Abstract
This study aimed to develop and evaluate five simplified AGW treatment options with or without low-cost ceramic filters, including direct ceramic filtration, alum coagulation–sedimentation, alum coagulation–sedimentation–ceramic filtration, alum coagulation–sedimentation–activated carbon, adsorption–sedimentation, and ceramic filtration–activated carbon adsorption–sedimentation. In raw AGW, concentrations of turbidity (14.8 NTU), TSS (24 mg/L), COD (63.2 mg/L), BOD (37.0 mg/L), and fecal coliform (884 MPN/100 mL) exceeded the greywater reuse standards for unrestricted irrigation, toilet flushing, and firefighting. In laboratory experiments, first three treatment options produced almost similar effluent with residual turbidity 1.5 NTU, COD 35 mg/L, and BOD 20 mg/L. Addition of the activated carbon to the last two treatment options enhanced the BOD and COD removals (< 12 mg/L). Multicriteria decision-making was performed to evaluate all the AGW treatment options against effluent water quality, treatment cost, land requirements, and need of skilled operators. Criteria weights were estimated using Entropy method, while the technique for order preference by similarity to ideal solution was used to final ranking of AGW treatment options. Results found direct filtration as the most feasible option to reuse AGW for restricted irrigation, and for unrestricted irrigation, toilet flushing, and firefighting, the ceramic filtration–activated carbon adsorption–sedimentation treatment process would be the more feasible option.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Most of the arid and semiarid countries in Gulf region, including the Kingdom of Saudi Arabia (KSA), are facing a serious threat of limited fresh water resources. Due to the development of these countries in every domain and an increasing population, this issue will become more critical in the near future. Consequently, significantly increased water demand will cause a decrease in the level of groundwater, the main source of fresh water in these regions (Ouda 2013). In arid and semiarid regions, renewable groundwater and surface water supplies are limited, and climate change is expected to further increase water demand (Hellegers et al. 2013).
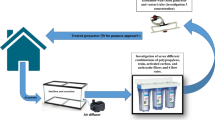
Due to the limited water resources in these regions, it is necessary to reconsider greywater reuse to reduce the demand of groundwater and other freshwater water resources. However, greywater treatment for reuse is a complex and expensive process, as the existing plumbing system makes it difficult to separate greywater from black water. Previous studies based on sustainability recommended separating the plumbing system for the collection and treatment of black and greywater (Otterpohl 2002). Greywater represents the major part of domestic wastewater, with low content of pathogens, organic material, and nutrients, and therefore can be treated and reused easily (Noutsopoulos et al. 2018). Ablution water is a kind of greywater that is produced from the washing of a certain part of the body (wudhu) before performing prayers for Muslims.
In most Middle East countries, mosques produce a large amount of ablution greywater (AGW). The quality of AGW is better than the domestic greywater produced from washing, cooking, and bathing (Suratkon et al. 2014; Mohamed et al. 2016). Therefore, AGW can be easily reused by introducing cost-effective and simple treatment methods (Suratkon et al. 2014) in order to contribute to sustainable development and water resource conservation without compromising public health and environmental pollution. In recent years, some Middle East countries, including KSA, have reconsidered reusing greywater for some restricted and unrestricted irrigation purposes. However, the process is limited by some major constraints, such as a lack of public and social awareness and sustainable treatment systems.
In most Middle Eastern countries, including KSA, the AGW from mosques is being discharged directly into sewerage drains. Generally, ablution water is neutral in pH (6.92–7.10) with COD varying between 50 and 70 mg/L, BOD varying between 20 and 40 mg/L, TSS varying between 5 and 146 mg/L, turbidity varying between 10 and 30 NTU, and E. coil varying between 100 and 1000 CFU/100 mL. (Al-Wabel 2011; Mohamed et al. 2016). The quality of ablution greywater suggests that a basic and simple treatment system could be applied to recycle and reuse for non-potable water applications, such as landscaping, toilet flushing, car washing, and irrigation (Suratkon et al. 2014).
Several studies have been conducted on the treatment of greywater produced from kitchens, baths, and laundry. Based on the required reuse application, different treatment technologies, including physical (March et al. 2004), chemical (Lin et al. 2005; Pidou et al. 2008), and biological (Fountoulakis et al. 2016; Atanasova et al. 2017) methods have been proposed and studied for greywater treatment. However, the current research on the treatment and recycling of AGW is limited.
To our knowledge, very few researches based on physical–chemical methods using sand filter for AGW treatment have been published (Prathapar et al. 2006; Al-wabel 2011; Al-Mughalles et al. 2012; Mohamed et al. 2016). However, those treatment systems possess some drawbacks, including being difficult to maintain and less effective to contaminant removal. For instance, sand filter is a low-cost technique for greywater treatment, but is restricted by low efficiency of BOD, COD, and TOC removal and frequent clogging problems (Li et al. 2009; Zipf et al. 2016). In our previous studies, a low-cost ceramic filter (made of clay soil and rice bran) was successfully applied to treat household wastewater and greywater (Hasan et al. 2011, 2015). This filter, combined the coagulation and flocculation processes, has also been applied to treat sand filter backwash water and produced high-quality recyclable water (Shafiquzzaman et al. 2018). Advantages of using a ceramic filter are that it is easy to maintain due to prolonged operation without clogging, has low sludge production due to chemical-free operation, is a simple backwash procedure, has low energy consumption, low operating cost, and does not need a very skilled professional to operate the system (Hasan et al. 2011, 2015; Shafiquzzaman et al. 2018). Therefore, using a low-cost ceramic filter for AGW treatment may have positive technological and environmental impacts and would open a new door to sustainable AGW management. Alum coagulation and activated carbon (AC) adsorption processes have been widely used for wastewater and greywater treatment because of their efficacy to remove wide range of pollutants (Lin et al. 2005; Pidou et al. 2008). Therefore, ceramic filtration integrating with alum coagulation or AC adsorption would be feasible treatment options for AGW. In addition to environmental and technical aspects, the economical evaluation of different AGW treatment systems, conducted in this study, will contribute the sustainability of AGW reuse. To the best of authors’ knowledge, the efficacy of ceramic filtration with or without alum coagulation and AC adsorption for treating AGW has not been evaluated, so far.
