Abstract
Excessive outflow of phosphate ions into closed water bodies such as lakes and sea coasts leads to water pollution due to eutrophication. In the present study, we attempted to prepare an activated carbon (AC) with excellent adsorption of phosphate ions. Polyacrylonitrile-based carbon fibers with high nitrogen content were activated with zinc chloride to produce an AC, and finally, the AC was heat-treated at 950 °C to convert nitrogen species such as pyridinic nitrogen (N-6) and pyrrolic nitrogen (N-5) into quaternary nitrogen (N-Q). A sample impregnated with raw material and zinc chloride in a 1:4 weight ratio, activated at 850 °C for 60 min and then heat-treated at 950 °C for 10 min (8.5Z4(60)-9.5HT10) showed the highest phosphate ion adsorption of 0.38 mmol/g. The physical properties of the samples were evaluated by measuring TG–DTA, specific surface area, elemental analysis and X-ray photoelectron spectroscopy. The results showed that the phosphate ion adsorption increased with the increase in the proportion of N-Q in the total nitrogen species. The effect of Langmuir adsorption isotherm and equilibrium solution pH (pHe) was also investigated to evaluate the adsorption properties. At phosphate concentrations below 1.0 mmol/L, the phosphate ion adsorption amount was comparable to that of a commercial anion exchange resin (HP555), and the maximum phosphate ion adsorption amount was 0.4 mmol/g at a neutral pHe value of 6.0. Furthermore, the adsorbent showed 70% phosphate ion adsorption performance at a dosage of 0.1 g/L for even actual environmental water, indicating that it could be used in practice.
Trial registration number and date of registration: JEST D 21 01466 11th May 2021 , retrospectively registered.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Currently, the demand for phosphorus fertilizer for food production continues to increase with the global population growth, and phosphorus resources will be depleted (Mew 2016). In addition, the concentration of phosphate ions in closed water areas such as lakes and ponds is increasing due to domestic wastewater and industrial wastewater flowing out from urbanized area and excessive application of phosphorus fertilizer (Paerl 2009). The abnormal proliferation of algae due to eutrophication causes algal blooms, which have a serious impact on the water environment such as deterioration of water quality and destruction of ecosystems (Toner and Catling 2020). In order to prevent such adverse effects on the environment and organisms, it is necessary to develop more effective phosphorus removal methods. Current phosphorus treatment methods mainly include the coagulation sedimentation method (Seckler et al. 1996), the biological dephosphorization method (Okada et al. 1991) and the adsorptive removal method using adsorbents such as activated carbons (Baktas et al. 2018). However, the coagulation sedimentation method is costly, and the biological dephosphorization method is difficult to obtain stable treatment efficiency and also a large amount of sludge is generated after treatment (Yamazaki et al. 2016). Adsorption using AC is the method of removing pollutants by adsorbing them onto AC, which is obtained by activating carbon precursors to develop their pore structure. There are typically two types of activation methods: a gas activation using superheated steam or carbon dioxide gas (Yoo et al. 2018; Gonzalez et al. 2009), and a chemical activation using zinc chloride or potassium hydroxide (Hui and Zaini 2015). Since the chemical activations can be applied under relatively lower temperature conditions than the gas activations and the specific surface area of the obtained AC is also high, it can be expected to show a high adsorption ability. Previous studies have shown that anions such as phosphate ions can be attracted to the positively charged quaternary nitrogen (N-Q) on AC surface, whereas they can be repelled by the slightly negatively charged pyridinic nitrogen (N-6) and pyrrolic nitrogen (N-5), or acidic oxygen functional groups such as negatively charged carboxy groups, due to the effect of non-covalent electron pair (Machida et al. 2019). It has also been reported that N-6 and N-5 are converted to N-Q by heat-treated carbon fibers containing nitrogen at 500 °C or higher (Pels et al. 1995). Therefore, in this study, commercially available polyacrylonitrile (PAN)-based carbon fiber with a high nitrogen content of more than 20% was applied