applications where phenolics are employed such as bioremediation, allelochemicals, plant growth stimulation and food additives as antioxidants (Bujor et al. 2015). Phenolic accumulation in stressed plants is a persistent feature that serves as a defence mechanism against a variety of abiotic stresses. Phenolics enable plant tolerance and adaptability under adverse environment (Andersen 2003; Lattanzio et al. 2009). This class contains many members with antioxidant properties increasing plant performance in stressful conditions (Oszmanski 1995). Plants communicate with their surroundings owing to several metabolites. Polyphenols, in particular, help in nutrient mobilization and signal transmission from root to shoot. The root secretion contains phenolic compounds that modify the physiochemical characteristics of the rhizosphere. Soil bacteria convert phenolics into compounds that help in the mineralization of nitrogen and the production of humus (Halvorson et al. 2009). Furthermore, Seneviratne and Jayasinghearachchi (2003) concluded that phenolics promote nutrient uptake by increasing active absorption sites and soil porosity by rapidly mobilizing mineral elements. Similarly, Rehman et al. (2018a, b) discovered that the increase in the content of phenolics and organic acids in wheat root secretions which aided in Zn, N and Ca nutrient movement and uptake was the result of Zn application and PGPRs (plant growth-promoting rhizobacteria) inputs. In legumes, phenolic compounds also contribute to the nitrogen fixation. Legumes produce and then excrete a large number of secondary metabolites, primarily flavonoid compounds (flavanols and isoflavonoids) through their roots which inhibit auxin transport and stimulate cell division and are hence important in the synthesis of Nod factors and the formation of infection thread during nodulation (Zhang et al. 2009). Flavonoids are required for the development of functional pollen which has been derived from a study in which a trace amount of flavonol, aglycones, kaempferol or quercetin was added during pollination to flowers, which restored fertility in mature pollen (van der Meer et al. 1992; Taylor and Grotewold 2005). Some phenolic compounds, for example, inhibit the enzymes prolyl aminopeptidase and phosphatase, which are required for germination in bean seeds (Shankar et al. 2009). High phenolic acid content, on the other hand, has been found to have a beneficial effect on seed germination. Chen et al. (2016) in canary grass reported a large increase of 1042%, 120% and 741% in free, bound and total phenolic acid contents, respectively, during germination. In Lycopersicon esculentum, polyphenol-rich spruce bark extract enhanced seed germination while inhibiting root elongation (Balas and Popa 2008). Phenolics were discovered to lower seed tegument thickness while increasing seed tegument porosity that assists in water absorption and therefore enhance germination rate (Tobe et al. 2001). Polyphenolic-rich spruce bark extracts boosted rate of photosynthesis by increasing chlorophyll a and b pigment production in Zea mays and Helianthus annuus (Tanase et al. 2015). Phenols reduced the energy required for ion transport by altering the structure of thylakoids and mitochondrial membranes (Moreland and Novitzky 1987). Phenolic compounds also act as antioxidants by scavenging reactive oxygen species (ROS), limiting the action of oxidizing enzymes and catalysing oxygenation processes via the formation of metallic complexes (Amarowicz and Weidner 2009).
In conclusion, polyphenols are generated in plants under ideal and (at higher levels) stress environment, and they play a significant role in development, such as signal transduction, cell reproduction, hormone regulation, photosynthetic activity regulation, seed germination and reproduction rate. Plants that develop more polyphenols in response to abiotic stresses are more adaptable to restrictive environments.
15.5.2 Role of Phenolics on Plants Under Heavy Metal Stress
For dealing with heavy metal stress, plants have a range of molecular and physiological strategies, including complex biochemical and genomic mechanisms. Many of these mechanisms in plants are constitutive since they are part of the homeostatic process. To defend themselves against heavy metals in the soil, plants employ a variety of strategies, including tolerance to them. Other pathways are exclusive that are only triggered in the presence of a certain metal toxicity. All reactions can be described as either avoidant or tolerant. When provoked by heavy metal toxicity, the plant’s first line of defence is to reduce metal absorption through the utilization of cells and root exudates. Root exudate increases efflux or biosorption to plant cell walls and keeps metals from entering the cell, therefore classified as an avoidance strategy. Many plants have distinct systems known as metal ion tolerance mechanisms wherein metal ions are housed in compartments and are not allowed to interact with delicate cell components. However, it should be noted that when any cell is constantly subjected to intense stress, its normal defensive systems may become depleted. At this period, metals may be chelated, transported, sequestered or detoxified in the vacuole of plants. Plants have numerous detoxification systems to survive. When any of these processes is activated in a plant, the production of stress-related proteins, hormones, antioxidants, signalling molecules and heat shock proteins begins. Stressed plants also establish symbiotic relationships with mycorrhizal fungi that store metals in the rhizosphere, thereby rendering them inaccessible to the plant. This is another approach for thriving under challenging conditions. Plants have highly developed and complicated defence and control signalling networks that engage several genes at the same time. The mitogen-activated protein kinase (MAPK) cascade is one of the most intrinsic and significant systems engaged in plant abiotic stress. All eukaryotic species have been shown utilizing this mechanism (Jonak et al. 2002; Tena et al. 2001). The MAPK cascade involves phosphorylation, leading to a variety of events such as cell division, differentiation, the production of some stress-related genes or the regulation of the activity of others. MAPK is also associated with hormone responses, which control gene expression in a variety of ways. Surprisingly, the quantity of trace metals in soil is a crucial factor in deciding whether the metals impede or stimulate plant development. Heavy metals typically accumulate in root cells as a result of Casparian strip obstruction or entrapment by root cell walls. Heavy metal accumulation in plants disrupts several biochemical, physiological and morphological activities, interfering with crop output (Shahid et al. 2015). Phenolics serve multiple of roles in plants. Under various environmental and stress situations, there is a rise in phenylpropanoid metabolism and the quantity of phenolic compounds (Diaz et al. 2001; Sakihama and Yamasaki 2002). Isoflavones and other flavonoids are synthesized when plants are injured or when temperatures and nutrition levels are low (Takahama and Oniki 2000; Ruiz et al. 2003). The bulk of them are antimicrobial in nature. To prevent UV-B entering plant’s interior tissues, plants store UV-absorbing flavonoids and other phenolics mostly in the vacuoles of epidermal cells (Kondo and Kawashima 2000). Roots of legume plants produce flavonoids that trigger genes in bacteria residing in root nodules (Winkel-Shirley 2002). It has been proven that phenolic compound production is stimulated in wheat and maize when exposed to nickel and aluminium toxicity, respectively (Diaz et al. 2001; Winkel-Shirley 2002). After being sprinkled with copper sulphate, Phaseolus vulgaris leaves accumulate soluble and insoluble phenolics; similarly Phyllanthus tenellus leaves were found to contain higher levels of phenolics than the control plants (Diaz et al. 2001). Enhanced levels of phenolics were shown to be linked to increased activity of phenolic compound metabolism enzymes, therefore indicating afresh phenolic biosynthesis under heavy metal stress. However, some research suggests that the rise in flavonoid