From this perspective, this study aimed to develop five simplified methods for the treatment of AGW using a low-cost ceramic filter, alum coagulation and activated carbon adsorption either individually or combining. The specific objectives are: (1) conduct laboratory scale continuous and batch experiments to investigate the contaminant removal efficiency of each treatment option for targeting reuse applications, (2) evaluate economic feasibility of the full scale unit for each AGW treatment option, and (3) evaluate and rank all the AGW treatment options against effluent water quality, treatment cost, land requirements, and need of skilled operators by performing multicriteria decision-making (MCDM).
Materials and methods
AGW production and sampling
A medium-sized mosque (Al-Ajaji mosque) located in Buraidah, KSA, was selected for this study. AGW was collected from Al-Ajaji mosque using a cylindrical container. AGW, mixed with black water, is currently being discharged into the municipal sewerage system. Ablution water flows out from the ablution room to the municipal sewer through a 100-mm drain pipe. A cylindrical container (diameter 400 mm and height 1.2 m) was installed at the outlet of the ablution drain pipe to estimate AGW production and collect AGW samples. The quantity of ablution greywater received by the cylinder was calculated by estimating the height of AGW level in the cylinder. The number of people who performed ablution was counted, and the amount of water received by the cylinder was measured separately for each of five daily prayers. The production of ablution greywater (L per prayer/person) was calculated by dividing the total number of people by the amount of ablution greywater received by the collection cylinder in one prayer. Twelve samples were collected at 12 different prayer times. After collection, AGW samples were transported to the laboratory and analyzed for physical, chemical, and biological parameters. Samples were also used for the experiments without any pre-treatment.
Low-cost ceramic filter
This research investigated the possible applications of a low-cost ceramic filter for the treatment of AGW. The low-cost ceramic filter was manufactured using locally available materials (clay soil and saw dust). Details of the manufacturing process of the filter are described in our previous study (Shafiquzzaman et al. 2011). In brief, a cylindrical filter was made using dough produced by mixing clay soil and sawdust with water at a ratio of 80:20 by weight. The dough was then cast in a cylindrical mold to make the filter. The filter was then fired at 900 °C for 4–5 h in a pottery kiln. The final filter was cylindrical in shape (Fig. 1), having a pore size of 1–5 μm. The manufacturing cost of filter was estimated to be 0.2–0.3 US$/filter (Shafiquzzaman et al. 2011).
Alum and activated carbon (AC)
Powdered aluminum sulfate hydrate (Al2 (SO4)3·12H2O) was purchased from WETICO—Water and Environment Technologies Co., Ltd., KSA, and used as the coagulant. Before each experiment, a fresh coagulant solution (10 mg/mL concentration) was prepared by mixing 10 g of alum powder in 1 L of distilled water. Activated carbon (AC) used in this study was obtained from a CTO-10 (Riverpure, Taiwan) carbon block cartridge filter that was purchased from the local market in Buraidah city, KSA. The activated carbon was extracted from the filter used in this study. The key characteristics of the activated carbon were: bulk particle density 372 kg/m3, moisture content 6.7%, and particle size 0.2–1.8 mm.
Development of treatment options for AGW
Laboratory experiments were conducted to investigate the efficiencies of five treatment options to treat AGW. Figure 2a–e shows the five treatment options investigated in the present study: (a) direct ceramic filtration (DF), (b) coagulation–sedimentation (CS), (c) coagulation–sedimentation–ceramic filtration (CSF), (d) coagulation–sedimentation–AC adsorption–sedimentation (CSAS), and (e) filtration–AC adsorption–sedimentation (FAS).
Schematic diagram of five alternatives for the treatment of ablution greywater. a Option 1—direct ceramic filtration (DF), b option 2—coagulation–segmentation (CS), c option 3—coagulation–sedimentation–ceramic filtration (CSF), d option 4—coagulation–sedimentation–AC adsorption–sedimentation (CSAS), and e option 5—filtration—AC adsorption–sedimentation (FAS)
The five treatment options were evaluated separately, and efficiencies were examined by monitoring the water quality of treated AGW for each process. As the main focus of this study was to evaluate the possible applications of a low-cost ceramic filter for AGW treatment, only the DF treatment process was monitored in detailed by measuring pH, TSS, TDS, turbidity, COD, BOD, PO4–P, NH4–N, and fecal coliform, while for the other four treatment options, pH, TDS, turbidity, COD, and BOD were monitored, because none of these parameters except turbidity and COD in raw AGW exceeded the greywater reuse guidelines for restricted irrigation in KSA. As ceramic filter is also used in CSF and FAS treatment options, the measured parameters in DF options are comparable with these two treatment options.