as a raw material for activated carbon. If the surface area of PAN–fiber precursor can be increased by zinc chloride activation and then N-6 and N-5 can be efficiently converted to N-Q by heat treatment, it is expected that the phosphate ion adsorption performance can be improved. In this study, the optimal activation conditions for phosphate ion adsorption were determined by preparing samples at different temperatures, activation times of zinc chloride and times of subsequent heat treatment at 950 °C. The physical properties of the samples were investigated by TG–DTA, BET method, elemental analysis and X-ray photoelectron spectroscopy. Langmuir adsorption isotherm, the effect of equilibrium solution pH (pHe) and the adsorption amount for environmental water were also investigated to evaluate the adsorption properties. In general, AC with metal oxides supported on the surface seems to increase the adsorption amounts of anions because the surface seems to be positively charged (Bacelo et al. 2020). However, in the sample prepared in the present study, the metal oxide has been removed by hydrochloric acid, so the adsorption sites should be only positively charged N-Q and the other sites. To the best of our knowledge, the phosphate ion adsorption amount of this sample is the highest among ACs without any metal oxides on the surface and they are considered to be effective for water treatment (Sakamoto et al. 2020; Miyazato et al. 2020).
Materials and methods
Preparation of activated carbons
Zinc chloride activation
It has been reported that when carbon materials are impregnated with zinc chloride solution and calcined in an inert gas atmosphere at high temperature, hydrogen and oxygen in the materials are removed as H2 and H2O by the strong dehydrating effect of zinc chloride in addition to the thermodynamical shift, and pores are also formed (Ma 2017). In the present study, PYROMEX (Teijin Ltd. PYR) was subjected to zinc chloride activation in order to develop pores. After drying 6 g of PYR cut into approximately 1 cm squares at 110 °C in air for 20 min, 4 times the PYR weight of zinc chloride was added to a beaker and 500 mL of distilled water, mixed well with PYR and dried completely at 110 °C in air to obtain sample Z4. The sample was placed in an electric tube furnace and heated from room temperature in nitrogen gas at 30 mL/min to 650–950 °C and held at each temperature for 30–120 min. The sample treated at 850 °C for 30 min was named 8.5Z4(30), and the sample treated at 850 °C for 60 min was named 8.5Z4(60). After the furnace temperature had been cooled down to near room temperature, the samples were removed from the furnace. In order to remove the residual zinc from the sample, a solution of 1 M HCl and boiling distilled water was added, mixed them well, washed for a few minutes and then filtered through an aspirator under reduced pressure. This process was repeated three times. Finally, the samples were washed overnight in a Soxhlet extractor to wash off the excess HCl and dried overnight at 110 °C in air to obtain samples.
Heat treatment
Heat treatment was carried out to convert N-6 and N-5, which are considered to inhibit phosphate adsorption, into positively charged N-Q, which contributes to the adsorption. The zinc chloride-activated samples were placed in an electric tube furnace and heated up to 950 °C in an atmosphere of 30 mL/min helium gas flow and held for 10–30 min after attaining 950 °C. The sample treated at 850 °C for 60 min and then at 950 °C for 10 min is named as 8.5Z4(60)-9.5HT10.
Properties of the prepared adsorbents
TG–DTA analysis
The thermogravimetric (TG) and differential thermal (DTA) curves of PYR and Z4 were measured by heating a 20 mg sample from room temperature to 1000 °C at a rate of 10 °C/min in a 30 mL/min helium gas atmosphere.
Specific surface area and pore distribution
The specific surface area and pore distribution of the samples were measured. The samples were dried in air at 110 °C overnight, vacuum degassed at 300 °C for 1 h, and then, nitrogen adsorption/desorption isotherms were measured in liquid nitrogen at − 196 °C. The specific surface area SBET [m2/g], average pore diameter Davg [nm] and pore volume Vtotal [cm3/g] were determined by the BET method. The micropore volume Vmicro [cm3/g] was also determined from the αs plot. The value of the mesopore volume Vmeso [cm3/g] was calculated from the difference between Vtotal and Vmicro (Fierro et al. 2008).
