Abstract
In the present work, selective magnetic solid-phase extraction speciation of Cr(VI) and Cr(III) from aqueous solution with synthesized magnetic nanoparticles modified with 1,5-diphenylcarbazide (DPC). Cr(VI) could be separated from Cr(III) due to its complexation with 1,5-diphenylcarbazide. Fe3O4@SiO2@DPC with an average size of 22 nm has been characterized by X-ray diffraction, field emission scanning electron microscopy, and Fourier transform infrared spectroscopy. The optimized conditions for adsorption were established by adjusting the parameters affecting speciation of Cr(VI) and Cr(III), such as pH, sample volume, metal ion concentration, adsorbent dosage, and time. The modified magnetic nanoparticles preferably adsorbed Cr(VI) at pH = 2.5 and made it possible to speciate it from Cr(III). Four times recovery of magnetic solid phase was investigated by eluting Fe3O4@SiO2@DPC with different concentrations of HCl, HNO3 and H2SO4 and it was found that 10 mL of 1 M HNO3 was the best eluent. The obtained data fitted with Langmuir isotherm of adsorption which indicates that the adsorption of Cr(VI) onto Fe3O4@SiO2@DPC is a type of monolayer sorption. Finally, magnetic nanoparticles were evaluated for chromium electroplating wastewater where it showed a sufficient ability to work in a real media.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution has increasingly become a serious environmental issue (Pyrzynska 2010). Toxic heavy metal ions in water hold a great harmful impact on the environment and human health (Becker 2005; Brown and Milton 2005; Simpson 2000). Among them, chromium is naturally occurring in the earth’s crust, but due to its extensive use in various industrial processes such as mining, leather tanning, textile dyeing, electroplating and wood preservatives, a widespread Cr contamination in the environment is induced. The World Health Organization (WHO) recommended a maximum allowable concentration in drinking water for Cr(VI) to be 0.05 mg L−1 (Cui et al. 2014). Chromium species exist principally in two different oxidation states in the environment, Cr(III) and Cr(VI). Their biological activity, chemical behavior and influences on the environment are very different. While Cr(III) is necessary for the living organisms especially for human as an essential nutrient and as an important agent in insulin functioning and thus carbohydrate, lipid and protein metabolism activity, Cr(VI) is a toxic species that attacks the liver, kidney and lungs (Bulut et al. 2007; Ma et al. 1998; Marques et al. 2000; Shanker et al. 2005; Wang et al. 2005).
The reduction in Cr(VI) is considered to serve as a detoxification process when it occurred at a distance from the target site while it may serve to activate chromium toxicity if it took place in or near the cell nucleus of target organs (Dayan and Paine 2001). In other words, if Cr(VI) was reduced to Cr(III) extracellularly, this form of the metal is not readily transported into cells and so toxicity is not observed. The balance that exists between extracellular Cr(VI) and intracellular Cr(III) is what ultimately dictates the amounts and rates at which Cr(VI) can enter cells and impart its toxic effects (Cohen et al. 1993; Mattia et al. 2004). Thus, Cr(VI) exerts many harmful effects in humans such as cancer and mutation in living cells, damage in DNA–protein cross-links, and break in the single-strand. On the other hand, Cr(III) is relatively less or nontoxic; hence, it is listed as an essential element for good health as well as a nutrition purpose for many organisms. Chromium is also essential for the metabolism of higher animals; for example, impaired carbohydrate metabolism seen in Cr-deficient humans can be corrected by administration of small amounts of the metal. Cr(III) is identified and partially characterized as the glucose tolerance factor (GTF) believed to be essential for the normal disposition of glucose loads (Gabriel and Salifoglou 2005).
Owing to the aforementioned toxicity, the accurate and sensitive determination of Cr(III) and Cr(VI) ions is of great significance (Bulut et al. 2007; Singh et al. 2011). Several technologies has been employed to remove Cr(VI) during the last decades such as reverse osmosis (Ozaki et al. 2002), electrochemical treatment techniques (Janssen and Koene 2002), ion exchange (Vilensky et al. 2002; Xing et al. 2007) and adsorption (Kadirvelu et al. 2000). In addition to these methods, solid-phase extraction (SPE) is now counted as an effective trace element removal method due to its simplicity, rapidity and low-cost operating (Narin et al. 2008; Zhai et al. 2010). Moreover, magnetic solid-phase extraction (MSPE) is a new development of SPE technique in which magnetic sorbents are added into the sample solution for extraction. With an additional magnetic field, magnetic sorbents can be very rapidly isolated from the bulk solution (Cui et al. 2014). Nanomagnets possess the advantages of large reactive surface area and high surface reactivity and consequently high chemical reaction efficiency for heavy metals removal. They would, however, tend to form aggregation, and it is difficult for them to be separated and recycled due to their strong attractive interparticle forces in nanoscale (Yao et al. 2016). To solve this problem, scientist found one-step method to fabricate monodispersed nanomagnets coated with SiO2 shell. Besides preventing aggregation, high porosity is another advantage of the SiO2 layer at the surface of nanomagnets. Furthermore, the presence of silanol groups makes the functionalization of Fe3O4 surface adjustable for more attractive environmental applications (Ghembaza et al. 2012).
