Abstract
Plastic waste, considered a great threat to the environment, requires an effective treatment process. The ability of the microbes to oxidize the polymeric chain (C–C bonds), hydrolyze and produce carbon dioxide and water as final products of degradation was studied. The process becomes complicated due to structural complexity of the polymer. The present study is the continuation of the LDPE degradation using the Winogradsky Column. The determination of metabolites formed on degradation is discussed. The FTIR analysis indicated the reduction in the intensity of the C-H, confirming the cleavage of the alkane chains in LDPE. The metabolites produced during the degradation resulted in the formation of smaller alkanes, which contain C32, C22, C16, C18 and aromatic compounds such as phenols and benzene dicarboxylic acid. The occurrence of terminal oxidation of the polymeric chain, cleavage, fragmentation and cyclization of the alkanes confirm the biodegradation process. The current research also focuses on the biodegradation of LDPE using bacterial strains isolated from dumpsite soil samples. The degraded LDPE was analyzed for its metabolite production using GC–MS. It enabled us to understand and hypothesize an overview pathway of LDPE degradation by bacterial strains. The hypothesized pathway indicated that bacterial strains performed fragmentation and cyclization of the long polymeric chain, followed by hydrogenation and oxidation, resulting in the formation of alcohols, aldehydes, ketones and carboxylic acid compounds leading to ester formation. The esters are then understood to enter the ꞵ-oxidation pathway or TCA cycle, producing carbon dioxide and water molecules.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The origin of the global threat (Plastics) dates back to 1907, which gained its importance with time. The resistivity of plastics to multiple factors made it an ideal alternative for cloth bags, leading to multiple applications (Zalasiewicz et al. 2016). The increase in demand encouraged plastic production, which led to the disposal and accumulation of single-use plastic. It was reported to be a 10% increase in plastic waste production in municipal solid wastes. Plastic accumulation has an ill impact on the entire ecosystem, leading to the death of living organisms. Plastics are reported to release monomers, oligomers and additives, toxic to the environment. Apart from being a source of toxic pollutants, it also acts as a carrier for Persistent Organic Pollutants (POPs), heavy metals and pathogens (Chen et al. 2019). Co-occurrence of PAH and microplastics due to chemical affinity leading to its persistence in the marine environment. This raises the need for an alternative method to degrade both PAHs and microplastics (Ali et al. 2024).
The degradation or treatment of plastics is widely studied by applying various techniques, such as photodegradation, thermal oxidation, biodegradation, and hydrolysis (Cai et al. 2018). The biodegradation of plastic wastes by bacteria and fungi is well reported. The weathering of polymeric chains to monomers and oligomers takes time (Song et al. 2017). The microbial community gets acclimatized on prolonged exposure to plastic wastes. Winogradsky Column (WC), used to study microbial diversity (site specific), provides knowledge on niche formation by various microbes based on their characteristics (Dworkin 2012). It serves as a tool to study soil microbial diversity and biogeochemical cycle balanced by the indigenous microbes (Lalla et al. 2021). Biodegradation of plastic waste involves degradation using bacterial strains, algae, fungi and other biological organisms. Duration of biodegradation relies on the structural complexity of the polymer. Microbes on prolonged exposure to plastic pollution gains resistance and consumes it as a carbon source (Jebashalomi et al. 2024). The aerobic degradation process involves breaking down organic compounds into small organic molecules, utilizing oxygen as an electron acceptor, and producing carbon dioxide and water as final products (Zeenat et al. 2021). Another process of degradation involves an anaerobic process. Microbes consume iron, sulfate, Mn, and nitrate as electron acceptors, producing methane, CO2, water and residual carbon compounds as byproducts. During the process of degradation, the polymers are insoluble in nature, which makes the uptake of polymers by the microbes difficult (Gu 2003). Non-pretreated plastics are utilized as a carbon source by forming biofilms on the plastic surface. It is reported to cause weight loss, surface morphological changes, and alter physical and chemical properties. Azeko et al. (2015) reported biodegradation of plastics by the following approaches—to identify the ability to degrade plastics and incubation of microbial cultures in the natural samples or in-situ process. The process of biodegradation is a cascade including modification of polymeric properties for effective degradation, fragmentation of polymers by hydrolysis forming intermediate products, bio assimilation of fragmented polymeric chains, and mineralization of the same, resulting in carbon dioxide and water formation as final products (Montazer, Habibi Najafi, and Levin 2020).
