Abstract
World produces 360 million tons of plastic every year, out of which only 7% plastic undergo recycling leaving majority of this waste lying around that get accumulated in the environment, thus posing a serious threat especially in the form of microplastics (MPs) to the large group of organisms on earth. Microplastics have been observed both in freshwater as well as in marine environment; however, recently, the occurrence of MPs in the fresh water that will ultimately lead to pollution in drinking water is emerging as one of the major concern for the scientific community. Till now, relative to the marine environment, the MPs pollution in the fresh water system is not very well understood and still an area of research that needs to be explored in detail. A detailed understanding of the origin, qualification, quantification, hazardous effects, etc. has be established to develop a proper waste management process for the MPs found in fresh water system. This review focuses on the sources, distribution, sampling methods, separation methods, methods of characterization and toxicological effects of microplastics in the freshwater environment. Various factors that affect the transportation and distribution of microplastics in fresh water system are reviewed. It is recommended that a standard protocol must be developed for identification of microplastics for scientific community across the world. The present study will help us to understand the source, transport, and effects of microplastics on the freshwater environment which would thereafter help to standardize the process for quantification and identification of microplastics in the freshwater environment. A combination of methods for the detection and prevention of microplastics in freshwater environment has also been suggested.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
After the discovery of the world’s first fully synthetic plastic in the form of Bakelite (American Chemical Society 1993), it has been an ideal material for a variety of uses (Andrady 2011). However, the increased production and wide use of plastic for several applications directly or indirectly have become a serious environmental problem. It is estimated that 8300 million metric tons of virgin plastic have been manufactured until 2015 and worldwide plastic production has reached 360 million metric tons per year in 2018 with an exponential tendency (Geyer et al. 2017; Plastics Europe 2019). Since 2014, nearly 300 million tons of plastic are being produced every year, 50% of which is single-use plastic (Plastics Europe 2019). According to a report, since 1950 to present, out of the total plastic produced to date, only 7% has been recycled (Geyer et al. 2017) (Scheme 1).
Plastics dumped in the environment has tendency to gradually decompose into smaller plastic particles with different sizes, among them the plastics formed in the range of the size from 0.1 μm to 5 mm are coined as MPs. The processes such as physical, chemical, and biological interactions, photo-degradation, and weathering action (Arthur et al. 2009; Barnes et al. 2009; Wagner et al. 2014) result in the degradation of the plastics into MPs particles. It was Thompson et al. 2004 who first realized the presence of MPs particles in the beach sediments and thereby coined the term MPs for the first time (Thompson et al. 2009; Browne 2015; Frias and Nash 2019; Xu et al. 2019). Although this was not the first time that small synthetic particles were reported (Carpenter and Smith 1972; Gregory 1977, 1978), MPs pallets have been reported in the marine ecosystem in 70s.
With each passing day, MPs pollution is increasing in marine, freshwater as well as terrestrial environments globally, because of (1) non-degradability which leads to accumulation (currently the only way to decompose plastic is by incineration) and (2) with the larger surface area other chemical pollutants and waterborne persistent organic pollutants (POPs) gets adsorbed on its surface (Mato et al. 2001; Hirai et al. 2011). It has been reported that (1) MPs are scattered everywhere and found ubiquitously, (2) sources of emission of MPs are heterogeneous, (3) MPs are being ingested by almost all organisms in one way or the other, and (4) impact of MPs on every organism and environment varies concerning the magnitude of pollution.
MPs contamination in the marine environment has been extensively studied in last 1 decade; however, for the fresh water contamination the studies are still limited. It has been observed that articles published for MP in marine environment is approximately six times higher than the articles reported for presence of MP contamination in fresh water. Since directly or indirectly fresh water system is the major source for the drinking water, therefore, proper studies must be carried out to investigate the MPs contamination in fresh water as well. Also, release of terrestrial MPs into the freshwater environment causes its transportation to the marine environment (Scheme 1) with the help of terrestrial ecosystem (Karbalaei et al. 2018), and if it is an isolated freshwater system (such as mountain lakes or ponds) it will stay there forever if not removed by any means and get degraded into micro- and nanosizes to pollute the whole ecosystem (Free et al. 2014).
With so much evidence for the presence of MPs in the freshwater system to date, there is no defined process for the monitoring of MPs in the freshwater system while most of the studies are focused on the marine environment (Browne et al. 2010; Cincinelli et al. 2017; Cózar et al. 2017). Given the above, we found that it is necessary to review the current research trends and methodologies for the detection of MPs, especially in the freshwater system. In this review, we have summarized: (1) magnitude of worldwide MPs pollution in freshwater environment along with the recent research associated with its occurrence, abundance, source and fate in freshwater environment; (2) sampling methods used in recent publications; (3) sample preparation and purification methods; (4) different identification and quantification techniques; (5) quality control, cross-contamination and pros and cons of avoiding cross-contamination; (6) toxicological effects of MPs; (7) MPs removal techniques from water; (8) interaction of MPs with emerging contaminants; (9) recommended control measures for the prevention of MPs pollution. Further, we have also recommended the suitable detection techniques for MPs in freshwater environment.
Microplastic sources in a freshwater environment
MPs are classified into two categories; primary MPs and secondary MPs and The presence of both primary as well as secondary MPs have been reported in freshwater (Browne et al. 2010; Vianello et al. 2013; Gall and Thompson 2015; Di and Wang 2018). The primary MPs are either manufactured plastics which are used as raw material (pellets, textile fiber, etc. (Fig. 1) used in textiles, medicines industries, construction industries, furniture industries, packaging industries) or plastics produced from the direct use in products (personal care products such as facial, body scrubs and microbeads for cosmetic (Fig. 1)) (Derraik 2002; Browne et al. 2010; Andrady 2011; Cole et al. 2011; Browne 2015; Driedger et al. 2015). This may be found nearby any freshwater system where human activities are prevalent. Secondary MPs are produced due to the physical and environmental degradation of the accumulated plastic in water bodies such as lakes and ponds (Andrady 2011, 2017; Cole et al. 2011). This accumulated plastic may undergo disintegration to form plastic particles to nano-scale, which possess a major threat to different exposed organisms (Free et al. 2014). These are also generated by other means such as tire erosion while driving, the release of synthetic textile fibers during laundry, road markings, city dust, marine coatings and so on (Gall and Thompson 2015; Lusher et al. 2015; Napper and Thompson 2016). Compared to primary MPs, a large amount of secondary MPs has been observed as major pollutant in water (Geyer et al. 2017; Talvitie et al. 2017b). Either plastic decomposes to release toxic chemicals that were initially used during manufacturing process or plastic adsorb these chemicals on its surfaces thus cause harm to the environment (Potthoff et al. 2017; Rios Mendoza et al. 2018).
A, B Microscopic images of the MPs particles (Baldwin et al. 2016). C Electron micrography image of the polyethylene beads extracted from a cosmetic product (Plymouth University, United Kingdom) (Katsnelson 2015). D Photographs of MPs obtained from the TGR, fiber, fragment, film, pellet, Styrofoam, and fading fiber (Di and Wang 2018)
Various studies have confirmed the presence of MPs in the areas where activities such as tourism and fishing are popular (Zhang et al. 2017; Wang and Wang 2018; Campanale et al. 2020; Li et al. 2020). Tourism leads to the disposal of single-use plastic such as water bottles, carry bags, straws, plastic sheets, and packaging plastic; this single-use plastic gets accumulated in isolated water bodies and disintegrates over time by weathering and temperature change. Activities like fishing lead to the disposal of the fishing net which is consumed by aquatic organisms. Free et al. (2014) reviewed that the prevalence of MPs in a freshwater environment mostly depends on human activities. Campanale et al. (2020) found MPs in Ofanto river of southeast Italy; they concluded that MPs pollution is mainly because of urbanization linked to seaside tourism and agricultural activities. One of the major sources of MPs entering domestic wastewater is by the washing of clothes that contains synthetic fiber (Browne 2015; Peng et al. 2017).When Hernandez et al. (2017) repeatedly did washing of clothes (containing synthetic fibers) in the washing machine by creating a household condition in the laboratory, they observed that the wastewater of the washing machine was containing a large number of fibers similar to MPs. According to a study conducted by Kalcikova and group (Kalčikova et al. 2017), an estimation around 100 ml of facial scrub can generate more than 1,300,000 particles. In a similar type of study (Carr et al. 2016), it was estimated that each toothpaste usage generates nearly 4000 polyethylene particles. Any form of plastic ends up either in the dumping yard or domestic/industrial waste ultimately reaches wastewater treatment plants and from there to the freshwater environment (Scheme 1).
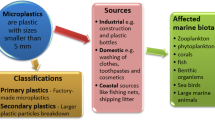
The major source of MPs pollution in freshwater environment is effluent from wastewater treatment plants which also includes domestic sewage (Magnusson and Norén 2014; Estahbanati and Fahrenfeld 2016; Murphy et al. 2016; Dyachenko et al. 2017; Mintenig et al. 2017). Although wastewater treatment plants can remove MPs (Talvitie et al. 2017b) and same particles (larger than 10 microns) can also be removed by tertiary treatment (Wardrop et al. 2016), even after this a large amount of MPs gets discharged into the freshwater system. It has been found that although the removal rate of MPs by the sewage treatment system is as high as 98%, everyday 65 million MPs still enter the water through sewage treatment facilities as stated by Carr and group (Carr et al. 2016) in his study. Bretas Alvim et al. (2020) stated that more than millions of MPs are released by municipal wastewater treatment plants per day. Several types of MPs sources are summarized in Fig. 2.
Rolsky et al. (2020) studied 14 articles related to MPs in sludge from wastewater treatment plants from twelve different countries and compared them; it was concluded that the Netherlands had the least count of 0.45 particles per gram, whereas Italy having the highest count of 113 particles per gram. Murphy et al. (2016) investigated a secondary wastewater treatment plant in Scotland and found that even with the efficiency of 98.41% for the removal of MPs, 6.5 × 107 particles per day are being discharged into the receiving water. These studies show that MPs originating from wastewater treatment plants can range from none to having concentrations as high as 9 × 104 particles/m3 in the effluent. The latest estimates place the total discharge of MPs from wastewater treatment plants at an average value of 2 × 106 particles/day (Sun et al. 2019), making wastewater treatment plants both a sink and a source of MPs. Interestingly, all these studies are mainly conducted in developed countries, having well-equipped facilities with the best wastewater management systems found to date. Developing countries and underdeveloped countries often lack proper wastewater management systems for all their freshwater systems; an abundance of MPs might be at a higher side even after considering a lower usage of plastic.
Further these studies also concluded that the major sources of MPs pollution in a freshwater environment are: (1) different human activities on the shoreline of rivers, lakes, ponds, etc. in terms of tourism, hydroponic activities, fishing, agriculture, urban movement (Free et al. 2014; Wang et al. 2018), (2) disposal of effluents from wastewater treatment plants (Cole et al. 2011; Browne 2015; Carr et al. 2016), (3) the presence of mining, chemical, etc. industries which leads to disposal of untreated wastewater into the water bodies (Zbyszewski and Corcoran 2011; Eriksen et al. 2013; Lechner et al. 2014), (4) disposal of untreated domestic sewage (which includes plastic particles in personal care products and domestic plastic) into the water bodies (Carr et al. 2016), (5) poor wastewater management nearby freshwater system (Free et al. 2014), (6) rural washing, (7) runoff from landfills and unmanaged waste dumps, (8) automotive tire wear, (9) weathering from construction sites, (10) sandblasting using polymer particles, (11) plastic film from agricultural processes, (12) stormwater and runoff, (13) atmospheric deposition, and (14) spillages and accidents.
The abundance, size, and type of polymers in any system represent the history, extent, causes of pollution in that system. For example, the presence of a mixed type of polymers indicates heavy human activities; the presence of single-use plastics indicates tourism activities (Retama et al. 2016). Similarly, the presence of a large amount of secondary MPs indicates the age of pollution. Urban river systems tend to be more polluted than non-urban systems. Phillips and Bonner (2015) found that in urban rivers, the most common type of MPs was film apart from other types such as fiber, pellets, and fragments. Water bodies close to densely populated or industrialized areas contain more MPs. Main river channels seem to accumulate plastic pollution and see higher concentrations of MPs than their tributary rivers. MPs concentration is also highly influenced by the weather system. Different types of MPs polymers used in various industries are summarized in Table 1.
MPs occurrence and abundance in fresh water system
MPs have been discovered across the globe from Asia to Antarctica via researchers in all types of mediums such as air, water, and soil (Wagner and Lambert 2018). It was found that from fish, biota or any other living organism to river bed sediments and surface water or drinking water, MPs have been detected in every stage of freshwater environment; though its distribution is very heterogeneous (Klein et al. 2018). Studies showed that MPs is more abundant in urban areas as compared to non-urban areas (where human activities are very less); however, MPs have also been observed even in remote areas such as mountain lakes (Free et al. 2014; Zhang et al. 2016). The abundance of MPs varies from zero to million pieces per cubic meter of water and this abundance depends on various factors such as sampling methods, the scale of sampling, least size consideration for sampling, site selection, human usage of the surrounding area (Eerkes-Medrano et al. 2015). MPs breakdown may be affected by several factors such as UV radiation, sunlight, atmospheric change, weathering, mechanical action, biofilm formation, hydration, the influence of additives such as anti-microbiological agents (Zhang et al. 2016; Wagner and Lambert 2018). Wei et al. (2020) reviewed that MPs in rural areas is very different from urban areas, they stated that it is more prevalent in remote rural areas. In a recent study (Deng et al. 2020) for China textile city, Shaoxing city, China Deng and group found the abundance of MPs ranging from 2.1 to 71.0 particles per liter in water and 16.7–1323.3 particles per kg (dry weight) in sediments which contained all kind of polymers. Mao further (Mao et al. 2020) found the presence of around 3.12 to 11.25 particles per liter in Wuliangsuhai lake, Bayannaoer city, and Inner Mongolia, and 0.3 to 1.9 particles per cubic meter in nine lakes, Argentine Patagonia and South America (Alfonso et al. 2020). Various studies conducted in freshwater systems of china have confirmed the presence of MPs in almost every investigated system with confirmation of every type of polymer with an abundance of zero to millions of particles per cubic meter. Similarly, various studies were conducted in Europe, the USA, Korea, and the presence of MPs in freshwater systems have been confirmed in various forms depending upon the location with an abundance of zero to a few thousand particles per cubic meter, the results have been summarized in Table 2 and Fig. 3.
Mapping of MPs occurrence in freshwater around the world (based on data in Table 2)
MPs sampling methods
A sampling of MPs depends on its distribution in the water column which is influenced by their physical properties, such as density, shape, size, adsorption of chemicals and biofouling, and by environmental conditions such as water density, wind, currents, and waves. Thus, the quantity and quality of MPs recovered are highly dependent on sampling location and depth. However, there is a large difference in the profile and types of MPs in every kind of environment, defined by different environmental factors.
Several studies have been performed on MPs in the last 2 decades (Table 3). However, there are no standard methodologies for the detection process including sampling, extraction, purification, identification, and quantification. There are significant variations in all the different studies which make it cumbersome to derive a standard methodology especially in the case of freshwater system.