Among the proposed functionalizations, diphenylcarbazide method is usually utilized for particularly determining trace amounts of chromium. 1,5-Diphenylcarbazide reacts in the acid medium with Cr(VI) ions to give a violet solution which is the basis of this sensitive method (Marczenko et al. 2000).
Based on the above review, our first goal in this study was to prepare 1,5-diphenylcarbazide (DPC) and SiO2-modified magnetic nanoparticles by the co-precipitation method and secondly, to establish a method of MSPE combined with FAAS for the speciation of Cr(VI) from Cr(III) in samples. The modified magnetic nanoparticles were characterized by XRD, FESEM and FTIR. The efficient parameters in the adsorption of the chromium species, such as pH, sample volume, the concentration of ion metals, extraction time, adsorbent dosage and eluent type for recovery were investigated, and the optimized conditions were successfully recognized in electroplating wastewater as a real water sample analysis.
Materials and methods
Standard solution and reagents
For the preparation of all samples, deionized water was used. All reagents were used without further purification. The stock solutions (1000 mg L−1) of Cr(III) and Cr(VI) were prepared by dissolving the appropriate amounts of Cr (NO)3·9H2O and K2Cr2O7. Polyethylene glycol 6000, iron(III) nitrate nonahydrate (Fe(NO3)·9H2O), iron(II) sulfate heptahydrate (FeSO4·7H2O), sodium hydroxide (NaOH), ammonia solution (25%), hydrochloric acid (HCl), nitric acid (HNO3), sulfuric acid (H2SO4), 1,5-diphenylcarbazide (DPC), trichloro(octadecyl)silane, ethanol and all other reagents used were of analytical grade from Merck (Germany).
Preparation of Fe3O4@SiO2@DPC magnetic nanoparticles
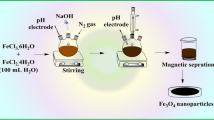
Modified magnetic nanoparticles with 1,5-Diphenylcarbazide were prepared by the following procedure: Fe3O4 was firstly synthesized by dissolving 4 g of Fe(NO3).9H2O and 1.4 g of FeSO4·7H2O in 100 mL of deionized water under helium atmosphere in three neck balloon with the aid of a magnetic stirrer. After 30 min, the solutions color changed to gloom. At this juncture, polyethylene glycol 6000 solution was prepared by dissolving 1 g of the powder in warm degassed deionized water with Heidolph homogenizer at 4000 rpm for 5 min and this solution was added dropwise to the first solution in 30 min duration; then, 40 mL ammonia solution (25%) was added apace. Finally, the precipitated black nanomagnets coated with polyethylene glycol were collected with an external magnetic field and washed with deionized water and ethanol. The cycle of washing and magnetic separation was repeated for several times. The product was dispersed in 100 mL ethanol for further use. Modification of magnetic nanoparticles with DPC was performed in the second step: to 10 mL ethanol containing 0.1 g DPC and 0.2 mL trichloro(octadecyl)silane, 1 drop of water and HCl (1 M) was added. The solution was stirred vigorously. This solution was then poured over the dispersed Fe3O4 nanoparticles solution. After 30 min, Fe3O4@SiO2@DPC was produced and collected with an external magnetic field and stand in oven for 2 h at 100 °C for fully drying.
Batch adsorption experiments
In order to evaluate the adsorption capacities of the Fe3O4@SiO2@DPC, batch experiments of chromium were conducted. Fifty milliliters of solution with different concentrations of Cr(III) and Cr(VI) (3–10 mg L−1) were prepared by dilution of the stock standard solution. Fe3O4@SiO2@DPC (0.05–0.12 g) was added to the above solutions (25–100 mL) under mechanical agitation (15–90 min). For all experiments, the initial pH value (2.5–10.5) of the samples was adjusted with HCl and NaOH solutions.
After the adsorption processes, Fe3O4@SiO2@DPC was separated by an external magnetic separation and the sustained ions were immediately analyzed by atomic absorption spectrometry (WFX-1F2, China).
To calculate the adsorption capacity and adsorption rate before and after adsorption, the following equations were employed:
where C0 and Ce are the initial and equilibrium concentrations (mg L−1) of Cr(VI) and Cr(III), respectively, V is the volume of Cr solution (L), and W is the weight of the Fe3O4@SiO2@DPC used (g). The Q is the sorption capacity, and E is percent adsorption (Li et al. 2014).
Results and discussion
Characteristics of the Fe3O4@SiO2@DPC
X-ray diffraction
X-ray powder diffraction (XRD) analysis was conducted on a D8-Advance (Product Cooperation Bruker AXS and Siemens) fitted with a Cu-Kα radiation tube. X-ray diffractograms of Fe3O4 and Fe3O4@SiO2@DPC magnetic nanoparticles are presented in Fig. 1a, b.