Jar tests for optimum doses of alum and AC
Optimum alum and AC doses were determined by a standard jar test procedure using a 1000-mL glass beaker. Coagulation tests were performed at 0, 5, 10, 20, 30, and 50 mg/L alum doses. A standard jar test was carried out to perform coagulation and flocculation experiments using a four stirrer A&F jar test apparatus (JM4, Nvatech International). Jar test was performed in three stages: 2 min rapid mixing at 100 revolutions per minute (rpm) in the initial first stage, followed by a slow mixing for 20 min at 40 rpm, and the final settling stage for another 30 min (Zheng et al. 2013). Optimum alum dose was determined by measuring the residual turbidity. AC-adsorption tests were performed at 0, 0.1, 0.2, 0.3, and 0.5 g/L of AC doses. In AC-adsorption tests, mixing (40 rpm) was performed for 20 min, followed by settling for 30 min, then residual COD were measured, and the optimum AC dose was determined.
Option 1: direct ceramic filtration (DF) experiments
In the DF experiments, raw AGW was directly fed to the filtration tank and filtration was performed using suction pumps (Fig. 2a). The ceramic filtration tank was made of a 12-mm-thick thermoplastic glass with dimensions 40 cm in length, 40 cm in width, and 60 cm in height. The ceramic filter was submerged in the filtration tank and placed horizontally on a plastic board to ensure the effective use of the entire surface of the filter for filtration. The permeate (final water) was obtained by suction pressure through the effluent tube to the effluent tank.
Filtration was conducted in two consecutive fluxes of 700 L/m2/day (pressure 1.5 Kpa) and 1400 L/m2/day (pressure 2.8 Kpa). The fluxes were chosen based on the range of AGW production (400–1200 L/day) at small- to medium-sized mosques in KSA found in the present study. Filtration was continuously performed for 6 days at 700 L/m2/day flux, after which the flux rate was changed to 1400 L/m2/day for 6 days. Before the second run started, the filter was cleaned with a soft brush and washed with tap water to remove accumulated foul materials on the filter. Therefore, the initial conditions for both runs were relatively similar. During filtration period, the flow rate and pressure were recorded on a daily basis to ensure the consistency of flux and pressure. Raw AGW and filter permeate were collected on a daily basis and analyzed for quality.
Option 2: coagulation–sedimentation (CS) experiments
In the CS experiments, coagulation was performed in a coagulation–sedimentation tank at 20 mg/L of optimum alum dose (Fig. 2b). Rapid mixing (100 RPM) was performed for 2 min, followed by slow mixing (40 RPM) for 20 min, and then settling for 30 min. Next, the suspension was transferred to the final effluent tank and analyzed for water quality. The experiments were performed on three consecutive days to ensure reproducibility.
Option 3: coagulation–sedimentation–ceramic filtration (CSF) experiments
The CSF experiment was carried out as continuous filtration combined with the coagulation process. Figure 2c presents a schematic view of the CSF experimental mode. The experimental setup consisted of a coagulation–sedimentation tank and a ceramic filtration tank. Coagulation–sedimentation were performed as described as in the CS experiments. The suspension of the coagulation–sedimentation tank was then feed to the ceramic filtration tank using suction pumps, and filtration was carried out continuously at a 1400 L/m2/day flux rate for 3 days. Raw AGW, suspension of sedimentation tank, and filter permeate were collected regularly, and water quality was measured.
Option 4: coagulation–sedimentation–AC-adsorption–sedimentation (CSAS) experiments
Figure 2d presents a schematic diagram of the CSAS experimental setup. The CSAS experiments consisted of a coagulation–sedimentation tank followed by an AC-adsorption–sedimentation tank. At the first stage, coagulation–sedimentation was performed as described in the CS experiments. The suspension of the coagulation–sedimentation tank was then fed to the AC-adsorption tank. A 0.2 g/L optimum AC dose (obtained from the AC-adsorption test) was added to the AC-adsorption tank and mixed at 40 RPM for 20 min, followed by settling for 30 min. Raw AGW, supernatant from the coagulation tank, and supernatant from the AC-adsorption tank were taken to measure water quality. The experiments were run for three consecutive days to ensure reproducibility.
Option 5: filtration–AC-adsorption–sedimentation (FAS) experiments
In the FAS experiments, at the first stage, direct filtration was performed at 1400 L/m2/day, as described in the DF experiments. The permeate was then fed to the AC-adsorption–sedimentation tank. AC-adsorption experiments were then performed, as described as in the CSAS experiments. The experiments were continuously run for 3 days. Water samples of raw AGW, filter permeate, and suspension of the AC-adsorption–sedimentation tank were collected to measure the treated water quality.