Hidalgo-Ruz et al. (2012) stated that sampling in a freshwater system (for sediments and water surface) can be categorized as selective, bulk and volume reduced. In selective sampling, direct extraction of the particles from the environment is done which can be identified by naked eyes; this method is suitable in the case of sediments on the shoreline of freshwater only. In bulk sampling, the entire volume of the sample is taken without reducing it by any means such as sieving. Bulk sampling is used at places where visual identification of MPs is difficult, an abundance of small size MPs in the sample, the actual scenario of contamination is required. In volume-reduced sampling, target-based sampling is done so that only the required material can be sampled and the rest can be discarded. This type of sampling is suitable at places where the study area is very large such as the marine environment and large rivers such as the Ganges and Thames, as in the case of greater area; bulk sampling will not produce effective results.
In a bulk sampling of water, a bucket, drum, bottles, etc. are used, whereas in volume-reduced sampling, trawling with the help of nets (such as manta net and plankton net) and sieves of different sizes during sampling or after the sampling may be used. In volume-reduced water sampling, mainly trawling has been used along with different types of nets such as manta, neuston, plankton, and bongo, other than trawling some researchers have used pumps (Fig. 4) along with a sieve of different mesh size (50–100 µm) (Mintenig et al. 2017). In bulk water, sampling water has been collected using stainless steel buckets, bottles, and some other containers along with a mesh with an upper size limit (2000–5000 µm) for avoiding bigger size particles. In some studies, volume reduction has been done using sieves of different sizes after bulk water sampling; this serves two purposes, volume reduction and particle size distribution. In most of the studies, the volume of surface water sampled using pumps ranges from 12 to 35 L. In bulk water sampling, collection of a small volume of water does not provide strong results and is not reliable. Therefore, for reliable results, high volume of water may be required as it provides a detailed idea of contamination for the selected site. This can be achieved by defining the requirement of the minimum volume of sample for the reliability of results. The volume of water sampled for bulk sampling in the freshwater system has not been standardized; it varies from 5 to 100 L, and higher volume sampled leads to strong and reliable results.
Figure showing a mobile pumping system for sampling designed by Mintenig et al. (2017)
In most of the studies for volume-reduced sampling, plankton net (Estahbanati and Fahrenfeld 2016; Su et al. 2016; Campanale et al. 2020; Dahms et al. 2020), Manta Net (Fig. 5) (Eriksen et al. 2013; Zhang et al. 2015, 2017; Anderson et al. 2017; Sighicelli et al. 2018; Liu et al. 2020b; Park et al. 2020; Wong et al. 2020), and pumps (Wang et al. 2017b, 2018, 2019; Rodrigues et al. 2018; Bordós et al. 2019; Jiang et al. 2019) has been used, and for bulk samplings, grab samplers such as water bottles, beaker or any other storage unit (Barrows et al. 2018; Eo et al. 2019; Yuan et al. 2019; Grbić et al. 2020; Han et al. 2020; Mao et al. 2020; Nan et al. 2020; Wang et al. 2020; Yin et al. 2020). Bordos et al. (Bordós et al. 2019) stated that certain conditions make the plankton net unsuitable sampling equipment for inland freshwater system because infrastructure is required for example, for river sampling, a bridge at a suitable location is needed. Sometimes the water body is not big or deep enough for sampling by a manta net and even using a flow meter does not guarantee the accuracy of filtered quantity. To avoid these difficulties, they designed a special type of apparatus which comprised of a jet pump (PedrolloJCRm 2A) operated by an aggregator (Honda EU20i), a PVC hose along with a brass foot valve, a 2 mm mesh size strainer. The size of this apparatus enabled the sampling process from a small boat and even from the shoreline. This can be used at a depth of 10 to 20 cm by connected to the pump.
Different methods adopted for sample collection A. using a bridge crane and B. by wading, and C. using backpack sprayer for washing of particles from the net (Baldwin et al. 2016)
In most of the studies, size of the minimum size of the trawling net considered is 330 µm to 500 µm. This limits the least size of MPs to 330 µm which affects the abundance of MPs and does not provide reliable results because in the case of freshwater environment, UV weathering is dominant which leads to degradation of MPs into smaller size particles which may be less than 330 µm and it is evident that MPs particles were more abundant in cases where bulk sampling was done or least size was reduced to 20 µm.
Wang et al. (2018) used a 12 V DC Teflon pump, and filtered water through a stainless steel sieve of mesh size 50-μm and collected 10-L water sample in each sampling process and mean abundance of particles found was 2867 particles per cubic meter, whereas Wong et al. (2020) used a manta net of mesh size 300 µm along with a Hydro-Bios mechanical flowmeter for the measurement of the amount of water flowing through the net and mean abundance of particles found was 83.7 ± 70.8 and 2.5 ± 1.8 particles per cubic meter. From this comparison, it is evident that the smaller the size of particles considered for a collection, rich will be the abundance of particles. They stated that particles of smaller size may be ignored by trawl mesh which leads to an underestimation of particle abundance in the sample. Mesh size and apparatus used for sampling play a key role in defining the abundance and type of MPs (Hidalgo-Ruz et al. 2012).
Vermaire et al. (2017) stated that a 100-µm mesh revealed concentrations nearly 100 times higher than a 333-µm mesh. Dris et al. (2018) concluded that 80-μm mesh size net increases the probability of sampling particles by 250 times as compared to 330-μm mesh size net. Hidalgo-Ruz et al. (2012) stated that a 100,000 times higher concentration of plastic fibers can be handled by 80-μm mesh than a 450-μm mesh. This shows that the abundance of MPs is much underestimated in the studies where the trawling method is used for sample collection. He et al. (2020) stated that diverse sampling and sample processing methods make it inconvenient to compare MPs occurrence and abundance patterns.
Sample preparation and purification methods
Depending upon the sampling method used, samples collected from freshwater contain many natural impurities such as microorganisms, silt, algae, micro-plants, and traces of non-plastic impurities. These impurities may be classified as organic and inorganic impurities. A sample may contain either organic as well as inorganic impurities or either of them depending upon the source of the sample collected. The type of impurities present in the sample influences the choice of sample analysis process. Sample analysis may be divided into four parts comprising of extraction, purification/digestion, quantification, and polymer identification.
Sample extraction
The most common processes used for sample extraction are screening, density separation, elutriation, pressurized fluid extraction, plastic sediment separator, filtration, etc. The process of screening involves a series of sieves of different sizes depending upon the targeted range of MPs (Hidalgo-Ruz et al. 2012; Rocha-Santos and Duarte 2015). The size of sieves may vary from 4.75 mm to 150 µm (Hidalgo-Ruz et al. 2012). The material used for these screens may be copper or stainless steel and screens are made up of a filter membrane (Everaert et al. 2018). In this process, sample is passed through these sieves and particles collected on each sieve are processed for further examination of the sample. This method is also used for the size range analysis of the sample (Desforges et al. 2014). This method is mostly adopted where drinking water is examined and for the volumetric reduction along with size categorization of the sample (Mathalon and Hill 2014; Lambert and Wagner 2016; Wu et al. 2016).
Density separation uses the principle of density difference between two materials. In this process, a salt solution is prepared to have a higher density than targeted polymers. Particles with lighter density tend to float in a denser solution and impurities with higher density tend to settle in the solution and the salt solution works as a separating media for the MPs. In this process, the sample is mixed with a liquid of defined density (in the case of MPs a saturated salt solution) (Andrady 2011; Hidalgo-Ruz et al. 2012). The mixture of sample and liquid is then shaken and stirred for a preset time and then the suspension is kept undisturbed until the heavy impurities are settled and only MPs remain on the surface of the solution and then the supernatant containing the MPs is collected into some other container for further examination. The density of MPs particles depends upon the type of polymers and their manufacturing process. As mentioned in Table 1, densities of MPs vary from 0.85 to 1.45 gm/cc and the density of other sediments is nearly 2.65 gm per cc (Hidalgo-Ruz et al. 2012). Selection of liquid solution depends on the type of targeted polymers for detection. The most commonly used solution is a saturated NaCl solution (density 1.2 gm/cc) due to its low cost and non-toxicity for humans (Bordós et al. 2019; Campanale et al. 2020; Dahms et al. 2020; Liu et al. 2020b), however, there are denser MPs such as PVC, PET CA, and PTFE which cannot be recovered with this solution because of their density difference. Other solutions are having a higher density which may be used to improve extraction efficiency such as calcium chloride (CaCl2; density 1.3 g/cc) (Grbić et al. 2020), sodium polytungstate (SPT, density 1.5 g/cc) (Frias et al. 2018), sodium iodide solution (NaI, density 1.8 g/cc) (Kedzierski et al. 2017), and zinc chloride solution (ZnCl2, density 1.6 g/cc) (Stolte et al. 2015; Imhof et al. 2016; Retama et al. 2016; Rodrigues et al. 2018; He et al. 2020; Yin et al. 2020). Shaking time may vary from 30 s to 2 h and the rest time for settling of heavy particles after shaking may vary from 2 min to 6 h. Marine strategy framework directive (MSFD) and national oceanic and atmospheric administration (NOAA) US has recommended the use of NaCl for the density separation. The selection of the density separator solution depends upon its cost, toxicity, separation efficiency, and targeted polymers. The efficiency of zinc chloride and sodium iodide solutions is very high but both are very hazardous, costly, and must be handled with care as both are harmful to the environment (Frias et al. 2018). Thus, recycling and reuse of both the solution are necessary. It was found that CaCl2 causes interference in the final results which makes it unsuitable for further use as a density separator (Stolte et al. 2015).
There are other methods which had been used by various researchers for MPs extraction such as pressurized fluid extraction which has a very high efficiency and particles upto 50 µm can be recovered by this method; however, there are some drawbacks which include morphological changes after extraction and difficulty in determining size distribution (Fuller and Gautam 2016). Another method is elutriation which has high efficiency but cannot be used in samples containing organic matter (Claessens et al. 2013; Kedzierski et al. 2017; Hengstmann et al. 2018). In elutriation-selected liquid, for example, water is directed at the bottom of the column which separates buoyant MPs and other sediments tend to settle at the bottom of the column. One of the advantages of this method is that it is cheap and it has high efficiency while the disadvantage is that it requires at least one hour per sample and particle size range by sieving (Claessens et al. 2013; Hengstmann et al. 2018). Ivleva et al. (2017) extracted MPs using the approach of density separation in combination with fluidization and floatation. It was observed that the sodium chloride solution when used with fluidization and then floated in sodium iodide solution provided an efficiency of 99% (Nuelle et al. 2014). An instrument for sediment separation was developed named Munich plastic sediment separator, when used with zinc chloride solution yields an efficiency of 95% in recovering MPs but it can only be used for sediment samples; however, this instrument is very expensive (Imhof et al. 2013; Mintenig et al. 2017).
Sample purification/digestion
Biological material may be a part of the environmental sample which may interfere with results and during analysis; it may be confused with MPs particles. This makes it important to create a standard method for the digestion of organic matters without damaging MPs particles chemically and structure-wise. The requirement of the digestion of organic matter depends on the quantity present in the sample. If the sample contains a very small amount of organic matter then this step becomes irrelevant. However, if the identification of MPs is to be done by visual inspection then this step becomes necessary. Sample purification may be divided into two categories: chemical purification and enzymatic digestion (Stock et al. 2019). Chemical purification may further be divided into the purification by acids, oxidizing agents, and alkaline (Löder et al. 2017).
Acid digestion may be used for purification of the sample but various researchers have shown that MPs particles are susceptive to acids and acidic digestion may change the chemical and structural appearance of the polymers (Rocha-Santos and Duarte 2015). Cole et al. (2014) stated that hydrochloric acid at low concentration and room temperature is not sufficient for digestion of organic matter and may result in a greater quantity of organic matter after degradation; further, they conducted digestion with strong oxidizing acids such as nitric acid and sulfuric acids and concluded that it may have the higher efficiency of digestion but it destroys most of the polymer particles in the process. It is fact that some polymers are less resistive to acids at high concentrations with high temperatures but there should be an optimum condition at which biological degradation may be achieved with the least disturbance to the polymer particles in a considerable amount of time (Cole et al. 2014).
Naidoo et al. (Naidoo et al. 2017) studied the effects of HNO3 on the different polymers and concluded that it has a very small amount of effects on nylon and PVC at room temperature. It leaves oil residue and causes the melting of most of the polymers such as PP, PE, PET, and PS. It may be concluded that the effects of acid on polymer depend on the amount of organic matter present in the sample and the temperature at which the sample is treated. Thereafter, acidic digestion may be less effective in the case of MPs samples as it may cause disintegration of polymer particles and may lead to underestimation of MPs pollution in the targeted vicinity (Cole et al. 2014; Thiele et al. 2019).
Alkali digestion has been used in some of the studies as an alternative to acidic digestion but it may also damage the polymer particles chemically and structurally and similar to acidic digestion it may also leave oil residue, tissue residue, and bone fragments on MPs particles. Munno et al. (2018) studied the effects of chemical digestion methods at the different temperatures on MPs particles and concluded that potassium hydroxide (KOH) may be used for digestion as it is very effective in organic digestion and has higher MPs recovery rates. Dahms et al. (2020) used a 10% KOH solution at room temperature for 18 h and recovered MPs particles from water samples with very little damage to the polymer particles. In all the studies conducted it was observed that the use of the alkaline solution for the digestion may damage the polymer particles in one way or another such as color change, structural damage, and degradation, and if used in less concentration, it may not be effective for digestion.
Oxidizing agents such as hydrogen peroxide (H2O2) have been used by various researchers in different concentration. It has been reported that usage of H2O2 with a concentration in the range of 30–35% has been more efficient as compared to the acidic and alkaline digestion with nearly no harm to polymer particles (Su et al. 2016; Wang et al. 2017a, 2019; Cheung et al. 2019; Deng et al. 2020; Han et al. 2020; Liu et al. 2020a; Mao et al. 2020; Wong et al. 2020; Yin et al. 2020). This is the most popular approach used for the digestion of organic matter; however, some of the researchers have also used 30–35% H2O2 in combination with the ferrous solution as a catalyst. Nuelle et al. (Nuelle et al. 2014) stated that almost every plastic polymer is resistive H2O2 with only loss of colors in MPs particles. Karami et al. (Karami et al. 2017) used 35% H2O2 at 50 0C for 96 h and found that nylon was degraded and some color loss in PET. National Oceanic and Atmospheric Administration (NOOA) have recommended the use of 30% H2O2 with Fenton’s reagent [Fe (II) solution (0.05 M)] at 75 °C for both water and sediment samples. It may be concluded that temperature is a deciding factor in the organic digestion process without harming MPs particles. Thus, it may be concluded that H2O2 is the most efficient in organic digestion chemically with next to zero impact on MPs particles.
Enzymatic digestion is another emerging approach for the digestion of organic matter and it is less hazardous as compared to chemical digestion. It may also have very little impact on MPs particles. In this method, MPs samples are incubated along with different enzymes such as proteinase, cellulose, chitinase, lipase, and amylase. It was also reported that marine samples can be treated with the help of proteinase-K (Cole et al. 2014). The use of this method is very limited due to its high cost (Stock et al. 2019). Enzymes are being used on a small scale because some of their methods require further treatment of H2O2 for the removal of undigested debris. It has not been used by many researchers for the digestion of freshwater samples.