Elemental analysis
The change in the nitrogen content of the samples due to surface treatment was investigated. A sample of 1.4–1.5 mg dried overnight at 110 °C in air was wrapped in tin foil and completely burnt, and the compositional content of C, H and N in the sample was determined in weight percent [wt%]. The sum of the C, H and N contents obtained by the analysis was subtracted from 100 wt% to be assumed to be the O content.
X-ray photoelectron spectroscopy
Nitrogen species and their abundances on the surface of the samples were determined by X-ray photoelectron spectroscopy (JPS-9030, JEOL) using the N1s spectra of nitrogen species proposed by Pels et al. (1995). That is, the N1s peaks of each sample were analyzed by classifying the surface nitrogen into N-6 (398.7 \(\pm\) 0.3 eV), N-5 (400 \(\pm\) 0.3 eV), N-Q (401.1 \(\pm\) 0.3 eV) and pyridine-N-oxide (N-X, 402–404 eV). The nitrogen content obtained by elemental analysis was considered to be the total amount of surface nitrogen.
Adsorption measurements
Thirty milligrams of the sample were added to 15 mL of a potassium dihydrogen phosphate solution with an initial concentration of 3.0 mmol/L, and the sample was shaken overnight at 100 rpm under room temperature. The equilibrium pH (pHe) of the solution was approximately 7.0. The solution was then filtered and diluted to a measurable concentration, and the phosphate ion concentration was determined by molybdenum blue absorption spectrophotometry using a UV–visible spectrophotometer. The equilibrium adsorption amount of phosphate ions, Qe (mmol/g), was calculated by the following equation.
where C0 and Ce are the initial and the equilibrium solution concentrations of phosphate ions (mmol/g), respectively, V is the solution volume (L) and W is the weight of sample (g).
Langmuir adsorption isotherms
Adsorption experiments were carried out using aqueous potassium dihydrogen phosphate solutions with initial concentrations of 0.1–10.0 mmol/L. The equilibrium concentration Ce (mmol/L) and the equilibrium adsorption capacity Qe (mmol/g) were measured at each initial concentration, and the following Eq. (2) was used to calculate each parameter (Foo and Hameed 2010). The coefficient of determination R2 was also calculated.
where Ce is the equilibrium solution concentration of phosphate ions, Qe and Xm are the equilibrium adsorption amount and the maximum adsorption amount (mmol/g), respectively, Ke is the Langmuir isotherm constant representing adsorption affinity (L/mmol).
Effect of equilibrium solution pH
The effect of equilibrium solution pH (pHe) on the adsorption performance of phosphate ion was investigated. Thirty milligrams of the sample were added to 15 mL of potassium dihydrogen phosphate solution at an initial concentration of 3.0 mmol/L with an initial pH adjusted to 2–13, and the sample was shaken overnight at 100 rpm under room temperature. The equilibrium concentration Ce (mmol/L) and the equilibrium adsorption amount Qe (mmol/g) were measured by the method previously described in “Adsorption measurements” section.
Adsorption in actual environmental water
The prepared samples were examined to determine their adsorption amount for phosphate ions in actual environmental water. As actual environmental water, we chose water from the Shin-kawa River, which flows into Lake-Inba, one of the most eutrophic lakes in Japan. The collected water from the Shin-kawa River was filtered to remove impurities, and then, the sample and water were added in different proportional amounts (1.0 g/L, 0.5 g/L, 0.2 g/L, 0.1 g/L) to Erlenmeyer flask for adsorption. Each flask was shaken at 100 rpm overnight at room temperature for adsorption experiments.