Analytical methods
Physical, chemical, and biological analyses of the water samples were conducted in the laboratory of Ministry of Environment, Water, and Agriculture, Buraidah, Al-Qassim, KSA. All water quality parameters were measured following the standard methods for the examination of water and wastewater analysis prescribed by American Public Health Association (APHA 2005). pH was measured using an Hach MP-6 (HACH, Loveland, CO, USA) portable pH meter. Turbidity was measured using a Hach 2100Q turbidity meter (2100Q, HACH, Loveland, CO, USA). Total dissolved solids (TDS) were measured using a Hach HQ411d TDS meter (HACH, Loveland, CO, US). Alkalinity was measured by titration with sulfuric acid. A Hach-DR5000 UV–Vis spectrophotometer was used to measure COD (reactor digestion method), PO4–P (acid persulfate digestion method), NH4–N (direct ISE method), Free Cl2 (USEPA DPD Method), and TSS (Photometric Method). Five-day BOD was measured by the dilution method in a BOD incubator. Fecal coliform (FC) was measured using a Quanti-Tray, which consists of 51 individually sealable cells. Oil and grease were measured by the hexane extractable gravimetric method.
Statistical analysis (t test) was performed using Microsoft Excel Analysis ToolPak to establish the significant variations of contaminates removal among the treatment options. The difference is considered to be statistically significant for p value < 0.05.
Cost estimation of the treatment processes
The cost of a wastewater treatment process generally includes investment, maintenance, and operation costs. The investment costs can be subdivided into construction costs, machinery and equipment costs, land costs, etc. The operation and maintenance costs of a wastewater treatment process include energy consumption costs, chemical consumption costs, sludge disposal costs, and personnel salaries. Nevertheless, the aim of the current study was to evaluate the economics of the treatment options. Therefore, different investment costs (including construction cost of tank, machinery and equipment cost and the filter cost), operating cost (electricity), and the cost of chemicals (Alum and AC) were considered for each process in the present study. The cost per m3 of AGW (US $/m3) was calculated based on the annualized investment cost, filter cost ($/year), chemical cost ($/year), and operating costs ($/year) (Nandi et al. 2009).
Evaluation of AGW treatment options
Each of the AGW treatment options described above may result in different water quality characteristics of the effluent. In addition, all the options require varying costs (construction and operation), land requirements, and, need of skilled operators. Figure 3 shows the overall hierarchy of the evaluation process. Therefore, the evaluation process of these treatment options comes out to be a multicriteria decision-making (MCDM) problem. In this research, Entropy method is used for assigning the weights to all the criteria, while the technique for order preference by similarity to ideal solution (TOPSIS) is used for aggregating the criteria performance scores and final ranking of AGW treatment options.
Entropy method for weights estimation
Entropy method investigates the irregularities among a given set of data. The value of Entropy increases with increasing level of irregularity. Higher value of entropy reflects smaller differences between the options in a particular criterion (i.e., criteria are less important) and results in smaller criteria weight (Abdullah 2013). The method is used to find out the criteria weights of different effluent quality parameters for the development of water quality index (WQI). Subsequently, it is employed for assigning weights to the top level criteria for evaluation of AGW treatment options (see Fig. 3).
All the criteria ‘j’ need to be normalized with respect to the option ‘i’ in a given decision matrix using the following equation (Hang and Yoon 1981):
The entropy Ej of the normalized matrix can be estimated with the help of the following equation:
where the constant α, for n number of options guaranteeing that all the values of Ej will range between ‘0’ and ‘1,’ is defined as:
The weight of the criteria can be estimated as:
where dj is the degree of diversity among the options in a given criterion j and is mathematically equal dj = 1 − Ej.
In this research, decision-makers also defined a priori for all the criteria. Subjective weights \(\hat{w}_{j}\) used in this research are 1 (very unimportant), 3 (unimportant), 5 (average important), 7 (important), and 9 (very important).
Final weights for each criterion are estimated for WQI and evaluation of AGW options using the following relationship:
TOPSIS method for developing the WQI and evaluating AGW treatment options
For aggregating the criteria scores, TOPSIS method is used to first develop WQI at the lower hierarchy level and then final ranking of AGW treatment options at the top of hierarchy in Fig. 3. The aggregated indices are based on the concept of similarity (i.e., relative closeness) to the positive ideal solution (PIS) and the remoteness from the negative-ideal solution (NIS). A step-by-step approach of the TOPSIS method is given below (Haider et al. 2016):
Step 1 Estimation of weights of water quality parameters and evaluation criteria.
Entropy method described above has been used to estimate the importance weights.
Step 2 Checking need for normalization.
As all the water quality parameters and evaluation criteria have different units, so there is a need for normalization using the following equation for benefit criteria:
where rij is the normalized value of xij and \(x_{i}^{*}\) is the maximum value of the jth criteria.
For cost criteria, firstly the criteria is transformed into the benefit criteria using the inverse function (i.e., 1/xij) and then the following equation has been used for normalization:
Step 3 Development of weighted matrix.
The weighted matrix has been developed as:
where vij is the weighted value of each criteria, wij is the corresponding weight of the parameters or criteria, and xij is the normalized value of each criteria.
Step 4 Identify positive-ideal and negative-ideal solutions.
In terms of weighted scores, X* is defined as the positive-ideal solution (PIS) and X− as the negative-ideal solution (NIS):
where J1 is the set of benefit criteria and J2 is a set of cost criteria. However, as all the criteria have been converted into benefit criteria, so the maximum values (i.e., vij) have been used for X* and the minimum values for X−.
Step 5 Compute the distance for each option from PIS and NIS.
The distances for all the criteria for each option are estimated by the n-dimensional Euclidean distance. The separation or distance of each criterion from the PIS has been calculated as:
and the distance for each criteria form the NIS can be calculated as:
Step 6 Aggregate final weighted scores to calculate WQI and rank the AGW treatment options by estimating the similarities to PIS.