concentration is mostly due to conjugate hydrolysis rather than de novo production (Parry et al. 1994). Rise in soluble phenolics, which are intermediates in the lignin production process, causes increased cell wall endurance and the formation of physical barriers that hinder heavy metals from acting adversely, which thus reflect the normal anatomical changes caused by stressors (Diaz et al. 2001). Metal stress causes the production of harmful ROS that leads to oxidative stress in plants resulting into toxicity and growth retardation (Guo et al. 2017; Pandey and Sharma 2002). The defence against this oxidative stress involves increased phenolic synthesis in metal-stressed plants (Handa et al. 2019; Kohli et al. 2018). Flavonoids can improve metal chelation, which helps reduce damaging hydroxyl radicals in plant cells (Mira et al. 2002), which is consistent with the discovery that metal excess raises flavonoid levels in plants (Kaur et al. 2017a, b). Metal poisoning promotes the production of flavonoids such as anthocyanins and flavonols that help plants defend themselves (Handa et al. 2019; Zafari et al. 2016). The buildup of phenolic compounds is caused by the upregulation of phenylalanine ammonia-lyase, chalcone synthase, shikimate dehydrogenase, cinnamyl alcohol dehydrogenase and polyphenol oxidase (Chen et al. 2019). Flavonoids also scavenge H2O2 and are expected to play an essential role in the phenolic/ascorbate-peroxidase cycle (Michalak 2006). According to Kovacik et al. (2009), the phenylpropanoid pathway precursors are synthesized by a process that requires two key enzymes, namely, shikimate dehydrogenase (SKDH) and glucose-6-phosphate dehydrogenase (G6PDH). There is yet another enzyme cinnamyl alcohol dehydrogenase (CADH) catalysing metabolic pathways that produce precursors for lignin synthesis. Heavy metals boost the activity of important biosynthetic enzymes such as PAL, SKDH, G6PDH and CADH, which promotes the phenylpropanoid production pathway in plants (Mishra and Sangwan 2019). Moreover, polyphenol oxidase (PPO) adds to the ROS scavenging process while also increasing a plant’s tolerance to abiotic stresses such as that of heavy metals (Mishra et al. 2014) (Table 15.2).
15.5.3 Antioxidant Action of Phenols
The notion of phenolic compound’s antioxidant activity is not unique (Bors et al. 1990). There have been several instances of increased buildup of phenolic compounds and peroxidase action in plants facing elevated metal concentrations. The ability of phenolics to chelate metals contributes to their antioxidant activity. Apart from having hydroxyl groups, phenolics have carboxyl groups that can attach to iron and copper (Jung et al. 2003). Plants exposed to heavy metals discharge substantial amounts of phenolics via their roots which can chelate and deactivate the iron (Fe) ions. It can also impede the superoxide-driven Fenton process, which is thought to be the primary source of ROS formation (Arora et al. 1998). Mn chelation protects tannin-rich plants (e.g. tea), which are resistant to Mn overload. Methanol preparations of Nymphaea rhizome revealed direct chelation, or adhering to polyphenols, for Cr, Pb and Hg (Lavid et al. 2001). Arora et al. (2000) have showed that flavonoids can change the order of lipid packing and hence affect peroxidation kinetics. Furthermore, they reduce free radical movement, minimize peroxidative reactions and in a concentration-dependent manner help in keeping membranes stable by reducing membrane fluidity (Blokhina et al. 2003). In yet another study by Verstraeten et al. (2003), it was revealed that flavanols and procyanidins can interact with polar head groups of membrane phospholipids via hydrogen bonding in addition to their well-known protein-binding capacity. As a result, these molecules can gather on the surface of the membranes, both within and outside the cells. They also proposed certain flavonoids help retain membrane integrity by preventing hazardous molecules from accessing the hydrophobic section of the lipid bilayer, such as those which may affect membrane rheology and cause damage to several constituents of membrane. In vitro studies have revealed that flavonoids may directly remove the following active oxygen molecular species: •O2 (superoxide), H2O2 (hydrogen peroxide) and 1O2 (singlet oxygen), OH (hydroxyl radical) or peroxyl radical. Their antioxidant functions are majorly dependent on their potential to transfer electrons or hydrogen atoms (Sakihama et al. 2000; Khan et al. 2000). The polyphenols contain an appropriate molecular chemistry required for this action and have been proven in vitro to be far more effective on a molar basis than vitamins E and C (Rice-Evans et al. 1997). Three structural features of flavonoids, according to Bors et al. (1990), are important drivers of their antioxidant potential:
-
(a)
The orto 3′,4′-dihydroxy structure in the B ring (e.g. in catechin and quercetin)
-
(b)
The 2,3-double bond in conjunction with the 4-oxo group in the C ring (allowing conjunction between the A and B rings, or electron delocalization)
-
(c)
The presence of a 3-OH group in the C ring and a 5-OH group in the A ring
Among these, the 3-OH group is the most essential predictor of electron-donating activity. When compared to aglycones, glycosylated flavonoids lose action (Rice-Evans et al. 1996). Flavonols were found to be oxidized in situ by hydrogen peroxide in epidermal strips of Vicia faba leaves, Tradescantia virginiana leaves and V. faba mesophyll cells (Takahama 1988). Plants have two kinds of peroxidases, which may be classified into two different groups: peroxidases (AP X) that employ ASC as an electron donor predominantly and others that preferentially employ phenolics. The AP X is predominantly found in chloroplasts, cytoplasm and peroxisomes, where it scavenges H2O2 produced in these cell organelles (Fig. 15.2) (Noctor and Foyer 1998). To detoxify H2O2 in these cellular organelles, AP X oxidizes ascorbate to the short-lived MDA (monodehydroascorbate) radical that gets degraded to ascorbate and DHA (dehydroascorbic acid). DHA is subsequently reduced to ascorbate by glutathione reductase (DHAR) a GHS-dependent enzyme (Sakihama et al. 2002). According to Noctor and Foyer (1998), MDA radical can be converted to ascorbate via the nonenzymatic ferredoxin (Fd) process or the NAD(P)-dependent enzymatic MDAR (monodehydroascorbate reductase). According to several studies, excessive amounts of heavy metals can impair the activity of AP X (Knorzer et al. 1996). Peroxidases that employ phenolics are classified into three types: soluble and cell wall-bound, apoplastic POXs and vacuolar. The POXs bound to the cell wall are thought to take part in the oxidation of lignin monomers, thereby supplying oxidized substrates for the lignin synthesis and other metabolic processes (de Obeso et al. 2003). According to Rai et al. (2004), peroxidases that contribute to lignin formation and may provide a protection against heavy metal toxicity are involved in the response of cadmium toxicity, wounding and pathogen attack. POXs, which are soluble and apoplastic, can scavenge H2O2 with the help of phenolics and ASC. It has been postulated that H2O2 can be detoxified by means of flavonols and phenylpropanoids that occur in vacuoles and the apoplast, by serving as electron donors for phenol peroxidases (guaiacol peroxidases) present in these compartments, resulting in the generation of phenoxyl radicals (Yamasaki et al. 1997). Peroxidases catalyse the initial step in antioxidant activity (reaction 1). Nonenzymatic interaction with ascorbate (reaction 2) can regenerate phytophenolics from phenoxyl radicals while limiting the generation of degradation products (Yamasaki et al. 1997). When monodehydroascorbate radicals develop in vacuoles, they are disproportionately converted to ascorbate and DHA (reaction 3), which can be transferred to the cytoplasm and reduced by DHAR (Takahama and Oniki 2000). Both ASC and DHA have been reported to be transported across tonoplast and between symplast and apoplast (Horemans et al. 2000). Sakihama et al. (2000) speculate that MDA reductase may function as a phenoxyl radical reductase in the apoplast to renew the redox state of phenols.