Understanding the degradation mechanism of plastics is sorted into three steps—initiation, propagation and termination process -. In the photo-oxidation process of degradation, the initiation involves the formation of free radicals by polymeric chain cleavage. This is only possible in plastics containing an unsaturated polymeric backbone (Grassie and Scott 1988). To increase the efficiency of biodegradation, high molecular plastics are converted into smaller compounds by an abiotic process, which makes them available for biotic degradation by bacterial strains. The polymer with methyl groups at the terminal is attacked by the microorganisms which enhances the degradation process (Gewert et al. 2015). The complex polymeric structures are cleaved into monomers and dimers, which undergo hydrolytic cleavage in the cell membrane, and the short-chained oligomers are transported to the cytoplasmic membrane. The compounds are reported to enter the β-oxidation or undergo degradation, then enter the TCA cycle (Mooney et al. 2006; Koutny et al. 2006; Shah et al. 2008; Sridharan et al. 2023).
Hence, the current report aims at biodegradation of LDPE using the Winogradsky column and bacterial strains isolated from Chennai dump yard soil. Lab scale biodegradation of LDPE was analyzed using weight loss % (preliminary method), FTIR and GC–MS analysis. The hypothesis of the LDPE biodegradation mechanism was deciphered based on GC–MS analysis. The pathway mechanism of LDPE degraded using the Winogradsky column followed fragmentation of the polymeric chain while the bacterial strains followed oxidation, dehydrogenation and esterification as a common mechanism. Thus, it shows that the efficiency is influenced by the mechanism of degradation and the method used for degradation. This study reports the unique mechanisms followed by each bacterial strain in LDPE degradation.
Materials and methods
Collection of soil samples and column construction
A random sampling method was used to collect soil samples from the Chennai dump yard and transported to the laboratory. The Winogradsky column (WC) constructed using the collected soil samples was reported, and the preliminary degradation analysis was mentioned in previous research work by Sridharan et al. (2021a, b). The chemicals used in this study was purchased from Merck, India and the agar agar was purchased from Himedia.
Isolation of plastic-degrading bacterial strains
The soil samples were collected from a dump yard (Otteri and Kodungaiyur) containing Municipal Solid Wastes (MSW). The samples were transported to the laboratory and enriched using Mineral Salts Medium (MSM g/L—NH4NO3 (0.1), MgSO4 (0.02), K2HPO4 (0.1), CaCl2 (0.01), KCl (0.015), yeast extract (0.01), FeSO4 (0.1 mg), ZnSO4 (0.1 mg), MnSO4 (0.1 mg)) (Ali et al. 2023). The enriched soil samples of about 1 mL were diluted in 9 mL of sterilized distilled water from 10−1 to 10−9 dilutions. About 100 µL of diluted soil samples from 10−6, 10−7 and 10−8 were spread-plated on MSM agar containing 100 mg/L of LDPE powder. The Petri dishes were then incubated for about 24 to 48 h (Afreen et al. 2020).
Biodegradation of LDPE using isolated bacterial strain
The bacterial strains which resulted in consistent growth on the MSM agar plates (pH 7) were used to degrade LDPE sheets. The bacterial strains at log phase (CFU 106/cm2) were inoculated in MSM broth of neutral pH supplied with two LDPE sheets (5×2 cm) in each batch (with an initial mass of 0.942 g) and incubated for 60 days. The experiment was performed in triplicates (Figure S1). The weight loss % of the LDPE sheets were monitored for every 15 days by retrieving the sheets from the culture broth, washing and disinfecting with 75% ethanol (Yao et al. 2022). The bacterial strains were molecularly characterized using 16S rRNA sequencing at Biokart.Ltd.