The overall water quality index and the score of each AGW treatment are calculated as:
The result of Eq. (13) was found in the form of ratio, so it was multiplied by 100 to rationally translate the WQI. For AGW treatment options, outcomes of Eq. (13) were directly used for final ranking.
Results and discussion
AGW produced at Al-Ajaji mosque
Around 13–35 persons perform ablution at Al-Ajaji mosque for five times in a day. Amount of AGW produced ranged between 3.2 and 5.5 L/person/prayer with an average of 3.6 L/person/prayer. Therefore, 490–1400 L/day (average 950 L/day) of ablution water is being produced at the Al-Ajaji mosque. These results are consistent with a previous study conducted by Prathapar et al. (2006) in which an average ablution greywater production ranging from 770 to 1940 L/day (with an average of 1220 L/day) was reported for a medium-sized mosque in Oman.
Raw AGW quality
Table 1 presents the concentrations of primary contaminants in AGW collected from the Al-Ajaji mosque. Values of pH ranging between 7.7 and 8.1 are suitable for any reuse application. Turbidity values in all the samples were found higher than 10 NTU and were higher than the reuse standard of KSA (MWE 2006). High variations were observed for COD and BOD values in AGW. The concentration of BOD was higher than 10 mg/L, i.e., KSA standards for unrestricted irrigation (see Table 1). BOD of 40 mg/L (i.e., KSA reuse standard for restricted irrigation) was found in 60% of raw AGW samples.
Table 1 shows that TDS levels in all the raw AGW samples were less than KSA’s unrestricted irrigation standards (Standard A). Free residual chlorine (Cl2) was not detected in any of the AGW samples. Fecal coliform (FC) levels were higher than reuse Standard A (see Table 1). The raw AGW quality measured in this study showed that the mean values of turbidity, TSS, COD, BOD, and fecal coliform were higher than the reuse Standard A. Therefore, the AGW needs to be treated for unrestricted irrigation.
Determination of optimal dose of alum and AC for AGW treatment
The results of jar test presented in Fig. 4a show that up to 95% turbidity removal was achieved at 20 mg/L of alum dose. The removal was constant and insignificant (p value < 0.05) while further increasing the alum doses of 30 and 50 mg/L. Based on these results, the optimal dose of alum was found to be 20 mg/L.
In AC-adsorption test (Fig. 4b), residual COD was achieved to be 14.1 mg/L at 0.1 g/L of AC dose and further decreased to 10.4 mg/L at 0.2 g/L dose. No significant change in residual COD was observed by increasing the AC dose to 0.3 and 0.5 g/L. Therefore, the optimum dose of AC was found to be 0.2 g/L. The optimum alum dose of 20 mg/L and AC dose of 0.2 g/L were used in further laboratory experiments to evaluate different treatment options.
Option 1: direct ceramic filtration (DF) system
Variations of flux and pressure during 12 days of direct filtration are presented in Fig. 5. None of the operating conditions showed any significant change of flux or pressure drop. These results indicate that the ceramic filter can be operated for a long time with the flux ranging between 700 and 1400 L/m2/day without noticeable fouling of the filter. These results well agree with a previous study conducted by Shafiquzzaman et al. (2018) which reported that the ceramic filter can be operated at 2000 L/m2/day continuously for 22 days without fouling while treating highly turbid sand filter backwash water. T test results showed no significant differences (p value > 0.05) for removing TDS, turbidity, COD, BOD, TSS, and fecal coliform for the two operating flux conditions.
Figure 6a presents the results of turbidity and TSS removal. Turbidity removal was found to be steady throughout the filtration operation for both fluxes and provided high turbidity removal. Effluent turbidity ranged from 0.5 to 2 NTU with an average value of 1.16 NTU. The results indicate that almost all the suspended particles causing turbidity were rejected by the low-cost microporous (1–5 µm) ceramic filter, while the colloidal particles might be absorbed onto the clay-based filter. Previous studies reported that micro-filtration (MF) membranes are highly effective at removing turbidity from greywater (Ramon et al. 2004; Kim et al. 2009). TSS removal was achieved with an average of 92%. TSS removal was consistent for both the operating fluxes, and effluent TSS ranged from 0.3 to 1.0 mg/L.
Figure 6b presents the removal performances of COD and BOD. Efficiency of the COD removal achieved 43.3%. The effluent of the filter produced an average of 32.0 mg/L (SD = ± 11.1 mg/L) COD, and almost all samples were below 35 mg/L (except on days 6 and 8). In the same manner, average BOD removal efficiency was 33.2% (Fig. 6b). The effluent of the filter produced an average of 19.8 mg/L (± 2.8 mg/L) BOD with no observations higher than 25 mg/L. The particulate forms of BOD and COD were removed through physical straining of the filter surface, whereas the parts of dissolved COD and BOD were adsorbed on to the natural clay-based ceramic filter used in this study. Natural clay has some sorption capabilities of organic from wastewater (Ogunmodede et al. 2014).
Figure 6c shows the removal of fecal coliform (FC) through low-cost ceramic filter from 882 MPN/100 mL in raw AGW to 180 MPN/100 in the treated effluent. FCs in about 60% of the effluent samples were found higher than 100 MPN/100 mL and in 40% of the samples below 100 MPN/100 mL. Although significant removal was achieved, FCs in the effluent were higher than the specified values in reuse regulations, such as those for unrestricted irrigation and toilet flushing (MWE 2006). Therefore, a disinfection process is recommended after filtration for reusing the AGW.