Similarity between APX action (in chloroplast and cytosol) and POX action (in apoplast and vacuole). MDA reductase, according to Sakihama et al. (2000), may act as a phenoxyl radical reductase in the apoplast to renew the redox state of phenols. POX detoxifies H2O2 by using phenolics as a substrate (drawn according to Sakihama et al. (2002), changed)
A scheme of four reactions is:
where reaction 4 is the sum of 1, 2 and 3.
When spin-stabilization effectors lengthen the lifespan of the radicals, the oxidized phenolic molecules (phenoxyl radicals) may display cytotoxic and prooxidant activities (Yamasaki and Grace 1998). This also applies to antioxidant compounds such as vitamin C, vitamin E and carotenoids (Rietjens et al. 2002). Because these radicals are unstable and swiftly degrade to non-radical compounds under normal physiological settings, they normally do not cause harm. In reality, they can be beneficial as prooxidants; for example, o-dihydroxy phenolics have anti-herbivore action under specific conditions (Barbehenn et al. 2003). However, due to their potential to generate free radical chain reactions in the membrane and their proclivity to cross-link with range of molecules, phenoxyl radicals are often harmful to biological systems (Sakihama et al. 2002). Metal ions have also been shown to stimulate prooxidant activity. Metal ions have the potential to affect the character of plant phenolics in vivo via modifying the lifespan of phenoxyl radicals. This might explain the harmfulness of metals like Al3+, Cd2+ and Zn2+ found in the root apoplast (Yamasaki and Grace 1998). Toxic, dark polymerization products of flavonoids may be formed irreversibly if ASC levels are low (Takahama et al. 1999).
15.6 Conclusion and Future Prospects
Plant phenolics are abundantly and ubiquitously found secondary metabolites, consisting of a huge pool of natural compounds with a vast and diverse spectrum of gene regulation and transport mechanisms. As an adaptation response to severe and unfavourable environmental circumstances such as heavy metal stress, in addition to physical injury, pathogen infection, mineral shortages and cold stress, plants build up phenolics in their tissues. Furthermore, these compounds, which are lignin and suberin precursors, undergo polymerization to form cell wall components. Despite a few research findings on the synthesis of phenolic compounds and their accumulation as an adaptive mechanism to heavy metal stress in plants, more research is needed to be done to comprehend their proper mechanism of accumulation, their interactions with other cell metabolites and their amplified expression and conferring tolerance in the face of heavy metal toxicity.
References
Alcantara E, Romera FJ, Cannete M, De la Guardia MD (1994) Effects of heavy metals on both induction and function of root Fe (III) reductase in Fe-deficient cucumber (Cucumis sativus L.) plants. J Exp Bot 45(12):1893–1898
Ali I, Jain CK (2004) Advances in arsenic speciation techniques. Int J Environ Anal Chem 84(12):947–964
Amarowicz R, Weidner S (2009) Biological activity of grapevine phenolic compounds. In: Grapevine molecular physiology & biotechnology. Springer, Dordrecht, pp 389–405
Andersen CP (2003) Source–sink balance and carbon allocation below ground in plants exposed to ozone. New Phytol 157(2):213–228
Arora A, Nair MG, Strasburg GM (1998) Structure–activity relationships for antioxidant activities of a series of flavonoids in a liposomal system. Free Radic Biol Med 24(9):1355–1363
Arora A, Byrem TM, Nair MG, Strasburg GM (2000) Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch Biochem Biophys 373(1):102–109
Aydinalp C, Marinova S (2009) The effects of heavy metals on seed germination and plant growth on alfalfa plant (Medicago sativa). Bulgarian J Agr Sci 15(4):347–350
Babula P, Adam V, Havel L, Kizek R (2009) Noteworthy secondary metabolites naphthoquinones-their occurrence, pharmacological properties and analysis. Curr Pharm Anal 5(1):47–68
Bachman GR, Miller WB (1995) Iron chelate inducible iron/manganese toxicity in zonal geranium. J Plant Nutr 18(9):1917–1929
Bakkaus E, Gouget B, Gallien JP, Khodja H, Carrot F, Morel JL, Collins R (2005) Concentration and distribution of cobalt in higher plants: the use of micro-PIXE spectroscopy. Nucl Instrum Methods Phys Res Sect B 231(1–4):350–356
Balas A, Popa V (2008) Bioactive compounds extracted from Picea abies bark. In: Proceedings of the 10th European workshop on lignocellulosics and pulp, Stockholm, Sweden, pp 25–28
Balestrasse KB, Benavides MP, Gallego SM, Tomaro ML (2003) Effect of cadmium stress on nitrogen metabolism in nodules and roots of soybean plants. Funct Plant Biol 30(1):57–64
Barbehenn RV, Poopat U, Spencer B (2003) Semiquinone and ascorbyl radicals in the gut fluids of caterpillars measured with EPR spectrometry. Insect Biochem Mol Biol 33(1):125–130
Barbehenn R, Dukatz C, Holt C, Reese A, Martiskainen O, Salminen JP, Constabel CP (2010) Feeding on poplar leaves by caterpillars potentiates foliar peroxidase action in their guts and increases plant resistance. Oecologia 164(4):993–1004
Basheer AA (2018) Chemical chiral pollution: impact on the society and science and need of the regulations in the 21st century. Chirality 30(4):402–406
Becker M, Asch F (2005) Iron toxicity in rice—conditions and management concepts. J Plant Nutr Soil Sci 168(4):558–573
Bell AA, Wheeler MH (1986) Biosynthesis and functions of fungal melanins. Annu Rev Phytopathol 24(1):411–451
Bennett GJ, Lee HH (1989) Xanthones from Guttiferae. Phytochemistry 28(4):967–998