Statistical analysis
The biodegradation significance of each bacterial strain was discerned using One-way analysis of variance (ANOVA) and post hoc comparisons using the Tukey HSD test (IBM SPSS statistical software).
Metabolites determination
The degraded LDPE sheets were washed with distilled water (5 min until the removal of adhered bacterial strain), Tween 20 (2 min) and 75% ethanol (5 min) to remove the adhered bacterial strains. The sheets were then cut into very small pieces of random sizes for effective dispersion of LDPE sheet, suspended in chloroform (Analytical grade – 99.0 to 99.4% purity) and sonicated for 2 h with a 5 min interval (amplitude 50 and pulse 2:2). The sonicated sheets were then air-dried for 24 h and re-suspended in chloroform and filtered using Whatman No.1 filter paper. The filtrate was analyzed using GC–MS (SHIMADZU, QP2010 PLUS at SRM Nanotechnology Research Center, Chennai, Tamil Nadu) (Kyaw et al. 2012). The oven temperature was set to rise from 40 °C in 3 min to 280 °C in 4 min at a rate of 10 °C/min. Using an HP5 column and helium gas, a gas chromatography-mass spectrometer was used to measure the degradation products of LDPE. The oven temperature was designed to be increased from 70 °C to 200 °C (maximum temperature − 250 °C at 15 °C/min, Injection liquid 1 µl). The components of a mass spectrometer include a tungsten filament electron source operating at 70 eV, a double focusing analyzer, and a photomultiplier tube acting as the detector with a maximum resolution of 5000 (Ambika, Lakshmi, and Hemalatha 2018).
Results and discussion
Results
Biodegradation of LDPE using Winogradsky column containing Kodungaiyur MSW
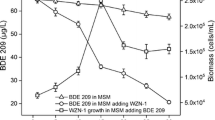
The metabolites formed during the biodegradation of LDPE were determined using GC–MS analysis. It determines the polymer breakdown and aids in analyzing the pattern of cleavage. The GC–MS chromatogram of non-degraded LDPE was reported in previous research by Sridharan et al. (2022) while Fig. 1 shows the degradation of LDPE using Kodungaiyur soil sample in the Winogradsky Column (WC).
Table 1 summarizes the changes observed in the LDPE treated using Kodungaiyur soil sample compared to Non-degraded LDPE. The new peaks were observed in the treated LDPE at 22.777, 25.093 and 30.055 RT. The disappearance of the peak at 17.37, 17.45, 18.93, 20.371, 21.341, 21.891, 24.953, 27.964, and 29.915 RT was observed in degraded LDPE. The electron ionization method was used for the mass spectra and the positive ion scanning mode determined the ions generated.
Table 1, shows the retention time, the metabolites and the peak area of the Non-degraded LDPE and treated LDPE. The mass spectral analysis of the GC peaks was detected using positive ions scanning mode while the MS of the treated LDPE is shown in Figure S2a for the GC peaks at 21.781 and 21.786 in non-degraded and degraded LDPE (figure S2b). In Non-degraded LDPE, the compound was observed to be 2-methyl octadecane (C19H40) while Dotriacontane (C32H66) was formed in degraded LDPE. Figure S3 a, b shows the mass spectrum of the compound Nonadecane (C19H40) in Non-degraded LDPE at 21.532 RT while the degraded LDPE shows the presence of 2-methyl-undecane (C12H26) at 22.262 RT. The mass spectrum in Figure S4 a, b shows the presence of the compound Octacosane (C28H58) in non-degraded LDPE and nonadecane (C19H40) in degraded LDPE. Figure S5 a, b shows the mass spectrum of RT 24.352 of non-degraded LDPE and 24.532 of degraded LDPE sheet. The non-degraded LDPE resulted in the presence of 2-methyl-undecane (C12H26) and degraded LDPE indicated the presence of nonadecane (C19H40).