Results and discussion
TG–DTA analysis
Figure 1 shows the TG/DTA curves of PYR and Z4. In case of PYR, the slope of the TG curve and the mass loss increased around 800 °C, and the peak of the DTA curve appeared. In case of Z4, a large endothermic peak for DTA and a significant decrease in mass for TG were observed at around 100 °C, which is due to the loss of moisture present in the sample (Sayğılı and Güzel 2016; Pietrzak and Wachowska 2004; Pereira et al. 2014). Similarly, there is a large decrease in peak and mass at 400–600 °C for Z4. This is due to the dehydration by ZnCl2 in the sample and the desorption of carbon (CO2/CO) and the volatilization of molten zinc chloride at 400–625 °C (Pereira et al. 2014).
Sample preparation conditions and adsorption amount
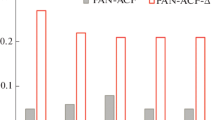
Figure 2 shows the Qe measurement results of each sample prepared by changing the activation temperature, activation time and heat treatment time. From Fig. 2a, Qe increased by raising the zinc chloride activation temperature from 650 °C, but the adsorption amounts no longer went up at the activation temperature above 850 °C. From Fig. 2b, when the zinc chloride activation temperature was fixed at 850 °C and the activation time was changed, the adsorption amount was higher in 60 min than that at 30 min, and it was almost the same at 60 min and 120 min. From Fig. 2c, when the zinc chloride activation temperature was fixed at 850 °C and the time was fixed at 60 min, the heat treatment time was changed, and the adsorption amount was significantly increased by the heat treatment. When the heat treatment time was extended from 10 to 30 min, the adsorption amount remained or slightly decreased. From the experimental results described above, it was determined that the best conditions for phosphate ion adsorption were activation treatment of zinc chloride at 850 °C for 60 min and heating treatment at 950 °C for 10 min.
Physical characteristics of samples
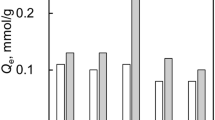
Table 1 shows the specific surface area, pore distribution and Qe measurement results of each sample. Zinc chloride activation increased the specific surface area of the sample from 9 m2/g to over 1500 m2/g. Although there are almost no mesopores in any of the samples, it was found that the volume of the micropores is proportional to the specific surface area. Moreover, when the heat treatment was performed at 950 °C, the specific surface area decreased, whereas the Qe increased; the values of the specific surface area and Qe were not in a proportional relationship. Therefore, large specific surface area does not necessarily mean a large adsorption amount. Table 2 shows the results of elemental analysis. By activating PYR with zinc chloride, hydrogen and nitrogen were decomposed during carbonization (Rahaman et al. 2007). Table 3 and Fig. 3 show the nitrogen content of each sample by XPS and the ratio of N-Q to the total nitrogen species, respectively. By increasing the activation time and performing heat treatment, the N content was decreased, and the amounts of N-6 and N-5 also decreased. However, since some of the N-6 and N-5 were converted to N-Q, the decreasing extent in N-Q was small compared to that in N-6 and N-5, leading to the increase in the adsorption amount of phosphate (Qe).
Adsorption isotherms
A commercially available anion exchange resin (IMAC HP555, Sumika Chemtex Co., Ltd.) was used as a reference adsorbent to compare the adsorption performance of the prepared samples. The structural formula of HP555 is shown in Fig. 4. As can be seen from the structural formula, HP555 has a large amount of positively charged quaternary ammonium cation that could be an anion adsorption site (Kino et al. 2020). The adsorption isotherms of the samples with initial phosphate concentrations of 0.1–10.0 mmol/L are shown in Fig. 5 with Langmuir isotherms, and the parameters of the Langmuir equation calculated from the experimental data are shown in Table 4. If the adsorption isotherm fits the Langmuir adsorption isotherm and the value of the dimensionless separation factor r of the Langmuir equation is within the range 0 < r < 1, the adsorbent would be suitable for equilibrium adsorption (Dada et al. 2012).