There are some other methods such as ultra-sonication in a combination of sodium dodecyl sulfate solution or deionized water but it has been observed that it may produce smaller MPs particles by the disintegration of brittle MPs samples (Cooper and Corcoran 2010; Enders et al. 2015; Mintenig et al. 2017).The use of microwaves is another digestion method but it may also damage MPs particles (Karlsson et al. 2017).
MPs identification and quantification techniques
After pretreatment of water sample containing MPs, the sample is filtered using vacuum filtration on the selected filter/petri dish and stored for drying of the sample. The MPs analysis comprises a four-dimensional challenge (1) the least size of MPs particles, (2) chemical composition of particle, (3) shape of every particle within a sample, and (4) abundance of any type of polymer particles in the sample (Hale 2017). The selection of filter material depends on the identification process to be used for the sample. Identification processes have been divided into four methods as shown in Fig. 6.
In most of the studies, reviewed identification and quantification of MPs are initially done by visual sorting directly or using a scanning electron microscope followed by chemical analysis of sorted particles using either spectroscopy or chromatography.
Visual sorting
It is done by reviewing filtered samples directly or using a microscope/stereoscope to sort the suspected MPs particles for further identification and analysis. Visual sorting of MPs is done based on the different physical characteristics for plastic polymers defined by various researchers such as particle size, shape, and color; however, visual sorting may be time-consuming and may provide misleading results due to the presence of similar particles from the organic and inorganic origin (Hidalgo-Ruz et al. 2012). The presence of such particles may lead to overestimation/underestimation depending upon the experience and knowledge of the individual conducting visual sorting. If the purification process mentioned above is not adopted properly or adopted at all then any organic/inorganic particle of similar shape and size may be confused with MPs particle; for example, some biologic material may be confused with black fragments. For further confirmation of confused particles, any additional approach may be required such as spectroscopy or chromatography. It has been observed that without any additional approach there is a large variation in the final results.
MPs are classified into four morphotypes: film, fiber, pellet, and fragment (Thompson et al. 2004, 2009). A film is a very thin and small layer or maybe a piece of bigger plastic debris; fiber is a MPs particle having a greatly elongated and slender appearance; pellets are round MPs particles having a spherical shape and every other type of particle is classified as a fragment when it cannot be termed as film, fiber or pellet. Han et al. (2020) stated that a particle should satisfy at least two criteria from the following mentioned points to avoid misidentification of particles: (1) any unnatural shapes for example a perfect sphere, (2) shiny or glass-like appearance of particle, (3) a homogeneous texture or material and whether the color of the particle is bright unnaturally, (4) particle does not have any organic structure or a visible cell, (5) absence of metallic luster, and (6) having fiber morphology (uniform diameter and 3D curvature). Norén 2007 stated various standardized criteria for the examination of MPs containing the following mentioned key points: (1) particles having any biological structure may be discarded for MPs, (2) particles having 3D structure may be considered as MPs, (3) homogeneously colored particles may be considered as MPs particles, and (4) particles transparent or whitish colored may be MPs particles and must be studied using fluorescence microscopy with high magnification. Li et al. (2018a) stated the drawbacks of the above-mentioned criteria and stressed that results of this method are largely affected because of various factors such as (1) quality of microscope, (2) individual’s factors for example carelessness, state of mind, fatigue, etc., (3) sample matrix, and (4) resolution of microscope limiting the size of particles to be counted. They observed an upto 70% rate of error in this method. Hidalgo-Ruz et al. (2012) mentioned that rate of error increases as the size of targeted particles decreases. Su et al. (2016) conducted a study for the presence of MPs in Taihu lake, china and analyzed the processed sample under a stereomicroscope and visually identified 1805 particles as per the physical characteristics of particles and randomly 113 particles were verified using micro-FTIR and 81 particles were confirmed MPs and rest were identified as non-plastic; this example can be seen as the case of overestimation of MPs and it becomes necessary to use chromatography/spectroscopy along with visual sorting method; however, visual sorting classifies MPs based on size, color, and shape which makes it easy to understand its origin.
The tagging method using staining dye
It was developed to make visual sorting convenient, in this method, a staining dye is used as a tool to label MPs particles fluorescently, and then these tagged particles can be visually identified using any microscope/stereoscope. Shim et al. (2016, Maes et al. (2017) and Tamminga (2017) demonstrated the use of staining method by dissolving Nile red dye in methanol with a concentration of 1 µg per milliliter and dying MPs particles (size range 20–1000 µm) using this solution; they analyzed these particles using microscope along with a LED for fluorescence. It provided very high recovery rates (nearly 95%). Highly hydrophobic MPs particles can be stained by Nile red molecules and Nile red becomes fluorescent in the presence of a hydrophobic environment; this makes hydrophobic MPs visible under a fluorescent microscope and may be counted by visual screening. This method may be useful for a large sample, which can be used for counting MPs particles in abundance. The tagging method is inexpensive, can be done with easily available equipment and it can be converted into semi-automation for getting throughput analysis of the sample. There may be some drawbacks of this method: (1) it requires a thorough pretreatment step since it may also stain any organic matter if present in the sample, hence, only this method cannot be used for visual sorting unless the sample is free of organic matter, (2) fluorescence signal is a variable of solvent polarity as Nile red dye is solvatochromic, (3) it may emit a weak fluorescent signal for some of the plastic particles such as PET, PVC, PUR, and PC. (4) Staining fibers and microfibers are very difficult. A failed use of some other staining methods has been reported such as Rose Bengal, Eosin B, oil red EGN, and Hostasol Yellow 3G as it becomes very difficult to stain plastic particles with other mentioned dyes (Maes et al. 2017).
Spectroscopy
It is one of the most commonly reported techniques; this can be done by either of the two methods which are Fourier transform infrared spectroscopy (FTIR) and Raman spectroscopy. Both these methods are vibrational spectroscopy methods, highly accurate, used for non-destructive analysis of samples, and provide spectra based on the interaction of the light beam with the targeted particles. In this method, MPs particles are exited using a light source with a specific wavelength and MPs particles achieve vibrations specific to their structures. This produces a spectrum with defined characteristics which is then compared with the fingerprint range available and it enables one to identify the nature of the material (plastic or non-plastic) also identification of polymer is done by comparing the reference spectrum and the obtained spectrum of the sample. Marine strategy framework directive (MSFD) technical subgroup has recommended that 10% MPs particles of size range 100–5000 µm and all the particles in the size lesser than 100 µm may be analyzed with any of these methods. Renner et al. (2018) stated that spectroscopic methods are most popular for chemical identification as these have been applied in more than 90% of the studies.
FTIR spectroscopy applies Fourier transform (a mathematical equation) for translation of raw data known as interferogram into the actual spectrum. It offers the possibility of identifying plastic particles based on their characteristic infrared spectra accurately (Reddy et al. 2006; Frias et al. 2010; Harrison et al. 2012; Vianello et al. 2013). FTIR mainly consists of two measurement modes which are transmittance and reflectance and both can be used for the detection of MPs particles each with different advantages and disadvantages. FTIR is an absorption-based spectroscopy method. In FTIR spectroscopy sample is subjected to an infrared light beam of defined wavelength range and this infrared light is absorbed by sample particles which enable the change in the permanent dipole moment of the chemical bond of the targeted sample particles; this is also a prerequisite condition which makes its use limited to the molecules having polar functional groups, for example, hydroxyl, carbonyl. The absorption of the infrared light is specific to the structure of the molecules. In FTIR spectroscopy, molecular vibration is excited by infrared radiation while interacting with the sample, these molecular vibrations highly depend on the molecular structure of the particles and have a very specific wavelength. The energy of infrared radiation exciting a specific molecular vibration is wavelength-specific which is absorbed by the molecule in a certain amount and this provides the characteristic IR spectra of the molecule. MPs polymers have a very specific infrared spectrum along with a distinct band pattern (Fig. 7) which makes the FTIR method very suitable for the identification of MPs (Hidalgo-Ruz et al. 2012). Different ranges of FTIR spectroscopy have been developed recently which are ATR-FTIR (attenuated total reflectance), Transmission FTIR, Reflection FTIR, and Micro-FTIR. ATR-FTIR is a spatial model of reflectance measurement and it requires contact of a crystal such as a diamond with the surface of the sample which implies that any sample with a hard surface may result in damaging the crystal. ATR-FTIR is used to characterize irregular shaped MPs particles along with opaque and thick particles. It may be used for the analysis of higher size particles > 500 µm because of the limitation of minimum contact area between sample and crystal required for analysis.
When a microscope is attached with FTIR, it is called µ-FTIR. Micro-FTIR is used for the analysis of smaller size particles upto 20 µm. It is a very good apparatus for getting a high-resolution map of the sample along with simultaneously mapping, visualizing, and collecting spectra. In micro-FTIR membrane filter can be used directly with only some preprocessing of the sample. Major drawbacks are (1) FTIR is very time-consuming, (2) it is difficult to detect particles less than 20 µm, (3) it is very effortful because it requires focusing on every particle, (4) since FTIR is used for the recording of transmission spectra of MPs particles directly on filters it requires IR transparent filters such as silica or aluminum oxide, (5) particles having non-transparency may be difficult to analyze using IR method, (6) this method requires the sample to be IR active, (7) instruments used for this method are expensive and requires skilled personnel for operation and analysis, and (8) IR active water must be removed from the sample before analysis.
Another recently developed FTIR method is the focal plane array detector along with FTIR imaging. It consists of a grid of detectors for example 32 × 32 which enables it to record simultaneously several thousand spectra. This facilitates the analysis of the entire sample on the filter simultaneously as compared to ATR-FTIR analyzing a single particle. Focal plane array detectors may be used in both transmittance and reflectance modes. This method is much faster as compared to single-particle FTIR but also more expensive and high processing power is required further it may analyze particles upto the size of 10 µm.
Raman spectroscopy is a scattering-based technique and it is proven and most effective for the identification of MPs. First, it was used in the mid-1980s for polymer identification and with time, it has advanced with great sensitivity increasing the ease of its usage in polymer identification and characterization. In this method, laser light having a single wavelength is directed towards the molecules for their excitation and a change in the polarity of the chemical bond is observed, this is also a prerequisite condition and it limits the use of this method to compounds having C–H, C=C double bonds and aromatic bonds. Each polymer comprises a unique and distinct set of vibrational modes that are used for chemical identification. If the MPs particle contains any other component or any foreign substance such as pigments or any other additive, it can be identified using Raman spectroscopy. A Raman spectrometer provides Raman spectra represented in the form of a graph showing relative wavenumber shift (in cm−1) on the x-axis and intensity on the y-axis (Fig. 8). Every quantized vibrational mode has different energy within the molecule, resulting in Raman spectra peak spanning anywhere from 0 to 4000 cm−1. Raman microscopy or µ-Raman spectroscopy is a spectroscopic method in which a microscope is coupled with a Raman spectrometer which enables us to collect spectra of distinct points from an image generated by the coupled microscope. This instrument is capable of analyzing the chemical and morphological properties of single particles like MPs. For MPs analysis, UV–Vis range lasers are used commonly which provides a spatial resolution of MPs particles in the micrometer range, providing a limiting size range. Certainty in the identification of polymers can be confirmed by comparing the recorded spectra with database spectra. Theoretically, a spatial resolution of 1 µm can be easily achieved by coupling optical microscope and Raman spectrometer; however, only Obmann et al. (2018) have reported the least size of 1 µm in his study, and Imhof et al. (2016) have reported a particle of diameter 5 µm. Filters used after purification of MPs should be low Raman background filter membranes; however, low Raman background filters are very expensive as compared to other filters for example a gold filter membrane is more than 10 times expensive as the cellulose filter membrane. Recent studies conducted using Raman microscope are summarized in Table 4.
The main drawbacks of this method are: (1) as stated earlier it is very expensive, (2) effort and time required for the processing and analysis of the sample, (3) availability of the equipment is very limited, (4) operations of the instrument and study of complex data requires an expert personal, (5) contaminants like biological residue may interfere with spectra which may result in producing a non-interpretable spectrum, (6) the automatic mapping of µ-Raman spectroscopy is yet to be developed, and (7) it is required to purify the sample before analysis.
Thermoanalytical methods
In pyrolysis–GC–MS and TGA-MS, the sample is thermally decomposed (pyrolyzed) with inert conditions and the gas is cryo trapped on the chromatographic column and is analyzed using a mass spectrometer, the recorded data are then compared with the reference data for information like polymer identity and its concentration (Dümichen et al. 2017; Eo et al. 2019; Campanale et al. 2020). This method may be used for the chemical characterization of a single MPs or a bulk sample. This method is destructive and it has a limitation of the size of MPs particles < 500 µm and this technique is not applicable for the samples having impurities with high concentration.
Another method is thermo-extraction and desorption coupled with GC–MS (TED-GC–MS) uses a combination of a thermo-gravimetric analysis (TGA) for thermal decomposition and solid-phase extraction for degradation of plastic products which are then analyzed using thermal desorption in GC–MS (for nearly 3 h) (Dümichen et al. 2017; Elert et al. 2017). The main advantage of this method over pyrolysis–GC–MS is that it can be used for relatively higher masses and it allows the measurement of complex heterogeneous matrices, which may result in the identification and quantification of MPs particles in the sample without any pre-selection.
Apart from gas chromatography, liquid chromatography also has been used for the quantification of MPs (Elert et al. 2017). In this method, an appropriate solvent is used for dissolving plastic polymers, for example, tetrahydrofuran for polystyrene (PS) and hexafluoroisopropanol for PET can be used as a dissolving agent and a polymer extract is prepared. This polymer extract is can be analyzes using size extrusion systems coupled with high-performance liquid chromatography (HPLC). This method has the advantage of analyzing relatively higher masses, provides improved representativeness, high recoveries, and can be used for quantification of MPs but this method is destructive, does not provide the size of MPs and it is yet to be applied to environmental samples.
Single-particle inductively coupled plasma mass spectrometry (ICP-MS) is one of the technique used recently by researchers for the MPs identification. It is mainly used for the detection and characterization of inorganic micro- and nanoparticles and its use for the analysis of carbon-based particles is very rare. In this method, the sample is ionized by the plasma, which results in breaking the particles to its atomic level enabling the measurement of its elements. Main application of this method is to analyze the trace metals; however, Bolea-Fernandez et al. (2020) and Laborda et al. (2021) developed a method by the detection of polystyrene microparticles in an aqueous solution of metal containing polystyrene beads by monitoring its 13C isotope at the dwell time of 50–100 µs. Bolea-Fernandez et al. (2020) analyzed the feasibility of detecting MPs particles using ICP-MS in single event mode. They stressed to use lower abundant 13 am nuclide at a low flow rate of 10 µL per minute to increase the signal to background ratio. Laborda et al. (2021) applied this method to screen MPs in personal care products and food packaging. Munier and Bendell (2018), Wijesekara et al. (2018) and Hildebrandt et al. (2020) used single-particle ICP-MS for the characterization of heavy metal contaminants on the surface of MPs particles since MPs plays a potential role in transportation of all kinds of contaminants (Primpke et al. 2020).
Pros and cons of avoiding cross-contamination
Cross-contamination is avoided to negate the false-positive results as MPs has been observed even in air. Due to cross-contamination MPs quantification might be overestimated and even any specific type of polymer might be falsely detected. Non-exclusion of cross-contamination may impact the conclusion of the work such as the sources of MPs pollution in selected vicinity, due to which the researcher may not be able to suggest an effective solution to MPs pollution in that area. Quantification and qualification results will not be reliable if suitable steps are not taken to avoid sample contamination due to the presence of MPs and similar elements in the testing environment which might cause interference in the results. A procedural blank allows understanding the extent of cross-contamination so that the same may be excluded from the consideration.