The mass spectrum shown in Figure S6 a, b) indicates the presence of 1-chloroheptacosane (C27H55Cl) in non-degraded LDPE and 1-heptadecanamine (C17H37N) in degraded LDPE. The formation of amino compounds is due to the addition of nitrogen sources in the Winogradsky column. This confirms that the LDPE polymeric chain underwent fragmentation by the bacterial strains in the Winogradsky column. The fragmentation of the polymeric chain was found to be uneven and the absence of alcohols, aldehydes, ketones and acids indicated the lack of oxidation of the polymer. The hypothesized pathway is given in Fig. 2. The LDPE sheet which is made of carbon and hydrogen was hypothesized to undergo fragmentation resulting in the formation of alkane compounds such as hexatriacontane (C24H50), octacosane (C28H58), 2-methyl tricosane (C24H50), 2,6,10,14-tetramethyl heptadecane (C21H44), and nonadecane (C19H40). This shows that the fragmentation of the LDPE has occurred, resulting in a smaller alkane chain formation.
The dump yard soil used to construct the WC and degraded LDPE was analyzed for the presence of degraded plastic waste metabolites as the MSW (Municipal Solid Waste) contained numerous plastic wastes (all types of plastics). Hence, the soil in the control WC (without LDPE) and test WC (with LDPE) was analyzed using GC–MS. The metabolites present in the control WC and test WC are tabulated in Table S1.
Compounds such as 2-chloro-2-methyl butane, 1,1-Diethoxy ethane, 2,4-dimethyl heptane, Decane, 4-methyl decane, Nonadecane, Hexadecane, Tetradecane, Octadecane are present in both control WC and test WC. The compounds that are formed by the degradation of the amended LDPE in the WC containing KN soil sample formed compounds such as 5-methyl decane (RT 9.593), 2-methyl decane (RT 11.161), 2-methyl undecane (RT 12.236), 2,6-dimethyl undecane (RT 13.046), 1-(Ethenyloxy) octadecane (RT 13.679), 3,7-dimethyl decane (RT 14.077), 2-methyl naphthalene (RT 14.593), 10-methyl Eicosane (RT 23.967), Tricosane (RT 28.126), and 2-methyl Nonadecane (RT 29.142). This confirms that the compounds formed during the degradation process are also released into the soil.
The FTIR spectrum shown in Fig. 3 depicts the increased peak intensity of the test soil WC compared to the control soil WC. The peak at 3000 cm−1 and 1000 cm−1 indicates the presence of C–H bond in the LDPE sheet. The higher intensity in the Test WC soil is due to the amendment of plastic sheets to determine the degradation. The presence of a weak O–H peak at 3600 cm−1 indicates the formation of water molecules by the bacterial strain or due to the moisture present in the soil sample. Thus, the increase in the peak intensity in the FTIR and increase in alkane formation as observed in the GC–MS confirms the cleavage of the polymeric chain. The degradation results also show the occurrence of oxidation at a slower rate as the presence of ether, aldehyde and acid formed in the degraded sheet spectrum is much less in comparison with the presence of alkanes. This might be due to the terminal oxidation which initiated the cleavage of the polymeric chain.
Isolation of LDPE degrading bacterial strains
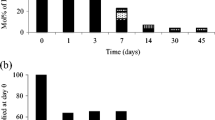
The isolated bacterial strains were grown on MSM agar containing LDPE. Among the isolated bacterial strains HB1KVG (S.homonis), HB2KVG, HB3KVG (L.massiliensis), KB4KVG (E. coli) and A5KVG (P. stutzeri). resulted in consistent growth on the culture plate while other bacterial strains showed minimum growth after subculture. The bacterial strain HB1KVG resulted in maximum weight loss percentage of 4.70 ± 0.45% followed by HB2KVG (2.32 ± 0.30%), A5KVG (1.27 ± 0.36%), KB4KVG (1.00 ± 0.51%) and HB3KVG resulted in minimum degradation of 0.62 ± 0.04% (Fig. 4). One-way ANOVA indicated that there is no statistically significant difference among the degradation performed using five bacterial strains at the end of 60 days of incubation (p-value < 0.05). The Tukey HSD test confirmed the significant effect of individual bacterial strains in LDPE degradation. The post hoc comparison of the LDPE degradation using individual bacterial strains is given in Table 2.