where r is the dimensionless separation factor, C0 is the initial solution concentration of phosphate ions and Ke is the Langmuir isotherm constant representing adsorption affinity (L/mmol). The values of r for HP555, 8.5Z4(60) and 8.5Z4(60)-9.5HT10 were 0.05–0.46, 0.07–0.47 and 0.09–0.48, respectively. Therefore, the samples prepared in this study are suitable for equilibrium adsorption of phosphate ions. If the adsorption isotherm fits the Langmuir model, a monolayer adsorption is formed on the surface of the adsorbent and equilibrium is attained above a certain concentration. In the previous study by Samatya et al., it was found that anion adsorption by N-Q on an AC surface occurs by ion exchange and approximates the Langmuir isotherm (Samatya et al. 2006). Therefore, 8.5Z4(60)-9.5HT10, which approximates the Langmuir model, is considered to contain as much N-Q as HP555. The equilibrium adsorption amount of 8.5Z4(60)-9.5HT10 was 0.15 mmol/g higher than that of 8.5Z4(60). This was probably due to the conversion of N-6 and N-5 to desirable N-Q by heat treatment at 950 °C. Moreover, looking at the low concentration region where Ce of the isotherm is less than 1.0 mmol/L, 8.5Z4(60)-9.5HT10 shows the same level of adsorption performance as HP555. In addition, since 8.5Z4(60)-9.5HT10 has high heat resistance and is resistant to acids and bases, high temperature hydrochloric acid can also be used for the desorption of phosphate ion and it can be regenerated more easily (Sakamoto et al. 2020).
Effect of equilibrium solution pH
The effect of equilibrium solution pH (pHe) on the adsorption of phosphate ions was investigated using HP555, 8.5Z4(60) and 8.5Z4(60)-9.5HT10. The results are shown in Fig. 6. The maximum Qe of 8.5Z4(60)-9.5HT10 was observed when pHe was about 6, and the Qe decreased with increasing pHe. In the lower pH range below pHe 4, the Qe decreased due to competitive adsorption of chloride ions by increasing HCl in the solution by adjusting pH. Similarly, in the higher range above pHe 7, the pH adjustment using NaOH solution increased the hydroxide ions competitive adsorption with phosphate ions leading to the decline of Qe. In the pH range of 5.8–8.6 (Kubo 1991), which is the standard value for discharging water into rivers and lakes, 8.5Z4(60)-9.5HT10 showed a high adsorption performance of 0.3–0.4 mmol/g. Therefore, 8.5Z4(60)-9.5HT10 is expected to have sufficient adsorption performance as a practical activated carbon adsorbent.
Adsorption in actual environmental water
The results of adsorption for phosphate ions in the Shin-kawa River water on 8.5Z4(60)-9.5HT10 are shown in Table 5. The concentration of phosphate ion in the filtered water of the Shin-kawa River was 0.043 mg/L. Adsorption experiment using 8.5Z4(60)-9.5HT10 indicated that the phosphate ion concentration was reduced to 0.013 mg/L, even at the lowest dosage of 0.1 g/L. These results indicate that the adsorbent has sufficient capacity to adsorb phosphate ions contained in actual environmental water and that a very small amount of the sample can be applied to purify a large amount of water.
Conclusion
In this study, several samples derived from commercially available flame resistant polyacrylonitrile (PAN) carbon fibers (PYR) were prepared by zinc chloride activation at 650–850 °C for 30–120 min, followed by heat treatment at 950 °C for 10–30 min. The most effective samples for the adsorption of phosphate ions were determined and their adsorption performance was investigated. The findings are summarized as follows.
-
1.
The mass of Z4 decreased significantly at around 100 °C due to the loss of moisture present in the sample and similarly at 400–600 °C due to the dehydration by ZnCl2 in the sample and the desorption of carbon (CO2/CO) and the volatilization of molten zinc chloride.
-
2.
Zinc chloride activation of PYR (Z4) at 850 °C for 30 min, followed by heating at 950 °C for 10 min after removing residual zinc by HCl, maximized the amount of phosphate adsorbed.
-
3.