Other parameters, such as pH, TDS, PO4–P, and NH4–N, were also regularly monitored during the filtration operations (data not shown). pH was observed to increase to an average 8.1 in the effluent from 7.5 in the raw water. TDS was not removed by filtration (no removal from an average 264 mg/L). PO4–P was little higher in raw water and increased at the effluent from an average 0.2 to 1.7 mg/L. A significant decrease in NH4–N (i.e., 25.2 ± 10.6% from 1.4 mg/L in raw AGW) was observed during the filtration process.
Option 2: coagulation–sedimentation (CS) treatment system
Figure 7a shows the removal of turbidity, COD, and BOD in the CS treatment process. Turbidity was reduced to an average of 1.5 (± 0.10) NTU. The results indicate that most of the particulate and colloidal particles were removed in the flocculation stage which resulted in low turbidity in the treated water. COD was reduced from 63 to 32.5 mg/L with a standard deviation (SD) of 4.2 mg/L, while the BOD value of 33.5 mg/L in raw AGW was reduced to 19.4 mg/L with a SD of 2 mg/L. The removals of turbidity, COD, and BOD in the CS treatment are almost similar to the removals observed in DF treatment process.
Option 3: coagulation–sedimentation–ceramic filtration (CSF)
In the combination of coagulation–sedimentation and ceramic filtration (CSF) experiments, the coagulation stage produced effluent with a very high removal of turbidity and moderate removals of COD and BOD (Fig. 7b). At the coagulation stage, the average residual turbidity, COD, and BOD were found to be 1.4 NTU (SD = ± 0.2 NTU), 35.9 mg/L (SD = ± 7.3 mg/L), and 22.6 mg/L (SD = ± 4.9 mg/L), respectively. After the filtration stage, residual turbidity, COD, and BOD remained relatively constant with effluent turbidity, COD, and BOD concentrations of 0.6 NTU, 32.5 mg/L, and 19.4 mg/L, respectively. The results indicate that the DF process can be a substitute of the CS process for the treatment of AGW. Nevertheless, the cost and the operating techniques of these two processes must be considered before selecting the best option.
Option 4: coagulation–sedimentation–AC adsorption–sedimentation (CSAS)
Figure 7c shows the removal performances of turbidity, COD, and BOD from AGW at each stage of CSAS treatment process. Analyses showed that after the coagulation stage, residual turbidity, COD, and BOD were 1.3 NTU (SD = ± 0.2NTU), 36.5 mg/L (SD = ± 7.1 mg/L), and 22.6 mg/L (SD = ± 4.8 mg/L) respectively. After the AC-adsorption stage, residual turbidity, COD, and BOD were further reduced 0.7 (± 0.3) NTU, 10.9 (± 0.3) mg/L, and 9.9 (± 1.0) mg/L, respectively, indicating that the AC stage greatly improved the treatment efficiency and contributed further to the reduction of COD and BOD. Previous studies also reported that activated carbon has high adsorption capacity, higher surface area, and microporous structure to remove BOD and COD from greywater (Sostar-Turk et al. 2005; Zipf et al. 2016).
Option 5: filtration–AC adsorption–sedimentation (FAS)
Figure 7d presents the removal of turbidity, COD, and BOD in each stage of the FAS treatment process. At the first stage after filtration, turbidity, COD, and BOD were reduced to 1.2 (± 0.8) NTU, 33.5 (± 3.8) mg/L, and 21.0 (± 1.9) mg/L, respectively, and further decreased to 0.65 (± 0.4) NTU, 11.4 (± 1.3) mg/L, and 9.2 (± 0.8) mg/L, respectively, at the second stage of AC adsorption. The residual turbidity, COD, and BOD values were almost identical to those of the CSAS with FAS treatment process.
Evaluation of AGW treatment options
Development of WQI for each AGW treatment option
The effluent quality (pH, TDS, turbidity, COD, and BOD) of the five treatment processes, investigated in this study, was compared using the t test with a 95% confidence interval. T test results verified whether there were significant differences between the water qualities treated by each system for each parameter. The turbidity values in the effluents of all the five treatment processes exhibited no significant differences (p value > 0.05).
The difference of COD and BOD removal in DF, CS, and CSF was also not significant (p value > 0.05). When comparing the CSAS and FAS treatment processes with the other three processes (DF, CS, and CSF), significant differences were seen in COD and BOD removal (p value < 0.05), indicating that AC enhanced COD and BOD removal from AGW. However, CSAS and FAS were not found significantly different from each other in terms of COD and BOD removal.
Table 2 shows the treated water quality of the five treatment options. Disinfection stage is necessary for recycling option A (Table 2). Raw AGW contains high concentrations of turbidity (14.1 NTU) and moderate levels of COD (63.2 mg/L), and BOD (37 mg/L). As per the KSA standards for recycling greywater, the raw ablution water cannot be recycled for any intended use (MWE 2006). Three treatment processes, DF, CS, and CSF, provided water quality with pH of 7.5–8.1, TDS of less than 500 mg/L, turbidity residuals of less than 1.5 NTU, COD residuals of less than 35 mg/L, and BOD residuals of less than 20 mg/L. The treated water with these three options is suitable for restricted irrigation (MWE 2006). In the CSAS and FAS treatment processes, a significant improvement in BOD and COD removals was achieved with average residual COD and BOD of less than 10 mg/L. The treated water from these two options is suitable for various intended uses after disinfection, including unrestricted irrigation, toilet flushing, street washing, and firefighting.