GC–MS analysis of bacterial degraded LDPE
The GC–MS chromatogram of biodegraded LDPE sheets was analyzed for metabolite formation. The LDPE sheets degraded by HB1KVG, HB2KVG, HB3KVG, KB4KVG and A5KVG bacterial strains were analyzed at a 15-day interval till day 60 (Table S2, S3, S4, S6 and S6) and Fig. 4.
LDPE bacterial degradation pathway prediction–a hypothesis
The LDPE sheet degraded by the HB1KVG bacterial strain resulted in the formation of smaller alkanes, which, on dehydrogenation, formed alkenes. The fragmented alkanes and alkenes oxidized to form alcohols. This might be due to the action of the Alkane hydroxylase enzyme produced by the bacterium during biodegradation. Simultaneously, the LDPE polymeric chain fragments underwent cyclization and aromatization to form aromatic hydrocarbons such as naphthalene and toluene—the aromatic compounds on oxidation formed alcohols (Fig. 5a).
Figure 5b summarizes the hypothesized LDPE biodegradation pathway by the HB2KVG bacterial strain. The degradation was initiated by the fragmentation of the LDPE sheet to form more minor alkane chain compounds.
The biodegradation pathway of LDPE by HB3KVG bacterial strain was hypothesized, as given in Fig. 5c. The degradation was initiated by the fragmentation of the LDPE polymeric chain into alkanes (small-chain compounds). The fragmented alkane undergoes dehydrogenation to form alkenes, which, on oxidation, form alcohols. LDPE, on degradation, forms aromatic compounds by aromatization process, which, on further oxidation using alcohol dehydrogenase, forms alcohols. The hydroxy compounds react with Phthalic acid to form esters via the esterification process at the end of the 60-day batch study. Similar trend was observed in LDPE degraded by HB1KVG, HB2KVG and HB3KVG bacterial strains.
The LDPE biodegradation pathway of the KBKVG4 bacterial strain was hypothesized to involve fragmentation to small alkanes, which, on further oxidation, formed alcohols (Fig. 5d). The polymeric chain also underwent an aromatization reaction to form aromatic hydrocarbon, which, on further oxidation, formed alcohols. The formation of alcohols might be aided by the monooxygenase enzyme produced by the bacterial strain. Further, the alcoholic compounds and the phthalic acid produced on further condensation reactions form esters via esterification reaction as the final degradation metabolites. A similar degradation pattern was observed in the LDPE degradation using the ABKVG5 bacterial strain (Fig. 5e).
Discussion
Plastic treatment processes are the need of the hour and multiple treatment techniques were analyzed by researchers to determine their efficiency. Biodegradation, a natural process of pollutant degradation has been focused recently to identify the occurrence of degradation. Even though, the literature suggests LDPE biodegradation using bacteria, algae, fungi, and other organisms, the process of occurrence is still a void. Many reports suggested the occurrence of biodegradation using weight loss %, FTIR, and SEM analysis, but the pathway of occurrence is still unclear. This study is a step forward in understanding the breakdown of the LDPE polymeric chain using the biodegradation process (Winogradsky column and bacterial strains). Hence, the current study is one of a kind that hypothesizes the LDPE degradation pathway as an overview. Winogradsky column by Sridharan et al. (2021a, b) was the first to report it as a tool for LDPE biodegradation. This current study provides a view of the process of LDPE polymeric chain cleavage using GC–MS analysis. Based on the mass spectrum of control LDPE, the fragmentation of LDPE polymeric chain to octacosane (C28H58), hexatriacontane (C24H50), 2-methyl tricosane (C24H50), 2,6,10,14-tetramethyl heptadecane (C21H44), and nonadecane (C19H40). The absence of alcohols, ketones, and carboxylic acid formation confirms that the polymeric chain was cleaved by the fragmentation process.