Quality control and avoiding cross-contamination
Due to the wide contamination of the environment and the presence of MPs in indoor environments, strict quality control measures should be taken to avoid contamination of samples even from the air (Dris et al. 2017). There are the following basic rules to avoid contamination of the sample, (1) use of metal and glass equipment in place of plastic equipment, (2) use of laminar flow hood for all the related laboratory works like sample processing and analysis, (3) surfaces should be cleaned using 70% ethanol and paper towel, (4) using filtered working solutions, (5) equipment should be washed with acid and ultrapure water, (6) 100% cotton lab coats should be preferred and use of synthetic textile may be avoided sample processing (Bergmann et al. 2017; Dris et al. 2017), (7) samples may be handled in a controlled air circulation room and sample at all times must be covered, (8) deionized water and any other salt solution may be used after filtration using a 20 µm pore size membrane filter in a fume hood (Torre et al. 2016; Wesch et al. 2017), (9) petri dishes may be used after a thorough cleaning and a check for the presence of MPs is possible, (10) all the working solutions must be prefiltered, (11) covering of all the samples using a seal lid or aluminum foil cap, and (12) a blank check test should always be performed at every stage to assess the degree of possible cross-contamination.
If these measures are not taken then the results may not be reliable as the results may show an increased amount of particle abundance due to cross-contamination which may be because of any of the reasons such as the use of plastic equipment and the presence of MPs particles in the water used for cleaning.
Interaction of MPs with emerging contaminants and its toxicological effects in freshwater environment
Presence of MPs is well established in the environment but apart from MPs there are other environmental contaminants such as heavy metals (arsenic, cadmium, lead, chromium, and mercury) and organic contaminants such as polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls phthalates (PCBs), polycyclic aromatic hydrocarbons (PAHs), and phenols. The major sources of these pollutants are different types of industries such as pharmaceutical, mining, petroleum, machinery, refining, textile, pesticide, and paper. Presence of these pollutants and MPs in the same environment may lead to their interaction by sorption and MPs particles may act as a spurious surface for the sorption, colonization of microbes (Zhao et al. 2020b) and other environmental contaminants. This combination of MPs with these environmental contaminants (such as arsenic, cadmium, lead, chromium, mercury, and persistent organic pollutants) may have a high toxicological effect to the freshwater environment (Fig. 9). Compared to the seriousness of this issue, there are very limited studies available with respect to the interaction of MPs and these contaminants. More research is needed in this area as MPs particles may act as a sink for the accumulation of other environmental contaminants.
Interaction of MPs with the contaminants depends upon its physiochemical properties (Scherer et al. 2017), which includes physical properties (such as shape, size, color, and density) (Frias et al. 2013; Song et al. 2014; Zhang 2017; Cheung et al. 2018; Naidu et al. 2018; Kokalj et al. 2018), chemical properties (such as Polarity, functional group, chemical stability, crystallinity, and surface charge) (Hung et al. 2010; Li et al. 2018b; Liu et al. 2019; Qiao et al. 2019), spatial distribution (Allen et al. 2019), temporal variation due to climate change (Herrera et al. 2018) and hydrophobicity of pollutants (Yu et al. 2019). These properties play a very critical role in determining the extent of interaction with the contaminants as bioavailability, sorption capacity of MPs and its consumption by other organisms is influenced by these properties. For example MPs of smaller size range have higher surface area which makes their bioavailability very high (Botterell et al. 2019; Yu et al. 2019) and highly toxic for the environment. Chen et al. (2020) stated that the bioavailability of MPs decides its influence on organisms. Thompson et al. (2004) concluded that the plastics can adsorb, transport and release chemicals into the environment, thus MPs becomes a pathway for the movement of other toxic chemical contaminants in the environment.
The nature of sorption may be categorized into two type, i.e., physical adsorption (Heinrich et al. 2020; Atugoda et al. 2020) and chemical adsorption (Zhang et al. 2018) which is defined by the degree of integration between the contaminants and MPs. In physical adsorption interaction between the two is defined by weak physical forces (Atugoda et al. 2020) which makes it reversible and chemical adsorption occurs in the presence of a strong chemical bond which makes it irreversible (Zhang et al. 2018). Various POPs (persistent organic pollutants) such as polyaromatic hydrocarbons, polychlorinated biphenyls, and hexachlorocyclohexane isomers have been found to be adsorbed on the MPs (Adjei et al. 2014; Yeo et al. 2017; Benson and Fred-Ahmadu 2020; Puckowski et al. 2021).
Zhao et al. (2020a) and coworkers reported that concentration of these emerging contaminants in MPs is hundred to thousands times higher as compared to their presence in the independent form. This combination of MPs and contaminants ingested by organisms may lead to accumulation of contaminants in a higher concentration, resulting in the increased toxicity of the MPs. Zhao and group (Zhao et al. 2020a) investigated the combined toxic effect of the BHA and MPs on zebrafish. They found that the toxicity concentration was enhanced in zebrafish larvae due to the combined effect of BHA and MPs. Hodson et al. (2017) and coworkers studied the combined effects of MPs and Zinc on the Lumbricus terrestris earthworms and they observed that the MPs may act as a vector which may increase the metal exposure in earthworms. Li et al. (2021) studied the adsorption behavior of tetrabromobisphenol-A on four types of MPs polymers (PE, PS, PP and PVC) in the presence of Ca2+ and humic acid. They observed that the sorption equilibrium of tetrabromobisphenol-A onto MPs could be achieved within 24 h. They also observed that Ca2+ acts as a catalyst for the sorption of tetrabromobisphenol-A on PE, PP and PS and humic acid had a negative effect on sorption behavior of tetrabromobisphenol-A (Li et al. 2021). Yu et al. (2020) and coworkers studied the adsorption process of tetrabromobisphenol-A on the pristine PE and microbeads, they observed that the sorption capacity of microbeads was fivefold higher than the pristine PE. They also studied their combined effect on zebrafish and it was observed that the combined effect was 3 times as compared to their individual effects.
Anderson et al. (2016) reviewed that there are different ways through which MPs toxicity may be classified: (1) physical damage such as clogging, intestinal blockage, cracking of villi and injury (Lei et al. 2018) due to ingestion and piling up of MPs within digestive tract, (2) leakage of pseudo feces and plastic additives such as plasticizers, (3) exposure of internal tissues and organs to MPs due to translocation inside the body, and (4) exposure to various adsorbed pollutants on MPs like persistent organic pollutants which is also known as mixture toxicity. Study of mixture toxicity may be classified based on its interaction approach with MPs. This approach may be classified into (1) additive effect, (2) synergistic effect, (3) antagonistic effect, and (4) potentiating effect. Therefore, it becomes an established fact that apart from having a direct impact on the freshwater environment MPs also has an indirect impact on the freshwater environment due to its chemical sorbing capacity which increases the health risk due to the higher toxicological effects in freshwater environment. Further, the possible adverse toxicological effect of MPs on human body is summarized in Fig. 9.
Methods for removal of MPs from water
Degradation using fungi
Russell et al. (2011) stated that fungi can be used for MPs degradation while MPs will act as nutrient for the fungi. They investigated the potential for the degradation PU MPs using Pestalotiopsis microspore under anaerobic conditions. Paço et al. (2017) used Zalerion maritimum (a marine fungi) for the degradation of PE MPs. They observed a positive correlation between the fungi weight gain and MPs weight loss with an efficiency of 43%. Sangale et al. (2019) used fungi for the degradation of polythene isolated from 12 different location along the west coast of India. They stated that the most efficient fungal isolates for the polythane degradation are MANGF1/WL (Aspergillus terreus strain) and PNPF15/TS (Aspergillus sydowii strain). Zhang et al. (2020) degraded PE MPs using the fungus named PEDX3 isolated from honeycomb or wax moth. It can be summarized that MPs can be degraded using the fungi; however, it requires a controlled surroundings and enzymatic presence. Due to slower reaction rate, plastic degradation takes a long time which may be reduced by various pretreatments such as photo oxidation, hydrolysis, and alcoholysis.
Using membrane-based techniques
For traditional drinking water treatment, processes used are sedimentation, coagulation, clarification, sand filters, skimming and various advanced tertiary treatment processes but none of them is solely responsible for MPs removal. Pivokonsky et al. (2018) stated that due to the similarities between the physical properties of suspended solid matter and MPs, signification amount of MPs can be reduced after filtration. It was observed that MPs enters the freshwater environment through the sludge disposal. Moslehyani et al. (2019) stated that the ultrafiltration process can be used as a feasible method for the water treatment. In this process, a U/f membrane of pore sizes 1–100 nm at low pressure. It has the capacity to filter microparticle of any composition from water in an economic manner. Talvitie et al. (2017a) stated that MPs can be removed from water efficiently using membranes along with some advantages such as ease of treatment and better effluent quality. Baiwen Ma et al. (2019) studied the effects of an iron-based coagulant along with ultrafiltration for the removal of PE. They observed a removal efficiency of 15%, but when they added Polyacrylamide for better coagulation removal efficiency was increased from 13 to 91% (Fig. 10). In a study reported by Li et al. (2018c), MPs was removed from synthetic wastewater using a dynamic membrane. It was observed that a better filtration of MPs can be achieved using dynamic membranes if the turbidity of influent is decreased from 195 NTU to 1 for the effluent (Horton and Dixon 2018). Lares et al. (2018) stated that membrane bioreactors have a better removal efficiency of MPs as compared dynamic membranes. Membrane techniques have a higher efficiency for MPs removal; however, they have a severe disadvantage of membrane fouling. An efficient strategy is required to deal with this problem of fouling. In a study conducted by (Enfrin et al. 2019) it was observed that during the treatment process various micro- and nanoparticles along with MPs are accumulated on the membrane surface thereby reducing the size of pores of membrane resulting in the clogging. This clogging results in higher energy consumption, reduced water flow, higher operation and maintenance cost, reduced efficiency of filtration.
Removal of MPs from drinking water using coagulation, sedimentation and ultrafiltration with Fe-based coagulants as described by Ma et al. (2019)
Magnetic extraction methods
In this method, magnetic seeds like iron nanoparticles and acid is used along with an external magnetic field for the improvement of separation speed. Iron-based nanoparticles were used because of its low cost availability, higher specific surface area and ferromagnetic properties. Coating of hexadecyltrimethoxysilane was used to ensure the hydrophobicity of nanoparticles (Grbic et al. 2019). Grbic et al. (2019) recovered medium sized (200 μm–1 mm) MPs from freshwater and sediments (84 and 78%, respectively). This method is suitable for small size particles; however, the presence of organic impurities and soil particles may damage the nanoparticles thereby reducing the efficiency of MPs removal, therefore, it may be recommended that this method is more suitable for the drinking water treatment (Grbic et al. 2019; Shen et al. 2020; Sun et al. 2020).
Electrocoagulation
It is an ecofriendly tertiary water treatment process in which metal electrodes are used to produce cations under the action of electric field. This process consists of three consecutive stages: (a) generation of electrons in the anode for the formation of hydroxides of Al3+ or Fe3+ also known as micro-coagulants, (b) due to these micro-coagulants suspended microparticles loses their stability, and (c) these suspended microparticles after collision to each other forms microflocs. Studies conducted by Millar et al. (2014) reported the use of this technique for the removal of other pollutants from water. The advantages of electrocoagulation are energy efficient, sludge minimization, economical (Zeboudji et al. 2013). In a recent study (Yavuz and Ögütveren 2018), it was observed that higher pollutant removal efficiency can be achieved in a higher neutral pH of water. Perren et al. (2018) studied the effects of water characteristics such as pH, conductivity, density, concentration and particle size of MPs on the removal efficiency of MPs under laboratory conditions (Fig. 11). It was observed that the electrocoagulation is very efficient for the removal of PE MPs with efficiency exceeding 90%. An optimal efficiency of 99.24% was achieved under the pH value of 7.5. It can be concluded that this method can efficiently remove MPs from water. This technique possesses the capacity to be converted from laboratory to industry because it is ecofriendly, economical and efficient.
Schematic diagram of bench-scale reactor set for removal of MPs from water using electrocoagulation by Perren et al. (2018)
Control measures to avoid microplastic pollution
The most reliable way to avoid MPs generation is to reduce the generation of MPs weather it is primary (pellets used for manufacturing) or secondary MPs (generate due to the force breakdown of primary products). The main pillars of MPs prevention are Reduce, Reuse, Recycling, Recover and Replace (Five Rs), Global action encouragement, Technological innovation and waste constraining. Picó and Barceló (2019) suggested the strategies to control MPs pollution is through a strong legislation, awareness programs for source control and by eliminating the MPs already present in the environment with the help of clean ups and remediation.
Pettipas et al. (2016) observed that various cleanup activities have been a part of mitigation strategy, which may help in reducing the force of MPs pollution in the environment. For limiting plastic pollution, Löhr et al. (2017) and Brennholt et al. (2018) suggested that the plastic litter should be reduced at source with a policy for better waste management. Schneider and Ragossnig (2015) stated that it is important to reduce the consumption of resources and energy along with limiting the release of harmful polymers which may be achieved by improving life cycle of plastic.
Plastic waste can be reduced at the production level be using some of the preventive measures (Thompson et al. 2009; Fendall and Sewell 2009; Liu et al. 2018; Bianchi et al. 2021) such as automation of plastic product designs for better life, reuse and recycling using life cycle assessment method; use of various alternative materials such as bio-based polymers (drop-in bioplastic) and curbing the production of single-use plastic.
Another way of reducing plastic waste is at consumer level. It can be achieved by creating awareness for avoiding the single-use plastic products such as packaging products, plastic water bottles, straws, plastic bags, cigarette butts, foam take-away containers, personal care products and their packaging tubes; this may lead to the plastic-free alternatives and may force the corporates to switch for alternatives and product redesigning.
It is a fact that every single-use product including food packaging and grocery packing save time and efforts but they are the root cause of the major waste produced on a daily basis by a consumer which also includes the packaging of these single-use products. It is also evident that there is always a leakage of single-use discarded products even in the most efficient waste management system. Banning these products is the most efficient way to reduce the generation of MPs. Convery et al. (2007) conducted a survey in Ireland related to taxes on plastic bags and concluded with a positive response from the costumers. This indicates that plastic production can be reduced by prohibition or by imposing a tax on easily replaceable plastic products such as single-use plastic and MPs in cosmetic products. Various international efforts have been made to reduce the plastic use by consumers. In 2018, the toy making company LEGO announced to replace petroleum-based material used for the manufacturing LEGO brick with the sustainable materials by 2030. In 2015, national games of India organized in Thiruvananthapuram a ban was imposed on disposable water bottles and usage of stainless steel tableware and flasks was witnessed avoiding the generation of nearly 120 tons of disposable waste (The Hindu 2015).
Plastic recycling is a very effective solution for the removal of plastic waste from the environment. Geyer et al. (2017) reported that since 1950, only 9% of total generated plastic have been recycled, and in 2015, around 20% of plastic was recycled. Plastic recycling is a tedious process which involves waste collection, particle sorting, washing to remove impurities, shredding and resizing into small pieces, identification and separation of plastics based on its polymer, color and compounding to produce the end product.