Bhattacharya A, Sood P, Citovsky V (2010) The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol Plant Pathol 11:705–719
Bishnoi NR, Dua A, Gupta VK, Sawhney SK (1993) Effect of chromium on seed germination, seedling growth and yield of peas. Agric Ecosyst Environ 47(1):47–57
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91(2):179–194
Bors W, Heller W, Michel C, Saran M (1990) Flavonoids as antioxidants: determination of radical-scavenging efficiencies. Methods Enzymol 186:343–355
Boudet AM (2007) Evolution and current status of research in phenolic compounds. Phytochemistry 68(22–24):2722–2735
Bujor OC, Talmaciu IA, Volf I, Popa VI (2015) Biorefining to recover aromatic compounds with biological properties. TAPPI J 14(3):187–193
Cakmak I, Marschner H (1993) Effect of zinc nutritional status on activities of superoxide radical and hydrogen peroxide scavenging enzymes in bean leaves. In: Plant nutrition—from genetic engineering to field practice. Springer, Dordrecht, pp 133–136
Cargnelutti D, Tabaldi LA, Spanevello RM, de Oliveira Jucoski G, Battisti V, Redin M, Schetinger MRC (2006) Mercury toxicity induces oxidative stress in growing cucumber seedlings. Chemosphere 65(6):999–1006
Chatterjee J, Chatterjee C (2000) Phytotoxicity of cobalt, chromium and copper in cauliflower. Environ Pollut 109(1):69–74
Chen Z, Yu L, Wang X, Gu Z, Beta T (2016) Changes of phenolic profiles and antioxidant activity in canaryseed (Phalaris canariensis L.) during germination. Food Chem 194:608–618
Chen S, Wang Q, Lu H, Li J, Yang D, Liu J, Yan C (2019) Phenolic metabolism and related heavy metal tolerance mechanism in Kandelia Obovata under Cd and Zn stress. Ecotoxicol Environ Saf 169:134–143
Clairmont KB, Hagar WG, Davis EA (1986) Manganese toxicity to chlorophyll synthesis in tobacco callus. Plant Physiol 80(1):291–293
Clijsters H, Van Assche F (1985) Inhibition of photosynthesis by heavy metals. Photosynth Res 7(1):31–40
Constabel CP, Barbehenn R (2008) Defensive roles of polyphenol oxidase in plants. In: Induced plant resistance to herbivory. Springer, Dordrecht, pp 253–270
Cornell HV, Hawkins BA (2003) Herbivore responses to plant secondary compounds: a test of phytochemical coevolution theory. Am Nat 161:507–522
Costa G, Morel JL (1994) Efficiency of H+-ATpase activity on cadmium uptake by four cultivars of lettuce. J Plant Nutr 17(4):627–637
Das P, Samantaray S, Rout GR (1997) Studies on cadmium toxicity in plants: a review. Environ Pollut 98(1):29–36
De Dorlodot S, Lutts S, Bertin P (2005) Effects of ferrous iron toxicity on the growth and mineral composition of an interspecific rice. J Plant Nutr 28(1):1–20
de Obeso M, Caparros-Ruiz D, Vignols F, Puigdomènech P, Rigau J (2003) Characterisation of maize peroxidases having differential patterns of mRNA accumulation in relation to lignifying tissues. Gene 309(1):23–33
Demirevska-Kepova K, Simova-Stoilova L, Stoyanova Z, Hölzer R, Feller U (2004) Biochemical changes in barley plants after excessive supply of copper and manganese. Environ Exp Bot 52(3):253–266
Desmet G, De Ruyter A, Ringoet A (1975) Absorption and metabolism of CrO42− by isolated chloroplasts. Phytochemistry 14(12):2585–2588
Diaz J, Bernal A, Pomar F, Merino F (2001) Induction of shikimate dehydrogenase and peroxidase in pepper (Capsicum annuum L.) seedlings in response to copper stress and its relation to lignification. Plant Sci 161(1):179–188
Dubey SK, Naik MM (2011) Lead-enhanced siderophore production and alteration in cell morphology in a Pb-resistant Pseudomonas aeruginosa strain 4EA. Curr Microbiol 62(2):409–414
Ebbs SD, Kochian LV (1997) Toxicity of zinc and copper to Brassica species: implications for phytoremediation. Am Soc Agron 26(3):776–781
Fiehn O (2002) Metabolomics—the link between genotypes and phenotypes. Funct Genom:155–171
Fodor E, Szabo-Nagy A, Erdei L (1995) The effects of cadmium on the fluidity and H+-ATPase activity of plasma membrane from sunflower and wheat roots. J Plant Physiol 147(1):87–92
Foy CD, Weil RR, Coradetti CA (1995) Differential manganese tolerances of cotton genotypes in nutrient solution. J Plant Nutr 18(4):685–706
Gajewska E, Skłodowska M, Słaba M, Mazur J (2006) Effect of nickel on antioxidative enzyme activities, proline and chlorophyll contents in wheat shoots. Biol Plant 50(4):653–659
Gamalero E, Lingua G, Berta G, Glick BR (2009) Beneficial role of plant growth promoting bacteria and arbuscular mycorrhizal fungi on plant responses to heavy metal stress. Can J Microbiol 55(5):501–514
Gimeno-García E, Andreu V, Boluda R (1996) Heavy metals incidence in the application of inorganic fertilizers and pesticides to rice farming soils. Environ Pollut 92(1):19–25
Goldbold DJ, Hutterman A (1986) The uptake and toxicity of mercury and lead to spruce (Picea abies) seedlings. Water Air Soil Pollut 31:509–515
Gonnelli C, Galardi F, Gabbrielli R (2001) Nickel and copper tolerance and toxicity in three Tuscan populations of Silene paradoxa. Physiol Plant 113(4):507–514
Grunhage L, Dammgen U, Jager HJ (1985) System zur flachendeckenden Erfassung von luftgetragenen Schadstoffen und ihren Wirkungen auf Pflanzen. Landschaftsökoklogisches Messen und Auswerten 1(2/3):95–105
Guo D, Mitchell RJ, Withington JM, Fan PP, Hendricks JJ (2008) Endogenous and exogenous controls of root life span, mortality and nitrogen flux in a longleaf pine forest: root branch order predominates. J Ecol 96(4):737–745
Guo H, Chen H, Hong C, Jiang D, Zheng B (2017) Exogenous malic acid alleviates cadmium toxicity in Miscanthus sacchariflorus through enhancing photosynthetic capacity and restraining ROS accumulation. Ecotoxicol Environ Saf 141:119–128