LDPE biodegradation using bacterial strains shows the formation of a lesser carbon chain on degradation. The non-degraded LDPE on GC–MS analysis resulted in the presence of a carbon chain from C8 to C35, after 15 days of degradation, the chain length ranged between C15–C22. At the end of 30 and 45 days of degradation, the carbon chains formed small compounds whose chain length ranged between C10–C21. After 60 days of incubation, the esters (C14–C24) were formed by the esterification process carried by the bacterial strains which could be due to the activity of esterases produced by bacteria during the degradation process. A recent report by Elsamahy et al. (2023) shows the results of biodegradation of LDPE using a yeast consortium isolated from termites. The consortium was reported to contain Sterigmatomyces halophilus, Meyerozyma guilliermondii, and Meyerozyma caribbica which utilized LDPE as a carbon source. Ritu et al. (2022) reported the biodegradation of LDPE using B. licheniformis isolated from waste dumpsite in Haryana. They reported that the isolate is efficient in consuming LDPE as a carbon source for 30 days. The GC–MS of the control was reported to contain ketones, carboxylic acids, and esters while the treated LDPE produced metabolites such as carboxylic acids and esters as new peaks in comparison with the control chromatogram. They reported further degradation of the carboxylic acids resulted in the formation of alkanes (lower and higher). Kyaw et al. (2012) studied LDPE film biodegradation using P. aeruginosa which is reputed in the presence of compounds containing functional groups such as esters, hydrocarbons, aldehydes, ether, ketones, oxygenated chemical compounds, fatty acids and other unreported compounds.
In this study, the overview degradation mechanism of LDPE was hypothesized, which confirms that the degradation pattern of LDPE using Winogradsky column and bacterial strains is unique. Despite a similar pathway mechanism followed by bacterial strains, reactions such as alkenes formation by dehydrogenation and cyclization of the linear polymeric chain unfold a new process of degradation. The current study does not emphasize enzymes involved in LDPE biodegradation, as the bacterial strains were not previously reported in the degradation of plastics and are yet to be studied in detail. The generally reported mechanism of polyethene biodegradation involves fragmentation and assimilation of the fragmented compounds by the microbes (Montazer, Habibi Najafi, and Levin 2019). There are also reports which suggest that microorganisms consume linear n-alkanes efficiently in lesser duration compared to branched alkanes (El-Shafei et al. 1998). Yoon et al. (2012) reported the degradation of linear n-alkane (such as hexadecane) as a model compound. The biodegradation was reported to be initiated by C–C bond hydroxylation which results in the production of alcohols (primary and secondary), and aldehydes, ketones and carboxylic acids on further oxidation. The generated carboxylated n-alkanes are compounds analogous to fatty acids which could be catabolized further by the beta-oxidation pathway (Jeon and Kim 2015; Yoon et al. 2012).
Conclusion
The research focuses on the degradation of LDPE using bacterial strains isolated from dumpsite soils and the degradation pathway mechanism prediction was performed based on the GC–MS chromatograms. This research holds a novel idea to utilize the indigenous microbes in plastic degradation. The application of this research provides and expands the scope of research in various fields such as bioinformatics, mathematics, and microbiology. The Winogradsky column is also used to understand the microbial diversity of soil in specific sites which does not require expertise to construct the column. The column was used as a tool to perform LDPE degradation. The enrichment of the indigenous microbes in the column enhances the process of degradation resulting in the formation of less complex metabolites compared to the parent polymeric compound. The oxidation of the terminal carbon in the polymeric chain aids in the degradation of LDPE. This study provides a scope to explore indigenous microbes in the biodegradation process. The Winogradsky column could also be utilized to treat various pollutants in the environment. The research widens the future scope to identify detailed pathways concerning molecular analysis by understanding the genetic makeup of the bacterial strains. Hence, the research provides a new perspective and lays a foundation for understanding the changes in the degradation pathway among various bacterial strains. The pathways predicted should be examined with caution and additional experiments are needed to validate the hypothesis in the near future.