This tedious process increases the overall cost of plastic recycling as compared virgin plastic production, further the recycled products have very limited sets of application with a weak strength as compared to virgin plastic. In general, this industry has a huge scope of improvement for example the efficiency of combustion system should be improved for the complete usage of waste plastic heat avoiding the pollution due to VOCs (volatile organic compounds) and dioxins (Zhong and Li 2020).
Another way of controlling plastic pollution is to replace petroleum-based plastic with the commercially available biodegradable/biocompatible plastics such as polybutylene succinate and polylactatide. According to Bioplastics (2020), out of 360 million tones around 2.1 million tons of bioplastic is produced globally which is set to increase upto 2.8 million tons in 2025. Pico and coworker (Picó and Barceló 2019) divided bioplastic into three groups: (1) partially bio-based and non-biodegradable such as polytrimethylene terephthalate, bio PET, PP, (2) simultaneously biodegradable such as polybutylene succinate, polylactic acid, and (3) fossil-based biodegradable such as polycaprolactone diol. All these bioplastic are biodegradable but require specific conditions for their degradation which rarely occurs in nature.
Further, the best possible strategies which may be recommended for controlling the MPs pollution in the environment may include:
-
a)
Reducing the demand of plastic from consumers by
-
i)
Implementing a strict legislation having strict ban and heavy taxes on plastic products.
-
ii)
Creating awareness among consumers for the harmful effects of single-use plastic via education, labeling and by suggesting a better ecofriendly alternative.
-
iii)
Providing various tax incentive schemes and cash payments for the consumers for the usage of reusable products.
-
b)
Replacing the single-use products with reusable product.
-
c)
Promoting the use of recyclable products and their production.
-
d)
Recovering the already leaked MPs into the system by planning and implementation of waste management system including sludge filtration for MPs before its disposal, planning and execution of cleanup drives.
-
e)
Replacing the existing plastic products with the bioplastic products.
Challenges and recommendations
Studies on the presence of MPs in freshwater environment are very limited to a handful of countries (such as China, USA, Australia, Germany, France, Canada, UK, and Italy) as compared to marine environment, which leaves us with a very limited data. More elaborated studies are required involving the major countries to form a comparative database to determine the fate, behavior and effects of MPs in freshwater environment all round the world.
In this study, we have discussed various sampling, filtration, quantification and identification methods of MPs in freshwater environment and most of them are similar to that of marine environment. It is observed that a combination of methods provides more reliable results which also depends on the least size of the sample. If the sample size varies from 100 to 1000 µm a combination of microscopic and spectroscopy analysis may be recommended and if the size is in the range of 10–300 µm then the micro-Raman spectroscopy is preferred.
There is a need to develop a standard and robust mechanism for the quantification and identification of MPs and the same should be verified to provide a better, comparable and reliable data to the researchers anywhere in the world. These results will then help us understand the role of MPs as a pollutant in the environment scientifically and the associated risk with it to formulate the suitable rules, regulations and accords worldwide for their enforcement.
We have reviewed the interaction of MPs with the other environmental pollutants and it is observed due to sorption and formation of biofilm on the MPs it becomes very challenging to differentiate the MPs from the natural polymers using spectroscopy specially in case of smaller particles and there are very limited studies on the interaction of freshwater contaminants with MPs and their toxicological effects on aquatic organisms. Studies may be conducted to assess their long-term effects along with the mixture toxicity.
It is recommended to determine decomposition rate of different polymer types found in the freshwater environment so that a correlation between primary and secondary MPs may be established, which will help in identification of MPs sources in freshwater; it will result in development of prevention approaches for the release of MPs in freshwater ecosystem. Further, a MPs monitoring protocol (with respect to chemical composition) at the municipal corporation level must be developed in every country to monitor growth of MPs in different parts of freshwater environment such as bank sediments, bank water, deep waters, bed sediments, biota, and fish.
Conclusion
MPs are one of the major pollutants in the environment. MPs footprints have been found all around the world in the marine environment, freshwater environment, even in the air and it is of grave concern because of the lack of knowledge about its impact on the humans and environment.
It was ascertained by Hidalgo-Ruz et al. (2012) that the MPs samples of the freshwater environment have undergone various degradation processes due to long time exposure such as thermal degradation, mechanical degradation, biodegradation, and photo-degradation due to UV exposure due to which various properties of the MPs particles have been altered including surface morphology. Various microorganisms and micro-plants may form a biofilm on the surface of the small size MPs which makes it troublesome to differentiate plastic particles and natural polymers like cellulose.
In this review article, an attempt has been made to summarize the current scenario of MPs contamination in freshwater environments such as rivers, lakes, and ponds all around the world. Different MPs analysis methods used by researchers around the world were also reviewed.
A detailed study was conducted for the MPs sampling methods to provide in-depth knowledge of both the methods (bulk sampling and volume-reduced sampling) to the researchers for their usage as per the applicability. A variety of methods used by the researchers for MPs extraction (like density separation) and sample purification (like enzymatic digestion) were discussed along with their benefits and drawbacks. MPs qualification and quantification methods such as FTIR, Raman spectroscopy, Tagging method, and chromatography were discussed along with their advantages and disadvantages. Quantification and identification of MPs become arduous with the use of any single analytical method and it becomes easier with the use of a combination of any of the analytical methods depending on the size of MPs to be analyzed. For the analysis of MPs, the size range of 1 mm to 200 µm, a combination of microscopy and FTIR spectroscopy may be used and for the size 200 µm–1 µm or less use of Raman microscopy may be recommended. If the sample is having negligible impurities after digestion and pretreatment, any chromatography technique may be recommended.
We have recognized that it is strenuous to quantify the MPs in a freshwater environment with the help of a single method. Many researchers have established that a combination of different quantification methods may be beneficial in the case of a freshwater environment.
Methods for pretreatment, quantification, and qualification of MPs in freshwater should be standardized and a standard protocol must be developed for identification of MPs in freshwater so that it may become convenient for researchers to verify and analyze the data of different researches in this area. The present review will be important to understand the challenges in the MP studies along with the possible pathways to further strengthen our knowledge, which will help to find out sustainable solution to this growing problem of MPs contamination.
Abbreviations
- PU:
-
Polyurethane
- PC:
-
Polycarbonate
- PS:
-
Polystyrene
- PE:
-
Polyethylene
- PP:
-
Polypropylene
- PVC:
-
Polyvinyl chloride
- PET:
-
Polyethylene terephthalate
- PA:
-
Polyamide (nylon)
- PMMA:
-
Polymethyl methacrylate
- PTFE:
-
Polytetrafluoroethylene
- RIC:
-
Resin identification code
- Ρ:
-
Density
- MPs:
-
Microplastics
References
Adjei IM, Sharma B, Labhasetwar V (2014) Nanoparticles: cellular uptake and cytotoxicity. In: Capco DG, Chen Y (eds) Nanomaterial: impacts on cell biology and medicine. Springer, Dordrecht, pp 73–91
Alfonso MB, Scordo F, Seitz C et al (2020) First evidence of microplastics in nine lakes across Patagonia (South America). Sci Total Environ 733:139385. https://doi.org/10.1016/j.scitotenv.2020.139385
Allen S, Allen D, Phoenix VR et al (2019) Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat Geosci 12:339–344. https://doi.org/10.1038/s41561-019-0335-5
Alves VEN, Figueiredo GM (2019) Microplastic in the sediments of a highly eutrophic tropical estuary. Mar Pollut Bull 146:326–335. https://doi.org/10.1016/j.marpolbul.2019.06.042
American Chemical Society (1993) Bakelite: The World’s First Synthetic Plastic. A Natl Hist Chem Landmarks 1–3
Anderson JC, Park BJ, Palace VP (2016) Microplastics in aquatic environments: implications for Canadian ecosystems. Environ Pollut 218:269–280. https://doi.org/10.1016/j.envpol.2016.06.074
Anderson PJ, Warrack S, Langen V et al (2017) Microplastic contamination in Lake Winnipeg, Canada. Environ Pollut 225:223–231. https://doi.org/10.1016/j.envpol.2017.02.072
Andrady AL (2011) Microplastics in the marine environment. Mar Pollut Bull 62:1596–1605. https://doi.org/10.1016/j.marpolbul.2011.05.030
Andrady AL (2017) The plastic in microplastics: a review. Mar Pollut Bull 119:12–22. https://doi.org/10.1016/j.marpolbul.2017.01.082
Arthur C, Baker J, Bamford H (2009) Proceedings of the international research workshop on the occurrence, effects, and fate of microplastic marine debris. Group 530
Atugoda T, Wijesekara H, Werellagama DRIB et al (2020) Adsorptive interaction of antibiotic ciprofloxacin on polyethylene microplastics: implications for vector transport in water. Environ Technol Innov 19:100971. https://doi.org/10.1016/J.ETI.2020.100971
Bagaev A, Khatmullina L, Chubarenko I (2018) Anthropogenic microlitter in the Baltic Sea water column. Mar Pollut Bull 129:918–923. https://doi.org/10.1016/j.marpolbul.2017.10.049
Baldwin AK, Corsi SR, Mason SA (2016) Plastic debris in 29 great lakes tributaries: relations to watershed attributes and hydrology. Environ Sci Technol 50:10377–10385. https://doi.org/10.1021/acs.est.6b02917
Ballent A, Corcoran PL, Madden O et al (2016) Sources and sinks of microplastics in Canadian Lake Ontario nearshore, tributary and beach sediments. Mar Pollut Bull 110:383–395. https://doi.org/10.1016/j.marpolbul.2016.06.037
Barnes DKA, Galgani F, Thompson RC, Barlaz M (2009) Accumulation and fragmentation of plastic debris in global environments. Philos Trans R Soc B Biol Sci 364:1985–1998. https://doi.org/10.1098/rstb.2008.0205
Barrows APW, Christiansen KS, Bode ET, Hoellein TJ (2018) A watershed-scale, citizen science approach to quantifying microplastic concentration in a mixed land-use river. Water Res 147:382–392. https://doi.org/10.1016/j.watres.2018.10.013
Benson NU, Fred-Ahmadu OH (2020) Occurrence and distribution of microplastics-sorbed phthalic acid esters (PAEs) in coastal psammitic sediments of tropical Atlantic Ocean. Gulf of Guinea Sci Total Environ 730:139013. https://doi.org/10.1016/J.SCITOTENV.2020.139013
Bergmann M, Wirzberger V, Krumpen T et al (2017) High quantities of microplastic in arctic deep-sea sediments from the HAUSGARTEN observatory. Environ Sci Technol 51:11000–11010. https://doi.org/10.1021/acs.est.7b03331
Bianchi FR, Moreschi L, Gallo M et al (2021) Environmental analysis along the supply chain of dark, milk and white chocolate: a life cycle comparison. Int J Life Cycle Assess 26:807–821. https://doi.org/10.1007/s11367-020-01817-6
Bioplastics E (2020) Facts and figures
Blair RM, Waldron S, Phoenix VR, Gauchotte-Lindsay C (2019) Microscopy and elemental analysis characterisation of microplastics in sediment of a freshwater urban river in Scotland. UK Environ Sci Pollut Res. https://doi.org/10.1007/s11356-019-04678-1
Bolea-Fernandez E, Rua-Ibarz A, Velimirovic M et al (2020) Detection of microplastics using inductively coupled plasma-mass spectrometry (ICP-MS) operated in single-event mode. J Anal at Spectrom 35:455–460. https://doi.org/10.1039/c9ja00379g
Bordós G, Urbányi B, Micsinai A et al (2019) Identification of microplastics in fish ponds and natural freshwater environments of the Carpathian basin, Europe. Chemosphere 216:110–116. https://doi.org/10.1016/j.chemosphere.2018.10.110
Botterell ZLR, Beaumont N, Dorrington T et al (2019) Bioavailability and effects of microplastics on marine zooplankton: a review. Environ Pollut 245:98–110. https://doi.org/10.1016/J.ENVPOL.2018.10.065
Brennholt N, Heß M, Reifferscheid G (2018) Freshwater microplastics: challenges for regulation and management BT—freshwater microplastics: emerging environmental contaminants? In: Wagner M, Lambert S (eds) Springer International Publishing. Springer International Publishing, Cham, pp 239–272
Bretas Alvim C, Bes-Piá MA, Mendoza-Roca JA (2020) Separation and identification of microplastics from primary and secondary effluents and activated sludge from wastewater treatment plants. Chem Eng J 402:126293. https://doi.org/10.1016/j.cej.2020.126293
Browne MA (2015) Sources and Pathways of Microplastics to Habitats. In: Bergmann M, Gutow L, Klages M (eds) Marine anthropogenic litter. Springer International Publishing, Cham, pp 229–244
Browne MA, Galloway TS, Thompson RC (2010) Spatial patterns of plastic debris along estuarine shorelines. Environ Sci Technol 44:3404–3409. https://doi.org/10.1021/es903784e
Bruge A, Dhamelincourt M, Lanceleur L et al (2020) A first estimation of uncertainties related to microplastic sampling in rivers. Sci Total Environ 718:137319. https://doi.org/10.1016/j.scitotenv.2020.137319
Campanale C, Stock F, Massarelli C et al (2020) Microplastics and their possible sources: the example of Ofanto river in southeast Italy. Environ Pollut 258:113284. https://doi.org/10.1016/j.envpol.2019.113284
Carpenter EJ, Smith KL (1972) Plastics on the Sargasso sea surface. Science (80-) 80(175):1240–1241. https://doi.org/10.1126/science.175.4027.1240
Carr SA, Liu J, Tesoro AG (2016) Transport and fate of microplastic particles in wastewater treatment plants. Water Res 91:174–182. https://doi.org/10.1016/j.watres.2016.01.002
Castro RO, Silva ML, Marques MRC, de Araújo FV (2016) Evaluation of microplastics in Jurujuba Cove, Niterói, RJ, Brazil, an area of mussels farming. Mar Pollut Bull 110:555–558. https://doi.org/10.1016/j.marpolbul.2016.05.037
Chen G, Feng Q, Wang J (2020) Mini-review of microplastics in the atmosphere and their risks to humans. Sci Total Environ 703:135504
Cheung LTO, Lui CY, Fok L (2018) Microplastic contamination of wild and captive flathead grey mullet (Mugil cephalus). Int J Environ Res Public Health. https://doi.org/10.3390/ijerph15040597
Cheung PK, Hung PL, Fok L (2019) River microplastic contamination and dynamics upon a rainfall event in Hong Kong, China. Environ Process 6:253–264. https://doi.org/10.1007/s40710-018-0345-0
Cincinelli A, Scopetani C, Chelazzi D et al (2017) Microplastic in the surface waters of the Ross Sea (Antarctica): Occurrence, distribution and characterization by FTIR. Chemosphere 175:391–400. https://doi.org/10.1016/j.chemosphere.2017.02.024
Claessens M, Van Cauwenberghe L, Vandegehuchte MB, Janssen CR (2013) New techniques for the detection of microplastics in sediments and field collected organisms. Mar Pollut Bull 70:227–233. https://doi.org/10.1016/J.MARPOLBUL.2013.03.009
Cole M, Lindeque P, Halsband C, Galloway TS (2011) Microplastics as contaminants in the marine environment: a review. Mar Pollut Bull 62:2588–2597. https://doi.org/10.1016/j.marpolbul.2011.09.025
Cole M, Webb H, Lindeque PK et al (2014) Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci Rep 4:1–8. https://doi.org/10.1038/srep04528
Convery F, McDonnell S, Ferreira S (2007) The most popular tax in Europe? Lessons from the Irish plastic bags levy. Environ Resour Econ 38:1–11. https://doi.org/10.1007/s10640-006-9059-2
Cooper DA, Corcoran PL (2010) Effects of mechanical and chemical processes on the degradation of plastic beach debris on the island of Kauai. Hawaii Mar Pollut Bull 60:650–654. https://doi.org/10.1016/j.marpolbul.2009.12.026
Cózar A, Martí E, Duarte CM et al (2017) The Arctic Ocean as a dead end for floating plastics in the North Atlantic branch of the Thermohaline Circulation. Sci Adv 3:1–9. https://doi.org/10.1126/sciadv.1600582
Crawford CB, Quinn B (2017) Microplastic pollutants.