Gupta VK, Ali I, Saleh TA, Nayak A, Agarwal S (2012) Chemical treatment technologies for waste-water recycling—an overview. RSC Adv 2(16):6380–6388
Hakeem KR, Sabir M, Ozturk M, Mermut A (2015) Soil remediation and plants: prospects and challenges. Elsevier, London, p 724
Halvorson JJ, Gonzalez JM, Hagerman AE, Smith JL (2009) Sorption of tannin and related phenolic compounds and effects on soluble-N in soil. Soil Biol Biochem 41(9):2002–2010
Handa N, Kohli SK, Sharma A, Thukral AK, Bhardwaj R, Alyemeni MN, Ahmad P (2018) Selenium ameliorates chromium toxicity through modifications in pigment system, antioxidative capacity, osmotic system, and metal chelators in Brassica juncea seedlings. S Afr J Bot 119:1–10
Handa N, Kohli SK, Sharma A, Thukral AK, Bhardwaj R, Abd-Allah EF, Ahmad P (2019) Selenium modulates dynamics of antioxidative defence expression, photosynthetic attributes and secondary metabolites to mitigate chromium toxicity in Brassica juncea L. plants. Environ Exp Bot 161:180–192
Harborne JB (1989) General procedures and measurement of total phenolics. Methods Plant Biochem 1:1–28
Harborne JB, Green PS (1980) A chemotaxonomic survey of flavonoids in leaves of the Oleaceae. Bot J Linn Soc 81(2):155–167
Harborne JB, Simmonds NW (1964) The natural distribution of the phenolic aglycones. In: Biochemistry of phenolic compounds, pp 77–127
Heil M (2008) Indirect defence via tritrophic interactions. New Phytol 178(1):41–61
Hernandez LE, Carpena-Ruiz R, Garate A (1996) Alterations in the mineral nutrition of pea seedlings exposed to cadmium. J Plant Nutr 19(12):1581–1598
Hewitt EJ (1953) Metal interrelationships in plant nutrition: I. effects of some metal toxicities on sugar beet, tomato, oat, potato, and marrowstem kale grown in sand culture. J Exp Bot 4(1):59–64
Horemans N, Foyer CH, Asard H (2000) Transport and action of ascorbate at the plant plasma membrane. Trends Plant Sci 5(6):263–267
Horiguchi T (1988) Mechanism of manganese toxicity and tolerance of plants: IV. Effects of silicon on alleviation of manganese toxicity of rice plants. Soil Sci Plant Nutr 34(1):65–73
Ishimaru K, Nonaka GI, Nishioka I (1987) Tannins and related compounds. LV. Isolation and characterization of acutissimins A and B, novel tannins from Quercus and Castanea species. Chem Pharm Bull 35(2):602–610
Izosimova A (2005) Modelling the interaction between calcium and nickel in the soil-plant system. Bundesforschungsanstalt fur Landwirtschaft (FAL)
Jonak C, Okresz L, Bogre L, Hirt H (2002) Complexity, cross talk and integration of plant MAP kinase signalling. Curr Opin Plant Biol 5(5):415–424
Jung C, Maeder V, Funk F, Frey B, Sticher H, Frossard E (2003) Release of phenols from Lupinus albus L. roots exposed to Cu and their possible role in Cu detoxification. Plant Soil 252(2):301–312
Kaur R, Yadav P, Sharma A, Thukral AK, Kumar V, Kohli SK, Bhardwaj R (2017a) Castasterone and citric acid treatment restores photosynthetic attributes in Brassica juncea L. under Cd (II) toxicity. Ecotoxicol Environ Saf 145:466–475
Kaur P, Bali S, Sharma A, Vig AP, Bhardwaj R (2017b) Effect of earthworms on growth, photosynthetic efficiency and metal uptake in Brassica juncea L. plants grown in cadmium-polluted soils. Environ Sci Pollut Res 24(15):13452–13465
Kaur P, Bali S, Sharma A, Vig AP, Bhardwaj R (2018) Role of earthworms in phytoremediation of cadmium (Cd) by modulating the antioxidative potential of Brassica juncea L. Appl Soil Ecol 124:306–316
Khan AG, Kuek C, Chaudhry TM, Khoo CS, Hayes WJ (2000) Role of plants, mycorrhizae and phytochelators in heavy metal contaminated land remediation. Chemosphere 41(1–2):197–207
Kısa D, Elmastas M, Ozturk L, Kayır O (2016) Responses of the phenolic compounds of Zea mays under heavy metal stress. Appl Biol Chem 59(6):813–820
Knorzer OC, Burner J, Boger P (1996) Alterations in the antioxidative system of suspension-cultured soybean cells (Glycine max) induced by oxidative stress. Physiol Plant 97(2):388–396
Kohli SK, Handa N, Sharma A, Kumar V, Kaur P, Bhardwaj R (2017) Synergistic effect of 24-epibrassinolide and salicylic acid on photosynthetic efficiency and gene expression in Brassica juncea L. under Pb stress. Turk J Biol 41(6):943–953
Kohli SK, Handa N, Sharma A, Gautam V, Arora S, Bhardwaj R, Ahmad P (2018) Interaction of 24-epibrassinolide and salicylic acid regulates pigment contents, antioxidative defense responses, and gene expression in Brassica juncea L. seedlings under Pb stress. Environ Sci Pollut Res 25(15):15159–15173
Kondo N, Kawashima M (2000) Enhancement of the tolerance to oxidative stress in cucumber (Cucumis sativus L.) seedlings by UV-B irradiation: possible involvement of phenolic compounds and antioxidative enzymes. J Plant Res 113(3):311–317
Kovacik J, Klejdus B, Hedbavny J, Stork F, Backor M (2009) Comparison of cadmium and copper effect on phenolic metabolism, mineral nutrients and stress-related parameters in Matricaria chamomilla plants. Plant Soil 320(1):231–242
Kukier U, Peters CA, Chaney RL, Angle JS, Roseberg RJ (2004) The effect of pH on metal accumulation in two Alyssum species
Kutchan T, Dixon RA (2005) Physiology and metabolism: secondary metabolism: nature’s chemical reservoir under deconvolution. Curr Opin Plant Biol 8:227–229
Lattanzio V (2013) Phenolic compounds: introduction. Nat Prod 50:1543–1580
Lattanzio V, Cardinali A, Di Venere D, Linsalata V, Palmieri S (1994) Browning phenomena in stored artichoke (Cynara scolymus L.) heads: enzymic or chemical reactions. Food Chem 50(1):1–7