References
Afreen B, Nouman Rasoo NR, Saima I (2020) Characterization of plastic degrading bacteria isolated from landfill sites. Intern J Clinic Microbiol BiochemTechnol. https://doi.org/10.29328/journal.ijcmbt.1001013
Ali S, Rehman A, Hussain SZ, Bukhari DA (2023) Characterization of plastic degrading bacteria isolated from sewage wastewater. Saudi J Biol Sci 30(5):103628
Ali, Mukhtiar, Dong Xu, Xuan Yang, and Jiangyong Hu. 2024. “Microplastics and PAHs mixed contamination an in-depth review on the sources, co-occurrence, and fate in marine ecosystems.” Water Research: 121622.
Ambika DK, Lakshmi B, Hemalatha K (2018) Degradation of low-density polythene by achromobacter denitrificans strain s1, a novel marine isolate. Int J Rec Sci Res 6(7):5454
Azeko ST, Etuk-Udo GA, Odusanya OS, Malatesta K, Anuku N, Soboyejo WO (2015) Biodegradation of linear low-density polyethylene by serratia marcescens subsp. marcescens and its cell-free extracts. Waste Biomass Valoriz 6:1047–1057
Cai L, Wang J, Peng J, Ziqing Wu, Tan X (2018) Observation of the degradation of three types of plastic pellets exposed to uv irradiation in three different environments. Sci Total Environ 628–629:740–747
Chen Q, Allgeier A, Yin D, Hollert H (2019) Leaching of endocrine disrupting chemicals from marine microplastics and mesoplastics under common life stress conditions. Environ Int 130:104938
Dworkin M (2012) Sergei Winogradsky: a founder of modern microbiology and the first microbial ecologist. FEMS Microbiol Rev 36(2):364–379
Elsamahy T, Sun J, Elsilk SE, Ali SS (2023) Biodegradation of low-density polyethylene plastic waste by a constructed tri-culture yeast consortium from wood-feeding termite: degradation mechanism and pathway. J Hazard Mater 448:130944
El-Shafei HA, Abd NH, El-Nasser AL, Kansoh, and Amal M. Ali. (1998) Biodegradation of disposable polyethylene by fungi and streptomyces species. Polym Degrad Stab 62(2):361–365
Gewert B, Plassmann MM, MacLeod M (2015) Pathways for degradation of plastic polymers floating in the marine environment. Environ Sci Process Impacts 17(9):1513–1521
Grassie, Norman, and Gerald Scott. 1988. Polymer Degradation and Stabilisation. CUP Archive.