Importance weights of each water quality parameter were estimating using the Entropy method described in Sect. 2. Using the values of subjective weights \(\hat{w}_{j}\), decision-makers established the priori for water quality parameters as follows: pH and TDS are average important, turbidity is important, and COD and BOD are very important. The corresponding weights for pH, turbidity, COD, BOD, and TDS were estimated to be 0.004, 0.140, 0.552, 0.263, and 0.042, respectively.
Table 2 presents the final results, while the detailed calculations for development of WQI are attached as “Appendix A” (see Supplementary Information). The results show that CSAS and FAS produced a high-quality effluent suitable for unrestricted irrigation. However, these options need to be further evaluated based on their cost, land requirements, and need of skilled staff for their efficient operations.
Evaluation and final ranking of AGW treatment options using MCDM
Table 3 presents the scoring matrix showing the estimated values for all the AGW treatment options against the set of evaluation criteria. WQI, cost, and land requirements are the objective criteria, while need of skilled operators was estimated subjectively based on expert opinion.
Tentative cost estimates for all the options, given in Table 3, show that CSAS is the most expansive treatment option while DF is the least cost option. Both the DF and FAS processes commonly consist of a filtration stage where a low-cost ceramic filter was employed instead of alum coagulation. Use of a low-cost ceramic filter would be more economical and sustainable than the use of alum coagulation. Other advantages of ceramic filter are: (1) easy to maintain due to prolonged operation without clogging and has low sludge production due to chemical-free operation, (2) low energy consumption due to less use of pumps, (3) low operating cost, and (4) does not require highly skilled operators (Shafiquzzaman et al. 2018). Instead of a conventional backwash process, a very simple and effective cleaning procedure using soft brush is recommended (Shafiquzzaman et al. 2018), which eventually reduces the cost of filter recovery process (cleaning or replacement).
Priori and importance weights established using Entropy method are given in second and third rows, while final ranks found by TOPSIS application are given in the last column of Table 3. Detailed calculations are attached as “Appendix B” (see Supplementary Information). It is evident from the MCDM results provided in Table 3 that DF is technically feasible and least cost option to reuse AGW for restricted irrigation. FAS being the top ranked is the most feasible treatment option for unrestricted irrigation and other purposes.
The overall study results demonstrate that the low-cost filter (i.e., DF) may be the most technically feasible and least cost option for landscape applications in the periphery of the mosques. However, to recycle treated AGW for wider intended uses, i.e., toilet flushing, landscaping, and floor washing, FAS can be used with minimal environmental impact (i.e., highest WQI) and optimal cost (i.e., less than CSAS) and relatively less need of highly skilled operators (due to less involvement of chemicals in the treatment process). The findings of present study clearly provide technical guidelines with sufficient experimental evidence for reuse of AGW in arid and semiarid environmental regions, such and KSA and rest of Gulf countries.
Conclusion
All five filtration processes tested in this study were found simple and effective for AGW treatment, as per the required standards for possible reuse applications. Overall, high removal efficiencies of turbidity, COD, and BOD were observed for all the treatment options. BOD (< 20 mg/L) and COD (< 35 mg/L) in the DF, CS, and CSF processes were found suitable for restricted irrigation purposes, but not for unrestricted irrigation.
The addition of an AC-adsorption stage with both the ceramic filtration (FAS treatment) and alum coagulation (CSAS treatment) options brought significant reductions to the enhancement of COD and BOD and suitable for unrestricted irrigation and other purposes.
Direct ceramic filtration (DF), alum coagulation–sedimentation (CS), and alum coagulation–sedimentation–ceramic filtration (CSF) processes can be alternatively used for contaminants removal from AGW, while CSAS and FAS processes can be alternatively used for contaminants removal from AGW. Results of MCDM verified that DF is technically feasible and least cost option to reuse AGW for restricted irrigation. FAS being the top ranked option to recycle treated AGW for wider intended uses (i.e., toilet flushing, unrestricted irrigation, and firefighting) can be used with minimal environmental impacts (i.e., highest WQI), optimal cost (i.e., less than CSAS), and relatively less need of highly skilled operators (due to less involvement of chemicals in the treatment process). A low-cost ceramic filter shall positively contribute toward sustainable AGW reuse applications. The findings of present study may provide technical guidelines for reuse of AGW in arid and semiarid environmental regions, such as KSA, other Gulf countries, and rest of the world.