Lattanzio V, Di Venere D, Linsalata V, Bertolini P, Ippolito A, Salerno M (2001) Low temperature metabolism of apple phenolics and quiescence of Phlyctaena vagabunda. J Agric Food Chem 49(12):5817–5821
Lattanzio V, Cardinali A, Ruta C, Fortunato IM, Lattanzio VM, Linsalata V, Cicco N (2009) Relationship of secondary metabolism to growth in oregano (Origanum vulgare L.) shoot cultures under nutritional stress. Environ Exp Bot 65(1):54–62
Lattanzio V, Lattanzio VM, Cardinali A (2006) Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochem Adv Res 661(2):23–67
Lavid N, Schwartz A, Yarden O, Tel-Or E (2001) The involvement of polyphenols and peroxidase activities in heavy-metal accumulation by epidermal glands of the waterlily (Nymphaeaceae). Planta 212(3):323–331
Le Bot J, Kirkby EA, Van Beusichem ML (1990) Manganese toxicity in tomato plants: effects on cation uptake and distribution. J Plant Nutr 13(5):513–525
Leng X, Jia H, Sun X, Shangguan L, Mu Q, Wang B, Fang J (2015) Comparative transcriptome analysis of grapevine in response to copper stress. Sci Rep 5(1):1–17
Lin D, Xiao M, Zhao J, Li Z, Xing B, Li X, Kong M, Li L, Zhang Q, Liu Y, Chen H, Qin W, Wu H, Chen S (2016) An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 21(10):1374
Loneragan JF (1988) Distribution and movement of manganese in plants. In: Manganese in soils and plants. Springer, Dordrecht, pp 113–124
Malik NJ, Chamon AS, Mondol MN, Elahi SF, Faiz SMA (2011) Effects of different levels of zinc on growth and yield of red amaranth (Amaranthus sp.) and rice (Oryza sativa, Variety-BR49). J Bangladesh Assoc Young Res 1(1):79–91
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Institute of Plant Nutrition University of Hohenheim, Germany
Marschner H, Römheld V, Kissel M (1986) Different strategies in higher plants in mobilization and uptake of iron. J Plant Nutr 9(3–7):695–713
Mathesius U (2008) Auxin: at the root of nodule development? Funct Plant Biol 35:651–668
Mathys W (1975) Enzymes of heavy-metal-resistant and non-resistant populations of Silene cucubalus and their interaction with some heavy metals in vitro and in vivo. Physiol Plant 33(2):161–165
Meharg AA (1994) Integrated tolerance mechanisms: constitutive and adaptive plant responses to elevated metal concentrations in the environment. Plant Cell Environ 17(9):989–993
Meharg AA, Macnair MR (1992) Suppression of the high affinity phosphate uptake system: a mechanism of arsenate tolerance in Holcus lanatus L. J Exp Bot 43(4):519–524
Michalak A (2006) Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol J Environ Stud 15:4
Mira L, Tereza Fernandez M, Santos M, Rocha R, Helena Florêncio M, Jennings KR (2002) Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity. Free Radic Res 36(11):1199–1208
Mishra B, Sangwan NS (2019) Amelioration of cadmium stress in Withania somnifera by ROS management: active participation of primary and secondary metabolism. Plant Growth Regul 87(3):403–412
Mishra B, Sangwan RS, Mishra S, Jadaun JS, Sabir F, Sangwan NS (2014) Effect of cadmium stress on inductive enzymatic and nonenzymatic responses of ROS and sugar metabolism in multiple shoot cultures of Ashwagandha (Withania somnifera Dunal). Protoplasma 251(5):1031–1045
Mohanpuria P, Rana NK, Yadav SK (2007) Cadmium induced oxidative stress influence on glutathione metabolic genes of Camellia sinensis (L.) O. Kuntze. Environ Toxicol 22(4):368–374
Moreland DE, Novitzky WP (1987) Effects of phenolic acids, coumarins, and flavonoids on isolated chloroplasts and mitochondria. In: Allelochemicals: role in agriculture and forestry. American Chemical Society, Washington, DC, pp 247–261
Moreno-Caselles J, Moral R, Pérez-Espinosa A, Pérez-Murcia MD (2000) Cadmium accumulation and distribution in cucumber plant. J Plant Nutr 23(2):243–250
Moss GP (2000) Nomenclature of lignans and neolignans (IUPAC recommendations 2000). Pure Appl Chem 72(8):1493–1523
Neelima P, Reddy KJ (2002) Interaction of copper and cadmium with seedling growth and biochemical responses in Solanum melongena. Nat Environ Pollut Technol 1(3):285–290
Nematshahi N, Lahouti M, Ganjeali A (2012) Accumulation of chromium and its effect on growth of (Allium cepa cv. Hybrid). Eur J Exp Biol 2(4):969–974
Nicaise V, Roux M, Zipfel C (2009) Recent advances in PAMP-triggered immunity against bacteria: pattern recognition receptors watch over and raise the alarm. Plant Physiol 150:1638–1647
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Biol 49(1):249–279
Oszmanski J (1995) Polyphenols as antioxidants in food. Przem Spoz 3:94–96
Ouzounidou G (1994) Copper-induced changes on growth, metal content and photosynthetic function of Alyssum montanum L. plants. Environ Exp Bot 34(2):165–172
Paivoke A (1983) The long-term effects of lead and arsenate on the growth and development, chlorophyll content and nitrogen fixation of the garden pea. In: Annales Botanici Fennici Finnish Botanical Publishing Board, pp 297–306
Palanisamy CP et al (2020) Phenolic allelochemicals from crops and weed management. In: Plant phenolics in sustainable agriculture. Springer, Singapore, pp 183–199
Pandey N, Sharma CP (2002) Effect of heavy metals Co2+, Ni2+ and Cd2+ on growth and metabolism of cabbage. Plant Sci 163(4):753–758
Parr PD, Taylor FG Jr (1982) Germination and growth effects of hexavalent chromium in Orocol TL (a corrosion inhibitor) on Phaseolus vulgaris. Environ Int 7(3):197–202
Parry AD, Tiller SA, Edwards R (1994) The effects of heavy metals and root immersion on isoflavonoid metabolism in alfalfa (Medicago sativa L.). Plant Physiol 106(1):195–202