Cutroneo L, Reboa A, Besio G et al (2020) Microplastics in seawater: sampling strategies, laboratory methodologies, and identification techniques applied to port environment. Environ Sci Pollut Res 27:8938–8952. https://doi.org/10.1007/s11356-020-07783-8
Dahms HTJ, van Rensburg GJ, Greenfield R (2020) The microplastic profile of an urban African stream. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2020.138893
Deng H, Wei R, Luo W et al (2020) Microplastic pollution in water and sediment in a textile industrial area. Environ Pollut 258:113658. https://doi.org/10.1016/j.envpol.2019.113658
Derraik JGB (2002) The pollution of the marine environment by plastic debris: a review. Mar Pollut Bull 44:842–852. https://doi.org/10.1016/S0025-326X(02)00220-5
Desforges JPW, Galbraith M, Dangerfield N, Ross PS (2014) Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Mar Pollut Bull 79:94–99. https://doi.org/10.1016/J.MARPOLBUL.2013.12.035
Di M, Wang J (2018) Microplastics in surface waters and sediments of the Three Gorges Reservoir, China. Sci Total Environ 616–617:1620–1627. https://doi.org/10.1016/j.scitotenv.2017.10.150
Di M, Liu X, Wang W, Wang J (2019) Manuscript prepared for submission to environmental toxicology and pharmacology pollution in drinking water source areas: Microplastics in the Danjiangkou Reservoir, China. Environ Toxicol Pharmacol 65:82–89. https://doi.org/10.1016/j.etap.2018.12.009
Driedger AGJ, Dürr HH, Mitchell K, Van Cappellen P (2015) Plastic debris in the Laurentian Great Lakes: a review. J Great Lakes Res 41:9–19. https://doi.org/10.1016/j.jglr.2014.12.020
Dris R, Gasperi J, Mirande C et al (2017) A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ Pollut 221:453–458. https://doi.org/10.1016/j.envpol.2016.12.013
Dris R, Gasperi J, Rocher V, Tassin B (2018) Synthetic and non-synthetic anthropogenic fibers in a river under the impact of Paris Megacity: sampling methodological aspects and flux estimations. Sci Total Environ 618:157–164. https://doi.org/10.1016/j.scitotenv.2017.11.009
Dümichen E, Eisentraut P, Bannick CG et al (2017) Fast identification of microplastics in complex environmental samples by a thermal degradation method. Chemosphere 174:572–584. https://doi.org/10.1016/j.chemosphere.2017.02.010
Dyachenko A, Mitchell J, Arsem N (2017) Extraction and identification of microplastic particles from secondary wastewater treatment plant (WWTP) effluent. Anal Methods 9:1412–1418. https://doi.org/10.1039/c6ay02397e
Eerkes-Medrano D, Thompson RC, Aldridge DC (2015) Microplastics in freshwater systems: a review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res 75:63–82. https://doi.org/10.1016/j.watres.2015.02.012
Elert AM, Becker R, Duemichen E et al (2017) Comparison of different methods for MP detection: What can we learn from them, and why asking the right question before measurements matters? Environ Pollut 231:1256–1264. https://doi.org/10.1016/j.envpol.2017.08.074
Enders K, Lenz R, Stedmon CA, Nielsen TG (2015) Abundance, size and polymer composition of marine microplastics ≥10 μm in the Atlantic Ocean and their modelled vertical distribution. Mar Pollut Bull 100:70–81. https://doi.org/10.1016/j.marpolbul.2015.09.027
Enfrin M, Dumée LF, Lee J (2019) Nano/microplastics in water and wastewater treatment processes—origin, impact and potential solutions. Water Res 161:621–638. https://doi.org/10.1016/J.WATRES.2019.06.049
Eo S, Hong SH, Song YK et al (2019) Spatiotemporal distribution and annual load of microplastics in the Nakdong River, South Korea. Water Res 160:228–237. https://doi.org/10.1016/j.watres.2019.05.053
Eriksen M, Mason S, Wilson S et al (2013) Microplastic pollution in the surface waters of the Laurentian Great Lakes. Mar Pollut Bull 77:177–182. https://doi.org/10.1016/j.marpolbul.2013.10.007
Estahbanati S, Fahrenfeld NL (2016) Influence of wastewater treatment plant discharges on microplastic concentrations in surface water. Chemosphere 162:277–284. https://doi.org/10.1016/j.chemosphere.2016.07.083
Plastics Europe (2019) Plastics—the facts 2019
Everaert G, Van Cauwenberghe L, De Rijcke M et al (2018) Risk assessment of microplastics in the ocean: modelling approach and first conclusions. Environ Pollut 242:1930–1938. https://doi.org/10.1016/j.envpol.2018.07.069
Fendall LS, Sewell MA (2009) Contributing to marine pollution by washing your face: microplastics in facial cleansers. Mar Pollut Bull 58:1225–1228. https://doi.org/10.1016/j.marpolbul.2009.04.025
Fischer EK, Paglialonga L, Czech E, Tamminga M (2016) Microplastic pollution in lakes and lake shoreline sediments—a case study on Lake Bolsena and Lake Chiusi (central Italy). Environ Pollut 213:648–657. https://doi.org/10.1016/j.envpol.2016.03.012
Free CM, Jensen OP, Mason SA et al (2014) High-levels of microplastic pollution in a large, remote, mountain lake. Mar Pollut Bull 85:156–163. https://doi.org/10.1016/j.marpolbul.2014.06.001
Frias JPGL, Nash R (2019) Microplastics: Finding a consensus on the definition. Mar Pollut Bull 138:145–147. https://doi.org/10.1016/j.marpolbul.2018.11.022
Frias JPGL, Sobral P, Ferreira AM (2010) Organic pollutants in microplastics from two beaches of the Portuguese coast. Mar Pollut Bull 60:1988–1992. https://doi.org/10.1016/j.marpolbul.2010.07.030
Frias J, Antunes J, Sobral P (2013) Local marine litter survey—a case study in Alcobaça municipality, Portugal. J Integr Coast Zo Manag 13:169–179. https://doi.org/10.5894/rgci395
Frias J, Pagter E, Nash R et al (2018) Standardised protocol for monitoring microplastics in sediments. JPI Oceans Baseman Proj. https://doi.org/10.13140/RG.2.2.36256.89601/1
Fuller S, Gautam A (2016) A procedure for measuring microplastics using pressurized fluid extraction. Environ Sci Technol 50:5774–5780. https://doi.org/10.1021/acs.est.6b00816
Gall SC, Thompson RC (2015) The impact of debris on marine life. Mar Pollut Bull 92:170–179. https://doi.org/10.1016/j.marpolbul.2014.12.041
Gasperi J, Dris R, Bonin T et al (2014) Assessment of floating plastic debris in surface water along the Seine River. Environ Pollut 195:163–166. https://doi.org/10.1016/j.envpol.2014.09.001
Geyer R, Jambeck JR, Law KL (2017) Production, use, and fate of all plastics ever made. Sci Adv 3:25–29. https://doi.org/10.1126/sciadv.1700782
Grbic J, Nguyen B, Guo E et al (2019) Magnetic extraction of microplastics from environmental samples. Environ Sci Technol Lett 6:68–72. https://doi.org/10.1021/acs.estlett.8b00671
Grbić J, Helm P, Athey S, Rochman CM (2020) Microplastics entering northwestern Lake Ontario are diverse and linked to urban sources. Water Res 174:115623. https://doi.org/10.1016/j.watres.2020.115623
Gregory MR (1977) Plastic pellets on New Zealand beaches. Mar Pollut Bull 8:82–84. https://doi.org/10.1016/0025-326X(77)90193-X
Gregory MR (1978) Accumulation and distribution of virgin plastic granules on New Zealand beaches. New Zeal J Mar Freshw Res 12:399–414. https://doi.org/10.1080/00288330.1978.9515768
Hale RC (2017) Analytical challenges associated with the determination of microplastics in the environment. Anal Methods 9:1326–1327. https://doi.org/10.1039/c7ay90015e
Han M, Niu X, Tang M et al (2020) Distribution of microplastics in surface water of the lower Yellow River near estuary. Sci Total Environ 707:135601. https://doi.org/10.1016/j.scitotenv.2019.135601
Harrison JP, Ojeda JJ, Romero-González ME (2012) The applicability of reflectance micro-Fourier-transform infrared spectroscopy for the detection of synthetic microplastics in marine sediments. Sci Total Environ 416:455–463. https://doi.org/10.1016/j.scitotenv.2011.11.078
He B, Goonetilleke A, Ayoko GA, Rintoul L (2020) Abundance, distribution patterns, and identification of microplastics in Brisbane River sediments. Australia Sci Total Environ 700:134467. https://doi.org/10.1016/j.scitotenv.2019.134467
Heinrich P, Hanslik L, Kämmer N, Braunbeck T (2020) The tox is in the detail: technical fundamentals for designing, performing, and interpreting experiments on toxicity of microplastics and associated substances. Environ Sci Pollut Res Int 27:22292–22318. https://doi.org/10.1007/s11356-020-08859-1
Hengstmann E, Tamminga M, vom Bruch C, Fischer EK (2018) Microplastic in beach sediments of the Isle of Rügen (Baltic Sea)—implementing a novel glass elutriation column. Mar Pollut Bull 126:263–274. https://doi.org/10.1016/J.MARPOLBUL.2017.11.010
Hernandez E, Nowack B, Mitrano DM (2017) Polyester textiles as a source of microplastics from households: a mechanistic study to understand microfiber release during washing. Environ Sci Technol 51:7036–7046. https://doi.org/10.1021/acs.est.7b01750
Herrera A, Asensio M, Martínez I et al (2018) Microplastic and tar pollution on three Canary Islands beaches: an annual study. Mar Pollut Bull 129:494–502. https://doi.org/10.1016/j.marpolbul.2017.10.020
Hidalgo-Ruz V, Gutow L, Thompson RC, Thiel M (2012) Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ Sci Technol 46:3060–3075. https://doi.org/10.1021/es2031505
Hildebrandt L, von der Au M, Zimmermann T et al (2020) A metrologically traceable protocol for the quantification of trace metals in different types of microplastic. PLoS ONE 15:1–18. https://doi.org/10.1371/journal.pone.0236120
The Hindu (2015) Green rules of the National Games. The Hindu 1
Hirai H, Takada H, Ogata Y et al (2011) Organic micropollutants in marine plastics debris from the open ocean and remote and urban beaches. Mar Pollut Bull 62:1683–1692. https://doi.org/10.1016/j.marpolbul.2011.06.004
Hodson ME, Duffus-Hodson CA, Clark A et al (2017) Plastic bag derived-microplastics as a vector for metal exposure in terrestrial invertebrates. Environ Sci Technol 51:4714–4721. https://doi.org/10.1021/acs.est.7b00635
Horton AA, Dixon SJ (2018) Microplastics: an introduction to environmental transport processes. Wires Water 5:1268. https://doi.org/10.1002/wat2.1268
Horton AA, Svendsen C, Williams RJ et al (2017) Large microplastic particles in sediments of tributaries of the River Thames, UK—abundance, sources and methods for effective quantification. Mar Pollut Bull 114:218–226. https://doi.org/10.1016/j.marpolbul.2016.09.004
Hung H-W, Lin T-F, Chiou CT (2010) Partition coefficients of organic contaminants with carbohydrates. Environ Sci Technol 44:5430–5436. https://doi.org/10.1021/es1004413
Hurley R, Woodward J, Rothwell JJ (2018) Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nat Geosci 11:251–257. https://doi.org/10.1038/s41561-018-0080-1
Imhof HK, Ivleva NP, Schmid J et al (2013) Contamination of beach sediments of a subalpine lake with microplastic particles. Curr Biol 23:R867–R868. https://doi.org/10.1016/j.cub.2013.09.001
Imhof HK, Laforsch C, Wiesheu AC et al (2016) Pigments and plastic in limnetic ecosystems: a qualitative and quantitative study on microparticles of different size classes. Water Res 98:64–74. https://doi.org/10.1016/j.watres.2016.03.015
Ivleva NP, Wiesheu AC, Niessner R (2017) Microplastic in aquatic ecosystems. Angew Chem Int Ed Engl 56:1720–1739. https://doi.org/10.1002/anie.201606957
Jiang C, Yin L, Wen X et al (2018) Microplastics in sediment and surface water of west dongting lake and south dongting lake: abundance, source and composition. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph15102164
Jiang C, Yin L, Li Z et al (2019) Microplastic pollution in the rivers of the Tibet plateau. Environ Pollut 249:91–98. https://doi.org/10.1016/j.envpol.2019.03.022
Kalčikova G, Alič B, Skalar T, et al (2017) Ac Sc. Ecsn
Kapp KJ, Yeatman E (2018) Microplastic hotspots in the snake and lower columbia rivers: a journey from the greater yellowstone ecosystem to the Pacific ocean. Environ Pollut 241:1082–1090. https://doi.org/10.1016/j.envpol.2018.06.033
Karami A, Golieskardi A, Choo CK et al (2017) A high-performance protocol for extraction of microplastics in fish. Sci Total Environ 578:485–494. https://doi.org/10.1016/j.scitotenv.2016.10.213
Karbalaei S, Hanachi P, Walker TR, Cole M (2018) Occurrence, sources, human health impacts and mitigation of microplastic pollution. Environ Sci Pollut Res 25:36046–36063. https://doi.org/10.1007/s11356-018-3508-7
Karlsson TM, Vethaak AD, Almroth BC et al (2017) Screening for microplastics in sediment, water, marine invertebrates and fish: Method development and microplastic accumulation. Mar Pollut Bull 122:403–408. https://doi.org/10.1016/j.marpolbul.2017.06.081
Karlsson TM, Kärrman A, Rotander A, Hassellöv M (2020) Comparison between manta trawl and in situ pump filtration methods, and guidance for visual identification of microplastics in surface waters. Environ Sci Pollut Res 27:5559–5571. https://doi.org/10.1007/s11356-019-07274-5
Katsnelson A (2015) News feature: microplastics present pollution puzzle. In: Proceedings of the National Academy of Sciences of the United States of America. p E3150
Kedzierski M, Le Tilly V, Bourseau P et al (2017) Microplastics elutriation system. Part A: numerical modeling. Mar Pollut Bull 119:151–161. https://doi.org/10.1016/J.MARPOLBUL.2017.04.060
Klein S, Worch E, Knepper TP (2015) Occurrence and spatial distribution of microplastics in river shore sediments of the rhine-main area in Germany. Environ Sci Technol 49:6070–6076. https://doi.org/10.1021/acs.est.5b00492
Klein S, Dimzon IK, Eubeler J, Knepper TP (2018) Analysis, occurrence, and degradation of microplastics in the aqueous environment. In: Wagner M, Lambert S (eds) Freshwater microplastics: emerging environmental contaminants? Springer International Publishing, Cham, pp 51–67