The total amount of nitrogen in the samples decreased with increasing the activation time and heat treatment, but desirable N-Q species was increased by converting undesirable N-6 and N-5 species resulting in rise in adsorption amount of phosphate.
-
4.
The Qe of the 8.5Z4(60)-9.5HT10 was high at the concentrations below 1.0 mmol/L, and the phosphate ion adsorption capacity was comparable to that of a commercial anion exchange resin (IMAC HP555) with excellent phosphate ion adsorption capacity.
-
5.
In the pH range of 5.8–8.6, which is the standard for the discharge of water into rivers and lakes, the sample of 8.5Z4(60)-9.5HT10 showed a phosphate ion adsorption capacity of more than 0.3 mmol/g. Therefore, the sample has sufficient adsorption performance as a practical activated carbon adsorbent.
-
6.
When 8.5Z4(60)-9.5HT10 was applied to adsorption for actual environmental water, the phosphate ion concentration decreased from 0.043 to 0.013 mg/L at a dose of 0.1 g/L.
References
Bacelo H, Pintor A, Santos S, Boaventura R, Botelho C (2020) Performance and prospects of different adsorbents for phosphorus uptake and recovery from water. Chem Eng J 381:122566. https://doi.org/10.1016/j.cej.2019.122566
Bektas TE, Angİn D, Gunes S (2018) Production and characterization of activated carbon prepared from orange pulp and utilization for the removal of phosphate ions. Fresenius Environ Bull 27:7973–7982
Dada AP, Olalekan AP, Olatunya AM, Dada O (2012) Langmuir, Freundlich, Temkin and Dubinin-Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J Appl Chem 3:38–45. https://doi.org/10.9790/5736-0313845
Fierro V, Torné-Fernández V, Montané D, Celzard A (2008) Adsorption of phenol onto activated carbons having different textural and surface properties. Microporous Mesoporous Mater 111:276–284. https://doi.org/10.1016/j.micromeso.2007.08.002
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10. https://doi.org/10.1016/j.cej.2009.09.013
Gonzalez JF, Roman S, Gonzalez-Garcia CM, Nabais JMV, Ortiz AL (2009) Porosity development in activated carbons prepared from walnut shells by carbon dioxide or steam activation. Ind Eng Chem Res 48:7474–7481. https://doi.org/10.1021/ie801848x
Hui TS, Zaini MAA (2015) Potassium hydroxide activation of activated carbon: a commentary. Carbon Lett 16:275–280. https://doi.org/10.5714/CL.2015.16.4.275
Kino K, Sakamoto T, Yuan J, Amano Y, Machida M (2020) Quaternary nitrogen functionalized carbonaceous adsorbents to remove nitrate from aqueous phase. Catal Today. https://doi.org/10.1016/j.cattod.2020.06.036
Kubo T (1991) Recent developments in wastewater management in Japan. Water Sci Technol 23:19–28. https://doi.org/10.2166/wst.1991.0397
Ma Y (2017) Comparison of activated carbons prepared from wheat straw via ZnCl2 and KOH activation. Waste Biomass Valorization 8:549–559. https://doi.org/10.1007/s12649-016-9640-z
Machida M, Yoo P, Amano Y (2019) Adsorption of nitrate from aqueous phase onto nitrogen-doped activated carbon fibers (ACFs). SN Appl Sci 1:323. https://doi.org/10.1007/s42452-019-0333-7
Mew MC (2016) Phosphate rock costs, prices and resources interaction. Sci Total Environ 542:1008–1012. https://doi.org/10.1016/j.scitotenv.2015.08.045
Miyazato T, Nuryono N, Kobune M, Rusdiarso B, Otomo R, Kamiya Y (2020) Phosphate recovery from an aqueous solution through adsorption-desorption cycle over thermally treated activated carbon. J Water Process Eng 36:101302. https://doi.org/10.1016/j.jwpe.2020.101302