Gu J-D (2003) Microbiological deterioration and degradation of synthetic polymeric materials: recent research advances. Int Biodeterior Biodegradation 52(2):69–91
Jebashalomi V, Charles PE, Rajaram R (2024) Microbial degradation of low-density polyethylene (LDPE) and polystyrene using Bacillus cereus (OR268710) isolated from plastic-polluted tropical coastal environment. Sci Total Environ 924:171580
Jeon HJ, Kim MN (2015) Functional analysis of alkane hydroxylase system derived from pseudomonas aeruginosa e7 for low molecular weight polyethylene biodegradation. Int Biodeterior Biodegradation 103:141–146
Koutny M, Lemaire J, Delort A-M (2006) Biodegradation of polyethylene films with prooxidant additives. Chemosphere 64(8):1243–1252
Kyaw BM, Champakalakshmi R, Sakharkar MK, Lim CS, Sakharkar KR (2012) Biodegradation of low-density polythene (LDPE) by pseudomonas species. Indian Microbiol 52(3):411–419
Lalla C, Calvaruso R, Dick S, Reyes-Prieto A (2021) Winogradsky columns as a strategy to study typically rare microbial eukaryotes. Eur J Protistol 80:125807
Montazer Z, Habibi MB, Najafi, and David B. Levin. (2019) Microbial degradation of low-density polyethylene and synthesis of polyhydroxyalkanoate polymers. Can J Microbiol 65(3):224–234
Montazer Zahra, Habibi Mohammad B, Najafi David B, Levin. (2020) Challenges with verifying microbial degradation of polyethylene. Polymers 12(1):123. https://doi.org/10.3390/polym12010123
Mooney A, Ward PG, O’Connor KE (2006) Microbial degradation of styrene: biochemistry, molecular genetics, and perspectives for biotechnological applications. Appl Microbiol Biotechnol 72(1):1
Ritu R, Jitender R, Poonam K, Pal SN, Rani SA (2022) Biodegradation and detoxification of low-density polyethylene by an indigenous strain bacillus Licheniformis SARR1. J Appl Biol Biotechnol 10(1):9–21
Shah AA, Hasan F, Hameed A, Ahmed S (2008) Biological degradation of plastics: a comprehensive review. Biotechnol Adv 26(3):246–265
Song YK, Hong SH, Jang Mi, Han GM, Jung SW, Shim WJ (2017) Combined effects of uv exposure duration and mechanical abrasion on microplastic fragmentation by polymer type. Environ Sci Technol 51(8):4368–4376
Sridharan R, Vetriselvan M, Krishnaswamy VG, Sagaya Jansi R, Rishin H, Thirumal Kumar D, Doss GP, C. (2021a) Integrated approach in LDPE degradation–an application using Winogradsky column, computational modeling, and pathway prediction. J Hazard Mater 412:125336
Sridharan R, Krishnaswamy VG, Senthil Kumar P (2021b) Analysis and microbial degradation of low-density polyethylene (Ldpe) in Winogradsky column. Environ Res 201:111646
Sridharan R, Sivamurugan V, Kumar P, Rangasamy G (2023) Degradation of LDPE using the winogradsky column containing otteri dumpsite soil: prediction of mechanism and metabolites determination. Current Anal Chem 19(6):489. https://doi.org/10.2174/1573411019666230719121536
Yao Z, Seong HJ, Jang Y-S (2022) Degradation of low-density polyethylene by bacillus species. Appl Biol Chem 65(1):1–9
Yoon M, Gyung HJ, Jeon MN, Kim, and Others. (2012) Biodegradation of polyethylene by a soil bacterium and alkb cloned recombinant cell. J Bioremed Biodegrad 3(4):1–8
Zalasiewicz J, Waters CN, Ivar di Sul JA, Corcoran PL, Barnosky AD, Cearreta A, Edgeworth M et al (2016) “The geological cycle of plastics and their use as a stratigraphic indicator of the anthropocene. Anthropocene 13:4–17
Zeenat AE, Bukhari DA, Shamim S, Rehman A (2021) Plastics degradation by microbes: a sustainable approach. J King Saud Univ - Sci 33(6):101538
Acknowledgements
Authors would like to thank Stella Maris College (Autonomous), Chennai, India for providing the research facilities and SEED funding to carry out this research work in time. We would like to acknowledge ICMR for providing financial support.
Author information
Authors and Affiliations
Contributions
Rajalakshmi Sridharan: Conceptualization; Investigation; Methodology; Validation; Writing original draft P. Senthil Kumar and Veena Gayathri K: Conceptualization; Investigation; Methodology; Supervision; Validation Gayathri Rangasamy: Conceptualization; Data curation; Formal analysis; Visualization.
Corresponding author
Additional information
Editorial responsibility: S. Mirkia.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sridharan, R., Kumar, P.S., Veenagayathri, K. et al. Pathway prediction of LDPE degradation using Winogradsky column and bacterial strains from municipal solid wastes. Int. J. Environ. Sci. Technol. (2024). https://doi.org/10.1007/s13762-024-05866-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13762-024-05866-4