Kokalj AJ, Kunej U, Skalar T (2018) Screening study of four environmentally relevant microplastic pollutants: uptake and effects on Daphnia magna and Artemia franciscana. Chemosphere 208:522–529. https://doi.org/10.1016/j.chemosphere.2018.05.172
Laborda F, Trujillo C, Lobinski R (2021) Analysis of microplastics in consumer products by single particle-inductively coupled plasma mass spectrometry using the carbon-13 isotope. Talanta 221:121486. https://doi.org/10.1016/j.talanta.2020.121486
Lambert S, Wagner M (2016) Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere 145:265–268. https://doi.org/10.1016/J.CHEMOSPHERE.2015.11.078
Lares M, Ncibi MC, Sillanpää M, Sillanpää M (2018) Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res 133:236–246. https://doi.org/10.1016/J.WATRES.2018.01.049
Lechner A, Keckeis H, Lumesberger-Loisl F et al (2014) The Danube so colourful: a potpourri of plastic litter outnumbers fish larvae in Europe’s second largest river. Environ Pollut 188:177–181. https://doi.org/10.1016/j.envpol.2014.02.006
Lei L, Liu M, Song Y et al (2018) Polystyrene (nano)microplastics cause size-dependent neurotoxicity{,} oxidative damage and other adverse effects in Caenorhabditis elegans. Environ Sci Nano 5:2009–2020. https://doi.org/10.1039/C8EN00412A
Li J, Liu H, Paul Chen J (2018a) Microplastics in freshwater systems: a review on occurrence, environmental effects, and methods for microplastics detection. Water Res 137:362–374. https://doi.org/10.1016/j.watres.2017.12.056
Li J, Zhang K, Zhang H (2018b) Adsorption of antibiotics on microplastics. Environ Pollut 237:460–467. https://doi.org/10.1016/j.envpol.2018.02.050
Li L, Xu G, Yu H, Xing J (2018c) Dynamic membrane for micro-particle removal in wastewater treatment: performance and influencing factors. Sci Total Environ 627:332–340. https://doi.org/10.1016/J.SCITOTENV.2018.01.239
Li Y, Lu Z, Zheng H et al (2020) Microplastics in surface water and sediments of Chongming Island in the Yangtze Estuary. China Environ Sci Eur. https://doi.org/10.1186/s12302-020-0297-7
Li S, Ma R, Zhu X et al (2021) Sorption of tetrabromobisphenol A onto microplastics: Behavior, mechanisms, and the effects of sorbent and environmental factors. Ecotoxicol Environ Saf 210:111842. https://doi.org/10.1016/j.ecoenv.2020.111842
Liu Z, Adams M, Walker TR (2018) Are exports of recyclables from developed to developing countries waste pollution transfer or part of the global circular economy? Resour Conserv Recycl 136:22–23
Liu P, Qian L, Wang H et al (2019) New insights into the aging behavior of microplastics accelerated by advanced oxidation processes. Environ Sci Technol 53:3579–3588. https://doi.org/10.1021/acs.est.9b00493
Liu P, Zhan X, Wu X et al (2020a) Effect of weathering on environmental behavior of microplastics: properties, sorption and potential risks. Chemosphere. https://doi.org/10.1016/j.chemosphere.2019.125193
Liu Y, Di ZJ, Cai CY et al (2020b) Occurrence and characteristics of microplastics in the Haihe River: an investigation of a seagoing river flowing through a megacity in northern China. Environ Pollut 262:114261. https://doi.org/10.1016/j.envpol.2020.114261
Löder MGJ, Imhof HK, Ladehoff M et al (2017) Enzymatic purification of microplastics in environmental samples. Environ Sci Technol 51:14283–14292. https://doi.org/10.1021/acs.est.7b03055
Löhr A, Savelli H, Beunen R et al (2017) Solutions for global marine litter pollution. Curr Opin Environ Sustain 28:90–99
Lusher AL, Tirelli V, O’Connor I, Officer R (2015) Microplastics in Arctic polar waters: the first reported values of particles in surface and sub-surface samples. Sci Rep 5:1–9. https://doi.org/10.1038/srep14947
Ma B, Xue W, Ding Y et al (2019) Removal characteristics of microplastics by Fe-based coagulants during drinking water treatment. J Environ Sci 78:267–275. https://doi.org/10.1016/J.JES.2018.10.006
Maes T, Jessop R, Wellner N et al (2017) A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci Rep 7:1–10. https://doi.org/10.1038/srep44501
Magnusson K, Norén F (2014) Screening of microplastic particles in and down-stream a wastewater treatment plant. IVL Swedish Environ Res Inst C 55:22
Mani T, Burkhardt-Holm P (2020) Seasonal microplastics variation in nival and pluvial stretches of the Rhine River—from the Swiss catchment towards the North Sea. Sci Total Environ 707:135579. https://doi.org/10.1016/j.scitotenv.2019.135579
Mao R, Hu Y, Zhang S et al (2020) Microplastics in the surface water of Wuliangsuhai Lake, northern China. Sci Total Environ 723:137820. https://doi.org/10.1016/j.scitotenv.2020.137820
Mathalon A, Hill P (2014) Microplastic fibers in the intertidal ecosystem surrounding Halifax Harbor, Nova Scotia. Mar Pollut Bull 81:69–79. https://doi.org/10.1016/J.MARPOLBUL.2014.02.018
Mato Y, Isobe T, Takada H et al (2001) Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ Sci Technol 35:318–324. https://doi.org/10.1021/es0010498
McEachern K, Alegria H, Kalagher AL et al (2019) Microplastics in Tampa Bay, florida: abundance and variability in estuarine waters and sediments. Mar Pollut Bull 148:97–106. https://doi.org/10.1016/j.marpolbul.2019.07.068
Millar GJ, Lin J, Arshad A, Couperthwaite SJ (2014) Evaluation of electrocoagulation for the pre-treatment of coal seam water. J Water Process Eng 4:166–178. https://doi.org/10.1016/j.jwpe.2014.10.002
Mintenig SM, Int-Veen I, Löder MGJ et al (2017) Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res 108:365–372. https://doi.org/10.1016/j.watres.2016.11.015
Moslehyani A, Ismail AF, Matsuura T et al (2019) Recent progresses of ultrafiltration (UF) membranes and processes in water treatment. Membr Sep Princ Appl Mater Sel Mech Ind Uses. https://doi.org/10.1016/B978-0-12-812815-2.00003-X
Munier B, Bendell LI (2018) Macro and micro plastics sorb and desorb metals and act as a point source of trace metals to coastal ecosystems. PLoS ONE 13:1–13. https://doi.org/10.1371/journal.pone.0191759
Munno K, Helm PA, Jackson DA et al (2018) Impacts of temperature and selected chemical digestion methods on microplastic particles. Environ Toxicol Chem 37:91–98. https://doi.org/10.1002/etc.3935
Murphy F, Ewins C, Carbonnier F, Quinn B (2016) Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ Sci Technol 50:5800–5808. https://doi.org/10.1021/acs.est.5b05416
Naidoo T, Glassom D, Smit AJ (2015) Plastic pollution in five urban estuaries of KwaZulu-Natal, South Africa. Mar Pollut Bull 101:473–480. https://doi.org/10.1016/j.marpolbul.2015.09.044
Naidoo T, Goordiyal K, Glassom D (2017) Are nitric acid (HNO3) digestions efficient in isolating microplastics from juvenile fish? Water Air Soil Pollut. https://doi.org/10.1007/s11270-017-3654-4
Naidu SA, Ranga Rao V, Ramu K (2018) Microplastics in the benthic invertebrates from the coastal waters of Kochi, Southeastern Arabian Sea. Environ Geochem Health 40:1377–1383. https://doi.org/10.1007/s10653-017-0062-z
Nan B, Su L, Kellar C et al (2020) Identification of microplastics in surface water and Australian freshwater shrimp Paratya australiensis in Victoria. Australia Environ Pollut 259:113865. https://doi.org/10.1016/j.envpol.2019.113865
Napper IE, Thompson RC (2016) Release of synthetic microplastic plastic fibres from domestic washing machines: effects of fabric type and washing conditions. Mar Pollut Bull 112:39–45. https://doi.org/10.1016/j.marpolbul.2016.09.025
Ng KL, Obbard JP (2006) Prevalence of microplastics in Singapore’s coastal marine environment. Mar Pollut Bull 52:761–767. https://doi.org/10.1016/j.marpolbul.2005.11.017
Norén F (2007) Small plastic particles in Coastal Swedish waters. KIMO Rep 1–11
Nuelle MT, Dekiff JH, Remy D, Fries E (2014) A new analytical approach for monitoring microplastics in marine sediments. Environ Pollut 184:161–169. https://doi.org/10.1016/j.envpol.2013.07.027
Oßmann BE, Sarau G, Holtmannspötter H et al (2018) Small-sized microplastics and pigmented particles in bottled mineral water. Water Res 141:307–316. https://doi.org/10.1016/j.watres.2018.05.027
Paço A, Duarte K, da Costa JP et al (2017) Biodegradation of polyethylene microplastics by the marine fungus Zalerion maritimum. Sci Total Environ 586:10–15. https://doi.org/10.1016/J.SCITOTENV.2017.02.017
Park TJ, Lee SH, Lee MS et al (2020) Occurrence of microplastics in the Han River and riverine fish in South Korea. Sci Total Environ 708:134535. https://doi.org/10.1016/j.scitotenv.2019.134535
Peng J, Wang J, Cai L (2017) Current understanding of microplastics in the environment: occurrence, fate, risks, and what we should do. Integr Environ Assess Manag 13:476–482. https://doi.org/10.1002/ieam.1912
Peng G, Xu P, Zhu B et al (2018) Microplastics in freshwater river sediments in Shanghai, China: a case study of risk assessment in mega-cities. Environ Pollut 234:448–456. https://doi.org/10.1016/j.envpol.2017.11.034
Perren W, Wojtasik A, Cai Q (2018) Removal of microbeads from wastewater using electrocoagulation. ACS Omega 3:3357–3364. https://doi.org/10.1021/acsomega.7b02037
Pettipas S, Bernier M, Walker TR (2016) A Canadian policy framework to mitigate plastic marine pollution. Mar Policy 68:117–122. https://doi.org/10.1016/j.marpol.2016.02.025
Phillips MB, Bonner TH (2015) Occurrence and amount of microplastic ingested by fishes in watersheds of the Gulf of Mexico. Mar Pollut Bull 100:264–269. https://doi.org/10.1016/j.marpolbul.2015.08.041
Picó Y, Barceló D (2019) Analysis and prevention of microplastics pollution in water: current perspectives and future directions. ACS Omega 4:6709–6719. https://doi.org/10.1021/acsomega.9b00222
Pivokonsky M, Cermakova L, Novotna K et al (2018) Occurrence of microplastics in raw and treated drinking water. Sci Total Environ 643:1644–1651. https://doi.org/10.1016/J.SCITOTENV.2018.08.102
Potthoff A, Oelschlägel K, Schmitt-Jansen M et al (2017) From the sea to the laboratory: characterization of microplastic as prerequisite for the assessment of ecotoxicological impact. Integr Environ Assess Manag 13:500–504. https://doi.org/10.1002/ieam.1902
Primpke S, Christiansen SH, Cowger W, et al (2020) Critical assessment of analytical methods for the harmonized and cost-efficient analysis of microplastics
Puckowski A, Cwięk W, Mioduszewska K et al (2021) Sorption of pharmaceuticals on the surface of microplastics. Chemosphere 263:127976. https://doi.org/10.1016/J.CHEMOSPHERE.2020.127976
Qiao R, Lu K, Deng Y et al (2019) Combined effects of polystyrene microplastics and natural organic matter on the accumulation and toxicity of copper in zebrafish. Sci Total Environ 682:128–137. https://doi.org/10.1016/j.scitotenv.2019.05.163
Reddy MS, Basha S, Adimurthy S, Ramachandraiah G (2006) Description of the small plastics fragments in marine sediments along the Alang-Sosiya ship-breaking yard, India. Estuar Coast Shelf Sci 68:656–660. https://doi.org/10.1016/j.ecss.2006.03.018
Renner G, Schmidt TC, Schram J (2018) Analytical methodologies for monitoring micro(nano)plastics: Which are fit for purpose? Curr Opin Environ Sci Heal 1:55–61. https://doi.org/10.1016/j.coesh.2017.11.001
Retama I, Jonathan MP, Shruti VC et al (2016) Microplastics in tourist beaches of Huatulco Bay, Pacific coast of southern Mexico. Mar Pollut Bull 113:530–535. https://doi.org/10.1016/j.marpolbul.2016.08.053
Rios Mendoza LM, Karapanagioti H, Álvarez NR (2018) Micro(nanoplastics) in the marine environment: Current knowledge and gaps. Curr Opin Environ Sci Heal 1:47–51. https://doi.org/10.1016/j.coesh.2017.11.004
Rocha-Santos T, Duarte AC (2015) A critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. TrAC—Trends Anal Chem 65:47–53. https://doi.org/10.1016/j.trac.2014.10.011
Rodrigues MO, Abrantes N, Gonçalves FJM et al (2018) Spatial and temporal distribution of microplastics in water and sediments of a freshwater system (Antuã River, Portugal). Sci Total Environ 633:1549–1559. https://doi.org/10.1016/j.scitotenv.2018.03.233
Rolsky C, Kelkar V, Driver E, Halden RU (2020) Municipal sewage sludge as a source of microplastics in the environment. Curr Opin Environ Sci Heal 14:16–22. https://doi.org/10.1016/j.coesh.2019.12.001
Russell JR, Huang J, Anand P et al (2011) Biodegradation of polyester polyurethane by endophytic fungi. Appl Environ Microbiol 77:6076–6084. https://doi.org/10.1128/AEM.00521-11
Sangale MK, Shahnawaz M, Ade AB (2019) Potential of fungi isolated from the dumping sites mangrove rhizosphere soil to degrade polythene. Sci Rep 9:5390. https://doi.org/10.1038/s41598-019-41448-y
Scherer C, Brennholt N, Reifferscheid G, Wagner M (2017) Feeding type and development drive the ingestion of microplastics by freshwater invertebrates. Sci Rep 7:17006. https://doi.org/10.1038/s41598-017-17191-7
Scherer C, Weber A, Stock F et al (2020) Comparative assessment of microplastics in water and sediment of a large European river. Sci Total Environ 738:139866. https://doi.org/10.1016/j.scitotenv.2020.139866
Schmidt LK, Bochow M, Imhof HK, Oswald SE (2018) Multi-temporal surveys for microplastic particles enabled by a novel and fast application of SWIR imaging spectroscopy—Study of an urban watercourse traversing the city of Berlin, Germany. Environ Pollut 239:579–589. https://doi.org/10.1016/j.envpol.2018.03.097
Schneider DR, Ragossnig AM (2015) Recycling and incineration, contradiction or coexistence? Waste Manag Res J Int Solid Wastes Public Clean Assoc ISWA 33:693–695