Peralta JR, Gardea-Torresdey JL, Tiemann KJ, Gomez E, Arteaga S, Rascon E, Parsons JG (2001) Uptake and effects of five heavy metals on seed germination and plant growth in alfalfa (Medicago sativa L.). Bull Environ Contam Toxicol 66(6):727–734
Petersen M, Strack D, Matern U (1999) Biosynthesis of phenylpropanoids and related compounds. In: Wink M (ed) Biochemistry of plant secondary metabolism, vol 2. Sheffield Academic Press, Sheffield, pp 151–222
Phillips DJ (1990) Arsenic in aquatic organisms: a review, emphasizing chemical speciation. Aquat Toxicol 16(3):151–186
Poonam RK, Bhardwaj R, Sirhindi G (2015) Castasterone regulated polyphenolic metabolism and photosynthetic system in Brassica juncea plants under copper stress. J Pharmacogn Phytochem 4(4):282–289
Prasad MNV, Malec P, Waloszek A, Bojko M, Strzałka K (2001) Physiological responses of Lemna trisulca L. (duckweed) to cadmium and copper bioaccumulation. Plant Sci 161(5):881–889
Quideau S (2009) Chemistry and biology of ellagitannins: an underestimated class of bioactive plant polyphenols. World Scientific
Quideau S, Deffieux D, Douat-Casassus C, Pouysegu L (2011) Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed 50(3):586–621
Rai V, Vajpayee P, Singh SN, Mehrotra S (2004) Effect of chromium accumulation on photosynthetic pigments, oxidative stress defense system, nitrate reduction, proline level and eugenol content of Ocimum tenuiflorum L. Plant Sci 167(5):1159–1169
Rashad Y, Aseel D, Hammad S (2020) Phenolic compounds against fungal and viral plant diseases. In: Plant phenolics in sustainable agriculture. Springer, Singapore, pp 201–219
Reddy AM, Kumar SG, Jyothsnakumari G, Thimmanaik S, Sudhakar C (2005) Lead induced changes in antioxidant metabolism of horsegram (Macrotyloma uniflorum (Lam.) Verdc.) and bengalgram (Cicer arietinum L.). Chemosphere 60(1):97–104
Rehman A, Farooq M, Naveed M, Nawaz A, Shahzad B (2018a) Seed priming of Zn with endophytic bacteria improves the productivity and grain biofortification of bread wheat. Eur J Agron 94:98–107
Rehman A, Farooq M, Naveed M, Ozturk L, Nawaz A (2018b) Pseudomonas-aided zinc application improves the productivity and biofortification of bread wheat. Crop Pasture Sci 69(7):659–672
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20(7):933–956
Rice-Evans C, Miller N, Paganga G (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci 2(4):152–159
Rietjens IM, Boersma MG, de Haan L, Spenkelink B, Awad HM, Cnubben NH, Koeman JH (2002) The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ Toxicol Pharmacol 11(3–4):321–333
Rout GR, Samantaray S, Das P (2000) Effects of chromium and nickel on germination and growth in tolerant and non-tolerant populations of Echinochloa colona (L.) Link. Chemosphere 40(8):855–859
Ruiz JM, Rivero RM, Lopez-Cantarero I, Romero L (2003) Role of Ca 2+ in the metabolism of phenolic compounds in tobacco leaves (Nicotiana tabacum L.). Plant Growth Regul 41(2):173–177
Runyon JB, Mescher MC, Felton GW, De Moraes CM (2010) Parasitism by Cuscuta pentagona sequentially induces JA and SA defence pathways in tomato. Plant Cell Environ 33:290–303
Sakihama Y, Yamasaki H (2002) Lipid peroxidation induced by phenolics in conjunction with aluminum ions. Biol Plant 45(2):249–254
Sakihama Y, Mano JI, Sano S, Asada K, Yamasaki H (2000) Reduction of phenoxyl radicals mediated by monodehydroascorbate reductase. Biochem Biophys Res Commun 279(3):949–954
Sakihama Y, Cohen MF, Grace SC, Yamasaki H (2002) Plant phenolic antioxidant and prooxidant activities: phenolics-induced oxidative damage mediated by metals in plants. Toxicology 177(1):67–80
Salt DE, Blaylock M, Kumar NP, Dushenkov V, Ensley BD, Chet I, Raskin I (1995) Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Biotechnology 13(5):468–474
Schmfger MEV (2001) Phytochelatins: complexation of metals and metalloids, studies on the phytochelatin synthase (Doctoral dissertation, PhD thesis, Munich University of Technology (TUM), Munich)
Seneviratne G, Jayasinghearachchi HS (2003) Mycelial colonization by bradyrhizobia and azorhizobia. J Biosci 28(2):243–247
Shahid M, Khalid S, Abbas G, Shahid N, Nadeem M, Sabir M, Dumat C (2015) Heavy metal stress and crop productivity. In: Crop production and global environmental issues. Springer, Cham, pp 1–25
Shankar SR, Girish R, Karthik N, Rajendran R, Mahendran VS (2009) Allelopathic effects of phenolics and terpenoids extracted from Gimelina arborea on germination of Black gram (Vigna mungo) and Green gram (Vigna radiata). Allelopath J 23(2):323–332
Sharma P, Dubey RS (2005) Lead toxicity in plants. Braz J Plant Physiol 17:35–52
Sharma P, Dubey RS (2007) Involvement of oxidative stress and role of antioxidative defense system in growing rice seedlings exposed to toxic concentrations of aluminum. Plant Cell Rep 26(11):2027–2038
Sinha S, Gupta M, Chandra P (1997) Oxidative stress induced by iron in Hydrilla verticillata (l.f.) Royle: response of antioxidants. Ecotoxicol Environ Saf 38(3):286–291
Smirnov OE, Kosyan AM, Kosyk OI, Taran NY (2015) Response of phenolic metabolism induced by aluminium toxicity in Fagopyrum esculentum Moench. plants. Ukr Biochem J 87(6):129–135
Smith JL, De Moraes CM, Mescher MC (2009) Jasmonate and salicylate-mediated plant defense responses to insect herbivores, pathogens, and parasitic plants. Pest Manag Sci 65:497–503
Stadtman ER, Oliver CN (1991) Metal-catalyzed oxidation of proteins: physiological consequences. J Biol Chem 266(4):2005–2008
Stiborova M, Ditrichova M, Brezinova A (1987) Effect of heavy metal ions on growth and biochemical characteristics of photosynthesis of barley and maize seedlings. Biol Plant 29(6):453–467