References
Abdullah L (2013) A new entropy weight for sub-criteria in interval type-2 fuzzy TOPSIS and its application. Int J Intell Syst Appl 02:25–33
Al-Mughalles MH, Rahman RA, Sula FB, Mahmud M, Abdullah SMS (2012) Greywater treatment using GAC biofilm reactor and sand filter system. Aust J Basic Appl Sci 6:283–292
Al-Wabel MI (2011) Simple system for handling and reuse of gray water resulted from ablution in Mosques of Riyadh City, Saudi Arabia. In: International conference on environment science and engineering, IPCBEE. IACSIT Press, Singapore, pp 42–45
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington, DC
Atanasova N, Dalmau M, Comas J, Poch M, Roda IR, Buttiglieri G (2017) Optimized MBR for greywater reuse system in hotel facilities. J Environ Manag 193:503–511
Fountoulakis MS, Markakis N, Petousi I, Manios T (2016) Single house on-site grey water treatment using a submerged membrane bioreactor for toilet flushing. Sci Total Environ 551–552:706–711
Haider H, Sadiq R, Tesfamariam S (2016) Inter-utility performance benchmarking model for small to medium sized water utilities: aggregated performance indices. J Water Resour Plan Manag 142(04015039):1–18
Hang CL, Yoon K (1981) Multiple attribute decision making: methods and applications. Springer, Berlin
Hasan MM, Shafiquzzaman M, Azam MS, Nakajima J (2011) Application of a simple ceramic filter to membrane bioreactor. Desalination 276:272–277
Hasan MM, Shafiquzzaman M, Nakajima J, Ahmed ATK, Azam MS (2015) Application of low cost ceramic filter to membrane bioreactor for greywater treatment. Water Environ Res 87:233–241
Hellegers P, Immerzeel W, Droogers P (2013) Economic concepts to address future water supply–demand imbalances in Iran, Morocco and Saudi Arabia. J Hydrol 50:62–67
Kim J, Song I, Oh H, Jong J, Park J, Choung Y (2009) A laboratory-scale graywater treatment system based on a filtration and oxidation process-characteristics of graywater from a residential complex. Desalination 238:347–357
Li F, Wichmann K, Otterpohl R (2009) Review of the technological approaches for grey water treatment and reuses. Sci Total Environ 407:3439–3449
Lin CJ, Lo SL, Kuo CY, Wu CH (2005) Pilot-scale electrocoagulation with bipolar aluminium electrodes for on-site domestic greywater reuse. J Environ Eng 131:491–495
March JG, Gual M, Orozco F (2004) Experiences on greywater re-use for toilet flushing in a hotel (Mallorca Island, Spain). Desalination 164:241–247
Mohamed RMSR, Adnan MN, Mohamed MA, Kassim AHM (2016) Conventional water filter (sand and gravel) for ablution water treatment, reuse potential, and its water savings. J Sustain Dev 9:35–43
MWE (2006) Technical guidelines for the use of treated sanitary wastewater in irrigation for landscaping and agricultural irrigation. Ministry of Water and Electricity, Riyadh
Nandi BK, Uppaluri R, Purkait MK (2009) Treatment of oily waste water using low-cost ceramic membrane: flux decline mechanism and economic feasibility. Sep Sci Technol 44:2840–2869
Noutsopoulos C, Andreadakis A, Kouris N, Charchousi D, Mendrinou A, Galanil A, Koumaki ME (2018) Greywater characterization and loadings-physicochemical treatment to promote onsite reuse. J Environ Manag 216:337–346
Ogunmodede OT, Adebayo OL, Ojo AA (2014) Enhancing adsorption capacity of clay and application in dye removal from waste water. Int Lett Chem Physiol Astron 39:35–51
Otterpohl R (2002) Options for alternative types of sewerage and treatment systems directed to improvement of the overall performance. Water Sci Technol 45:149–158
Ouda OKM (2013) Towards assessment of Saudi Arabia public awareness of water shortage problem. Resour Environ 3:10–13
Pidou M, Avery L, Stephenson T, Jeffrey P, Parsons SA, Liu S, Memon FA, Jefferson B (2008) Chemical solutions for greywater recycling. Chemosphere 7:147–155
Prathapar SA, Ahmed M, Al-Adawi S, Al-Sidiari S (2006) Design, construction and evaluation of an ablution water treatment unit in Oman: a case study. Int J Environ Stud 63:283–292
Ramon G, Green M, Semiat R, Dosoretz C (2004) Low strength graywater characterization and treatment by direct membrane filtration. Desalination 170:241–250
Shafiquzzaman M, Hasan MM, Nakajima J, Mishima I (2011) Development of a simple and effective arsenic removal filter based on ceramic filtration. J Water Environ Technol 9:333–347
Shafiquzzaman M, Al-Mahmoud A, Al-Saleem SS, Haider H (2018) Application of a low cost ceramic filter for recycling sand filter backwash water. Water 10:150
Sostar-Turk S, Petrinic I, Simonic M (2005) Laundry wastewater treatment using coagulation and membrane filtration. Resour Conserv Recycl 44:185–196
Suratkon A, Chan CM, AbRahman TST (2014) Smart WUDHU: recycling ablution water for sustainable living in Malaysia. J Sustain Dev 7:150
Zheng WY, Nigel G, Hui-juan L, Jiu-hui Q (2013) Comparison of FeCl3 and alum pre-treatment on UF membrane fouling. Chem Eng J 234:158–165
Zipf MF, Pinheiro IG, Conegero MG (2016) Simplified greywater treatment systems: slow filters of sand and slate waste followed by granular activated carbon. J Environ Manag 176:119–127
Acknowledgements
Assistance from Jesteen Joseph and Sultan Al-Qusayier for their cooperation in completion of water analysis and laboratory experiments is acknowledged gratefully.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: J Aravind.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shafiquzzaman, M., Alharbi, S.K., Haider, H. et al. Development and evaluation of treatment options for recycling ablution greywater. Int. J. Environ. Sci. Technol. 17, 1225–1238 (2020). https://doi.org/10.1007/s13762-019-02537-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-019-02537-7