Shen M, Song B, Zhu Y et al (2020) Removal of microplastics via drinking water treatment: current knowledge and future directions. Chemosphere 251:126612. https://doi.org/10.1016/J.CHEMOSPHERE.2020.126612
Shim WJ, Song YK, Hong SH, Jang M (2016) Identification and quantification of microplastics using Nile Red staining. Mar Pollut Bull 113:469–476. https://doi.org/10.1016/j.marpolbul.2016.10.049
Sighicelli M, Pietrelli L, Lecce F et al (2018) Microplastic pollution in the surface waters of Italian Subalpine Lakes. Environ Pollut 236:645–651. https://doi.org/10.1016/j.envpol.2018.02.008
Slootmaekers B, Catarci Carteny C, Belpaire C et al (2019) Microplastic contamination in gudgeons (Gobio gobio) from Flemish rivers (Belgium). Environ Pollut 244:675–684. https://doi.org/10.1016/j.envpol.2018.09.136
Song YK, Hong SH, Jang M et al (2014) Large accumulation of micro-sized synthetic polymer particles in the sea surface microlayer. Environ Sci Technol 48:9014–9021. https://doi.org/10.1021/es501757s
Sruthy S, Ramasamy EV (2017) Microplastic pollution in Vembanad Lake, Kerala, India: the first report of microplastics in lake and estuarine sediments in India. Environ Pollut 222:315–322. https://doi.org/10.1016/j.envpol.2016.12.038
Stock F, Kochleus C, Bänsch-Baltruschat B et al (2019) Sampling techniques and preparation methods for microplastic analyses in the aquatic environment—a review. TrAC Trends Anal Chem 113:84–92. https://doi.org/10.1016/J.TRAC.2019.01.014
Stolte A, Forster S, Gerdts G, Schubert H (2015) Microplastic concentrations in beach sediments along the German Baltic coast. Mar Pollut Bull 99:216–229. https://doi.org/10.1016/j.marpolbul.2015.07.022
Strand J, Lassen P, Shashoua Y, Anderson JH (2013) Microplastic particles in sediments from Danish waters. ICES Annu Sci Conf 23–27:4000
Su L, Xue Y, Li L et al (2016) Microplastics in Taihu Lake, China. Environ Pollut 216:711–719. https://doi.org/10.1016/j.envpol.2016.06.036
Sun J, Dai X, Wang Q et al (2019) Microplastics in wastewater treatment plants: detection, occurrence and removal. Water Res 152:21–37. https://doi.org/10.1016/j.watres.2018.12.050
Sun C, Wang Z, Chen L, Li F (2020) Fabrication of robust and compressive chitin and graphene oxide sponges for removal of microplastics with different functional groups. Chem Eng J 393:124796. https://doi.org/10.1016/J.CEJ.2020.124796
Talvitie J, Mikola A, Koistinen A, Setälä O (2017a) Solutions to microplastic pollution—removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res 123:401–407. https://doi.org/10.1016/J.WATRES.2017.07.005
Talvitie J, Mikola A, Setälä O et al (2017b) How well is microlitter purified from wastewater? A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant. Water Res 109:164–172. https://doi.org/10.1016/j.watres.2016.11.046
Tamminga M (2017) Nile red staining as a subsidiary method for microplastic quantifica-tion: a comparison of three solvents and factors influencing application reliability. SDRP J Earth Sci Environ Stud. https://doi.org/10.15436/jeses.2.2.1
Tamminga M, Stoewer S-C, Fischer EK (2019) On the representativeness of pump water samples versus manta sampling in microplastic analysis. Environ Pollut 254:112970. https://doi.org/10.1016/j.envpol.2019.112970
Tan X, Yu X, Cai L et al (2019) Microplastics and associated PAHs in surface water from the Feilaixia Reservoir in the Beijiang River, China. Chemosphere 221:834–840. https://doi.org/10.1016/j.chemosphere.2019.01.022
Thiele CJ, Hudson MD, Russell AE (2019) Evaluation of existing methods to extract microplastics from bivalve tissue: adapted KOH digestion protocol improves filtration at single-digit pore size. Mar Pollut Bull 142:384–393. https://doi.org/10.1016/J.MARPOLBUL.2019.03.003
Thompson RC, Moore CJ, Saal FSV, Swan SH (2009) Plastics, the environment and human health: current consensus and future trends. Philos Trans R Soc B Biol Sci 364:2153–2166. https://doi.org/10.1098/rstb.2009.0053
Thompson RC, Olsen Y, Mitchell RP, et al (2004) Lost at sea: where is all the plastic? https://doi.org/10.1126/science.1094559
Torre M, Digka N, Anastasopoulou A et al (2016) Anthropogenic microfibres pollution in marine biota. A new and simple methodology to minimize airborne contamination. Mar Pollut Bull 113:55–61. https://doi.org/10.1016/j.marpolbul.2016.07.050
Vermaire JC, Pomeroy C, Herczegh SM et al (2017) Microplastic abundance and distribution in the open water and sediment of the Ottawa River, Canada, and its tributaries. Facets 2:301–314. https://doi.org/10.1139/facets-2016-0070
Vianello A, Boldrin A, Guerriero P et al (2013) Microplastic particles in sediments of Lagoon of Venice, Italy: First observations on occurrence, spatial patterns and identification. Estuar Coast Shelf Sci 130:54–61. https://doi.org/10.1016/j.ecss.2013.03.022
Wagner M, Lambert S (2018) Freshwater microplastics—the handbook of. Environ Chem 58:302. https://doi.org/10.1007/978-3-319-61615-5
Wagner M, Scherer C, Alvarez-Muñoz D et al (2014) Microplastics in freshwater ecosystems: what we know and what we need to know. Environ Sci Eur 26:1–9. https://doi.org/10.1186/s12302-014-0012-7
Wang W, Wang J (2018) Investigation of microplastics in aquatic environments: an overview of the methods used, from field sampling to laboratory analysis. TrAC—Trends Anal Chem 108:195–202. https://doi.org/10.1016/j.trac.2018.08.026
Wang J, Peng J, Tan Z et al (2017a) Microplastics in the surface sediments from the Beijiang River littoral zone: composition, abundance, surface textures and interaction with heavy metals. Chemosphere 171:248–258. https://doi.org/10.1016/j.chemosphere.2016.12.074
Wang W, Ndungu AW, Li Z, Wang J (2017b) Microplastics pollution in inland freshwaters of China: a case study in urban surface waters of Wuhan, China. Sci Total Environ 575:1369–1374. https://doi.org/10.1016/j.scitotenv.2016.09.213
Wang W, Yuan W, Chen Y, Wang J (2018) Microplastics in surface waters of Dongting Lake and Hong Lake, China. Sci Total Environ 633:539–545. https://doi.org/10.1016/j.scitotenv.2018.03.211
Wang Z, Qin Y, Li W et al (2019) Microplastic contamination in freshwater: first observation in Lake Ulansuhai, Yellow River Basin, China. Environ Chem Lett 17:1821–1830. https://doi.org/10.1007/s10311-019-00888-8
Wang G, Lu J, Tong Y et al (2020) Occurrence and pollution characteristics of microplastics in surface water of the Manas River Basin. China Sci Total Environ 710:136099. https://doi.org/10.1016/j.scitotenv.2019.136099
Wardrop P, Shimeta J, Nugegoda D et al (2016) Chemical pollutants sorbed to ingested microbeads from personal care products accumulate in fish. Environ Sci Technol 50:4037–4044. https://doi.org/10.1021/acs.est.5b06280
Wei S, Luo H, Zou J et al (2020) Characteristics and removal of microplastics in rural domestic wastewater treatment facilities of China. Sci Total Environ 739:139935. https://doi.org/10.1016/j.scitotenv.2020.139935
Wesch C, Elert AM, Wörner M et al (2017) Assuring quality in microplastic monitoring: about the value of clean-air devices as essentials for verified data. Sci Rep 7:1–8. https://doi.org/10.1038/s41598-017-05838-4
Wessel CC, Lockridge GR, Battiste D, Cebrian J (2016) Abundance and characteristics of microplastics in beach sediments: Insights into microplastic accumulation in northern Gulf of Mexico estuaries. Mar Pollut Bull 109:178–183. https://doi.org/10.1016/j.marpolbul.2016.06.002
Wijesekara H, Bolan NS, Bradney L et al (2018) Trace element dynamics of biosolids-derived microbeads. Chemosphere 199:331–339. https://doi.org/10.1016/j.chemosphere.2018.01.166
Willis KA, Eriksen R, Wilcox C, Hardesty BD (2017) Microplastic distribution at different sediment depths in an urban estuary. Front Mar Sci 4:1–8. https://doi.org/10.3389/fmars.2017.00419
Wong G, Löwemark L, Kunz A (2020) Microplastic pollution of the Tamsui River and its tributaries in northern Taiwan: Spatial heterogeneity and correlation with precipitation. Environ Pollut 260:1–12. https://doi.org/10.1016/j.envpol.2020.113935
Wu CC, Bao LJ, Tao S, Zeng EY (2016) Significance of antifouling paint flakes to the distribution of dichlorodiphenyltrichloroethanes (DDTs) in estuarine sediment. Environ Pollut 210:253–260. https://doi.org/10.1016/J.ENVPOL.2015.12.012
Xiong X, Wu C, Elser JJ et al (2019) Occurrence and fate of microplastic debris in middle and lower reaches of the Yangtze River—from inland to the sea. Sci Total Environ 659:66–73. https://doi.org/10.1016/j.scitotenv.2018.12.313
Xu J-L, Thomas KV, Luo Z, Gowen AA (2019) FTIR and Raman imaging for microplastics analysis: state of the art, challenges and prospects. TrAC Trends Anal Chem 119:115629. https://doi.org/10.1016/j.trac.2019.115629
Yan M, Nie H, Xu K et al (2019) Microplastic abundance, distribution and composition in the Pearl River along Guangzhou city and Pearl River estuary, China. Chemosphere 217:879–886. https://doi.org/10.1016/j.chemosphere.2018.11.093
Yavuz Y, Ögütveren B (2018) Treatment of industrial estate wastewater by the application of electrocoagulation process using iron electrodes. J Environ Manage 207:151–158. https://doi.org/10.1016/J.JENVMAN.2017.11.034
Yeo BG, Takada H, Hosoda J et al (2017) Polycyclic aromatic hydrocarbons (PAHs) and hopanes in plastic resin pellets as markers of oil pollution via international pellet watch monitoring. Arch Environ Contam Toxicol 73:196–206. https://doi.org/10.1007/s00244-017-0423-8
Yin L, Wen X, Du C et al (2020) Comparison of the abundance of microplastics between rural and urban areas: a case study from East Dongting Lake. Chemosphere. https://doi.org/10.1016/j.chemosphere.2019.125486
Yonkos LT, Friedel EA, Perez-Reyes AC et al (2014) Microplastics in four estuarine rivers in the chesapeake bay, USA. Environ Sci Technol 48:14195–14202. https://doi.org/10.1021/es5036317
Yu F, Yang C, Zhu Z et al (2019) Adsorption behavior of organic pollutants and metals on micro/nanoplastics in the aquatic environment. Sci Total Environ 694:133643. https://doi.org/10.1016/J.SCITOTENV.2019.133643
Yu Y, Ma R, Qu H et al (2020) Enhanced adsorption of tetrabromobisphenol a (TBBPA) on cosmetic-derived plastic microbeads and combined effects on zebrafish. Chemosphere 248:126067. https://doi.org/10.1016/J.CHEMOSPHERE.2020.126067
Yuan W, Liu X, Wang W et al (2019) Microplastic abundance, distribution and composition in water, sediments, and wild fish from Poyang Lake, China. Ecotoxicol Environ Saf 170:180–187. https://doi.org/10.1016/j.ecoenv.2018.11.126
Zbyszewski M, Corcoran PL (2011) Distribution and degradation of fresh water plastic particles along the beaches of Lake Huron, Canada. Water Air Soil Pollut 220:365–372. https://doi.org/10.1007/s11270-011-0760-6
Zeboudji B, Drouiche N, Lounici H et al (2013) The influence of parameters affecting boron removal by electrocoagulation process. Sep Sci Technol 48:1280–1288. https://doi.org/10.1080/01496395.2012.731125
Zhang H (2017) Transport of microplastics in coastal seas. Estuar Coast Shelf Sci 199:74–86
Zhang K, Gong W, Lv J et al (2015) Accumulation of floating microplastics behind the Three Gorges Dam. Environ Pollut 204:117–123. https://doi.org/10.1016/j.envpol.2015.04.023
Zhang K, Su J, Xiong X et al (2016) Microplastic pollution of lakeshore sediments from remote lakes in Tibet plateau, China. Environ Pollut 219:450–455. https://doi.org/10.1016/j.envpol.2016.05.048
Zhang K, Xiong X, Hu H et al (2017) Occurrence and characteristics of microplastic pollution in xiangxi bay of three gorges reservoir, China. Environ Sci Technol 51:3794–3801. https://doi.org/10.1021/acs.est.7b00369
Zhang H, Wang J, Zhou B et al (2018) Enhanced adsorption of oxytetracycline to weathered microplastic polystyrene: kinetics, isotherms and influencing factors. Environ Pollut 243:1550–1557. https://doi.org/10.1016/j.envpol.2018.09.122
Zhang J, Gao D, Li Q et al (2020) Biodegradation of polyethylene microplastic particles by the fungus Aspergillus flavus from the guts of wax moth Galleria mellonella. Sci Total Environ 704:135931. https://doi.org/10.1016/J.SCITOTENV.2019.135931
Zhao S, Zhu L, Wang T, Li D (2014) Suspended microplastics in the surface water of the Yangtze Estuary System, China: First observations on occurrence, distribution. Mar Pollut Bull 86:562–568. https://doi.org/10.1016/j.marpolbul.2014.06.032
Zhao S, Zhu L, Li D (2015) Microplastic in three urban estuaries, China. Environ Pollut 206:597–604. https://doi.org/10.1016/j.envpol.2015.08.027
Zhao HJ, Xu JK, Yan ZH et al (2020a) Microplastics enhance the developmental toxicity of synthetic phenolic antioxidants by disturbing the thyroid function and metabolism in developing zebrafish. Environ Int 140:105750. https://doi.org/10.1016/J.ENVINT.2020.105750
Zhao L, Rong L, Xu J et al (2020b) Sorption of five organic compounds by polar and nonpolar microplastics. Chemosphere 257:127206. https://doi.org/10.1016/j.chemosphere.2020.127206
Zhong W, Li S (2020) Microplastic pollution control strategy. IOP Conf Ser Earth Environ Sci. https://doi.org/10.1088/1755-1315/546/3/032046
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: Samareh Mirkia.
Rights and permissions
About this article
Cite this article
Gupta, D.K., Choudhary, D., Vishwakarma, A. et al. Microplastics in freshwater environment: occurrence, analysis, impact, control measures and challenges. Int. J. Environ. Sci. Technol. 20, 6865–6896 (2023). https://doi.org/10.1007/s13762-022-04139-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-022-04139-2