Swain T (1975) Evolution of flavonoid compounds. In: The flavonoids. Springer, Boston, pp 1096–1129
Takahama U (1988) Oxidation of flavonols by hydrogen peroxide in epidermal and guard cells of Vicia faba L. Plant Cell Physiol 29(3):433–438
Takahama U, Oniki T (2000) Flavonoids and some other phenolics as substrates of peroxidase: physiological significance of the redox reactions. J Plant Res 113(3):301–309
Takahama U, Hirotsu M, Oniki T (1999) Age-dependent changes in levels of ascorbic acid and chlorogenic acid, and activities of peroxidase and superoxide dismutase in the apoplast of tobacco leaves: mechanism of the oxidation of chlorogenic acid in the apoplast. Plant Cell Physiol 40(7):716–724
Tanase C, Bara CI, Popa VI (2015) Cytogenetical effect of some polyphenol compounds separated from industrial by-products on. Cell Chem Technol 49:799–805
Tanase C, Bujor OC, Popa VI (2019) Phenolic natural compounds and their influence on physiological processes in plants. In: Polyphenols in plants. Academic, pp 45–58
Taylor LP, Grotewold E (2005) Flavonoids as developmental regulators. Curr Opin Plant Biol 8(3):317–323
Tena G, Asai T, Chiu WL, Sheen J (2001) Plant mitogen-activated protein kinase signaling cascades. Curr Opin Plant Biol 4(5):392–400
Tkalec M, Stefanic PP, Cvjetko P, Sikic S, Pavlica M, Balen B (2014) The effects of cadmium-zinc interactions on biochemical responses in tobacco seedlings and adult plants. PLoS One 9(1):e87582
Tobe K, Zhang L, Qiu GY, Shimizu H, Omasa K (2001) Characteristics of seed germination in five non-haplotypic Chinese desert shrub species. J Arid Environ 48:101
Tomas-Barberan FA, Clifford MN (2000) Dietary hydroxybenzoic acid derivatives–nature, occurrence and dietary burden. J Sci Food Agric 80(7):1024–1032
Van Assche F, Clijsters H (1983) Multiple effects of heavy metal toxicity on photosynthesis. In: Effects of stress on photosynthesis. Springer, Dordrecht, pp 371–382
Van Assche F, Cardinaels C, Clijsters H (1988) Induction of enzyme capacity in plants as a result of heavy metal toxicity: dose-response relations in Phaseolus vulgaris L., treated with zinc and cadmium. Environ Pollut 52(2):103–115
van der Meer IM, Stam ME, van Tunen AJ, Mol JN, Stuitje AR (1992) Inhibition of flavonoid biosynthesis in petunia anthers by antisense RNA: a novel way to engineer nuclear male sterility. In: Angiosperm pollen and ovules. Springer, New York, pp 22–27
Vazquez-Olivo G et al (2020) Phenolics from agro-industrial by-products. In: Plant phenolics in sustainable agriculture. Springer, Singapore, pp 331–346
Veitch NC, Grayer RJ (2008) Flavonoids and their glycosides, including anthocyanins. Nat Prod Rep 25(3):555–611
Vermerris W, Nicholson R (2006) Phenolic compound biochemistry. Springer, Dordrecht
Verstraeten SV, Keen CL, Schmitz HH, Fraga CG, Oteiza PI (2003) Flavan-3-ols and procyanidins protect liposomes against lipid oxidation and disruption of the bilayer structure. Free Radic Biol Med 34(1):84–92
Villiers F, Ducruix C, Hugouvieux V, Jarno N, Ezan E, Garin J, Bourguignon J (2011) Investigating the plant response to cadmium exposure by proteomic and metabolomic approaches. Proteomics 11(9):1650–1663
Wagay NA et al (2020) Phenolics: a game changer in the life cycle of plants. In: Plant phenolics in sustainable agriculture. Springer, Singapore, pp 241–275
Warne MSJ, Heemsbergen D, Stevens D, McLaughlin M, Cozens G, Whatmuff M, Penney N (2008) Modeling the toxicity of copper and zinc salts to wheat in 14 soils. Environ Toxicol Chem 27(4):786–792
Winkel-Shirley B (2002) Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol 5(3):218–223
Wu SH (1994) Effect of manganese excess on the soybean plant cultivated under various growth conditions. J Plant Nutr 17(6):991–1003
Wu S, Chappell J (2008) Metabolic engineering of natural products in plants; tools of the trade and challenges for the future. Curr Opin Biotechnol 19(2):145–152
Yamasaki H, Grace SC (1998) EPR detection of phytophenoxyl radicals stabilized by zinc ions: evidence for the redox coupling of plant phenolics with ascorbate in the H2O2-peroxidase system. FEBS Lett 422(3):377–380
Yamasaki H, Sakihama Y, Ikehara N (1997) Flavonoid-peroxidase reaction as a detoxification mechanism of plant cells against H2O2. Plant Physiol 115(4):1405–1412
Zafari S, Sharifi M, Chashmi NA, Mur LA (2016) Modulation of Pb-induced stress in Prosopis shoots through an interconnected network of signaling molecules, phenolic compounds and amino acids. Plant Physiol Biochem 99:11–20
Zeid IM (2001) Responses of Phaseolus vulgaris chromium and cobalt treatments. Biol Plant 44(1):111–115
Zhang WH, Tyerman SD (1999) Inhibition of water channels by HgCl2 in intact wheat root cells. Plant Physiol 120(3):849–858
Zhang J, Subramanian S, Stacey G, Yu O (2009) Flavones and flavonols play distinct critical roles during nodulation of Medicago truncatula by Sinorhizobium meliloti. Plant J 57(1):171–183
Zipfel C (2008) Pattern-recognition receptors in plant innate immunity. Curr Opin Immunol 20:10–16
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Magray, J.A., Sharma, D.P., Deva, M.A., Thoker, S.A. (2023). Phenolics: Accumulation and Role in Plants Grown Under Heavy Metal Stress. In: Lone, R., Khan, S., Mohammed Al-Sadi, A. (eds) Plant Phenolics in Abiotic Stress Management. Springer, Singapore. https://doi.org/10.1007/978-981-19-6426-8_15
Download citation
DOI: https://doi.org/10.1007/978-981-19-6426-8_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-6425-1
Online ISBN: 978-981-19-6426-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)