Okada M, Murakami A, Lin CK, Ueno Y, Okubo T (1991) Population dynamics of bacteria for phosphorus removal in sequencing batch reactor (SBR) activated sludge processes. Water Sci Technol 23:755–763. https://doi.org/10.2166/wst.1991.0526
Paerl HW (2009) Controlling eutrophication along the freshwater–marine continuum: dual nutrient (N and P) reductions are essential. Estuaries Coasts 32:593–601. https://doi.org/10.1007/s12237-009-9158-8
Pels JR, Kapteijn F, Moulijn JA, Zhu Q, Thomas KM (1995) Evolution of nitrogen functionalities in carbonaceous materials during pyrolysis. Carbon 33:1641–1653. https://doi.org/10.1016/0008-6223(95)00154-6
Pereira RG, Veloso CM, Silva NM, Sousa LF, Bonomo RCF, Souza AO, Souza M, Fontan R (2014) Preparation of activated carbons from cocoa shells and siriguela seeds using H3PO4 and ZnCl2 as activating agents for BSA and α-lactalbumin adsorption. Fuel Process Technol 126:476–486. https://doi.org/10.1016/j.fuproc.2014.06.001
Pietrzak R, Wachowska H (2004) Thermal analysis of oxidised coals. Thermochim Acta 419:247–251. https://doi.org/10.1016/j.tca.2004.02.014
Rahaman MSA, Ismail AF, Mustafa A (2007) A review of heat treatment on polyacrylonitrile fiber. Polym Degrad Stab 92:1421–1432. https://doi.org/10.1016/j.polymdegradstab.2007.03.023
Sakamoto T, Amano Y, Machida M (2020) Phosphate ion adsorption properties of PAN-based activated carbon fibers prepared with K2CO3 activation. SN Appl Sci 2:702. https://doi.org/10.1007/s42452-020-2465-1
Samatya S, Kabay N, Yüksel Ü, Arda M, Yüksel M (2006) Removal of nitrate from aqueous solution by nitrate selective ion exchange resins. React Funct Polym 66:1206–1214. https://doi.org/10.1016/j.reactfunctpolym.2006.03.009
Sayğılı H, Güzel F (2016) High surface area mesoporous activated carbon from tomato processing solid waste by zinc chloride activation: process optimization, characterization and dyes adsorption. J Clean Prod 113:995–1004. https://doi.org/10.1016/j.jclepro.2015.12.055
Seckler MM, Leeuwen MLJ, Bruinsma SL, Rosmalen GM (1996) Posphate removal in a fluidized bed-II. Process Optim Water Res 30:1589–1596. https://doi.org/10.1016/0043-1354(96)00017-6
Toner JD, Catling DC (2020) A carbonate-rich lake solution to the phosphate problem of the origin of life. PNAS 117:883–888. https://doi.org/10.1073/pnas.1916109117
Yamazaki Y, Gettongsong T, Mikawa M, Amano Y, Machida M (2016) Adsorptive removal of phosphate from water by ammonia gas activated polyacrylonitrile fiber. J Fiber Sci Technol 72:237–243. https://doi.org/10.2115/fiberst.fiberst.2016-0035
Yoo P, Amano Y, Machida M (2018) Adsorption of nitrate onto nitrogen-doped activated carbon fibers prepared by chemical vapor deposition. Korean J Chem Eng 35:2468–2473. https://doi.org/10.1007/s11814-018-0151-4
Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (KAKENHI Grant No. JP20K05187). The authors thank the center for analytical instrumentation Chiba University for supporting elemental analysis. They are also grateful to Prof. Dr. Fumio Imazeki, the head of Safety and Health Organization, Chiba University, for his financial support on our study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Editorial responsibility: Samareh Mirkia.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Matsuzawa, F., Amano, Y. & Machida, M. Phosphate ion adsorption characteristics of PAN-based activated carbon prepared by zinc chloride activation. Int. J. Environ. Sci. Technol. 19, 8159–8168 (2022). https://doi.org/10.1007/s13762-021-03695-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-021-03695-3