Chemical composition determined using X’pert High Score for Fe3O4 (Fig. 1a) is confirmed by JCPD file (card No 85-1436). Here, the match of Fe3O4 peaks with Fe3O4@SiO2@DPC peaks (Fig. 1b) reveals that coating process did not result in the phase change in Fe3O4.
Crystallite size (D) was calculated from Scherrer equation:
where D is crystallite size, λ is the wavelength of X-ray, β is the modified FWHM, and θ is the reflection angle. Crystallite size obtained for Fe3O4 was 11.63 nm. No difference was observed, as SiO2@DPC did not affect the XRD pattern.
Surface morphology
The morphology and size of the Fe3O4 and its modified form with DPC were determined using field emission scanning electron microscopy (FESEM) by employing a digital scanning microscope (Mira 3-XMU Simaging Detectors). The samples were coated with gold prior to FESEM analysis.
The image shown in Fig. 2a indicates that Fe3O4 exhibits a spherical morphology and good dispersity. The size distribution average was less than 17 nm in diameter. From Fig. 2b, it could be comprehended that after modifying the surface of the magnetic nanoparticles by DPC, no significant change has occurred in shape and size (maintaining spherical shape and the size was less than 22 nm). Actually, a slight increase in the size of the modified Fe3O4 indicates that a thin and uniform layer of DPC properly covered the surface of nanoparticles.
Figure 2c shows the particle size analysis of Fe3O4@SiO2@DPC magnetic nanoparticles by TEM (Philips CM30 300 kV). Magnetic nanoparticles were distributed, with a diameter less than 30 nm.
Fourier transform infrared spectroscopy
Fourier transform infrared spectroscopy (FTIR) spectra of Fe3O4 and Fe3O4@SiO2 @DPC are shown in Fig. 3a, b. In Fig. 3a, sharp peaks at 452.57 and 536.22 cm−1 can be ascribed to magnetic Fe3O4 nanoparticles. The weakening in the intensity of Fe3O4 peaks (Fig. 3b) and the occurrence of spectra in 1630, 1080 and 799 cm−1 can be attributed to SiO2 coating layer. Some peaks around, 1486, 1442 and 1406 cm−1 could be assigned to benzene cycle and amine in DPC molecules. These results demonstrate that SiO2 and DPC had been doped in the surface of F3O4 magnetic nanoparticles (Zhai et al. 2010).
Optimization of MSPE conditions
Effect of pH
Among the tested variables, pH was found to be the most critical factor that ruled the adsorption of metal ions on the surface of Fe3O4@SiO2@DPC because the initial pH is known to govern the surface charge of the adsorbent as well as the metal ions. The evaluation of pH effect on the speciation of Cr(VI) and Cr(III) was accomplished by adjusting the pH of the 50 mL sample solutions containing 5 mg L−1 of Cr(VI) and Cr(III) ions to fit in the range of 2.5–10.5, and the results are given in Fig. 4a. Clearly, the adsorption behavior of metal ions was sensitive to the pH changes. As we know, several forms of Cr(VI) ion exist in aqueous solution which are namely chromate (CrO42−), dichromate(Cr2O72−) and hydrogen chromate(HCrO4−). These ion forms are related to the solution pH and total chromate concentration (Li et al. 2009; Weckhuysen et al. 1996). At lower pH (1 < pH < 7), HCrO4− is the major form of Cr(VI), but it will change into Cr2O72− when the pH is above 7.0 (Fan et al. 2010; WooáLee and BináKim 2011). In the range of 2.5–7.5 and 7.5–10.5, Cr(VI) and Cr(III) exhibited totally different adsorption behaviors (Fig. 4a). While the adsorption percentage of Cr(VI) decreased (94–52%) with the increase in the pH in the range 2.5–7.5, adsorption of Cr(III) increased (8–48%). In this range of pH, the amino groups of 1,5-diphenylcarbazide mainly existed in the form of NH2+ on the surface of Fe3O4@SiO2@DPC, whereas Cr(VI) mainly existed in the form HCrO4−. Therefore, the adsorption percentage of Cr(VI) was higher than that of Cr(III) and the maximum adsorption percentage was obtained for Cr(VI). At pH 7.5, Cr(VI) and Cr(III) showed similar adsorption percentages because Cr(VI) in the form of HCrO4− was to end and Cr(III) in the form of Cr(OH)3 was to start. The adsorption percentage of Cr(III) rapidly increased with the further increase in pH from 7.5 to 10.5 (approximately 98%), while Cr(VI) could be quantitatively adsorbed in this range. This range of pH could be comfortable for specific speciation of Cr(III) from Cr(VI).
a Effect of pH on the adsorption percentage of Cr(III) and Cr(VI) on the Fe3O4@SiO2@DPC (0.1 g), concentration 5 mg L−1, extraction time 60 min, sample volume 50 mL, b the effect of adsorbent amount in the range of 0.05–0.12 g, metal ion concentration 5 mg L−1, pH = 2.5, extraction time 60 min, sample volume 50 mL and (c) amount of Q(mg g-1) for Cr(VI) and Cr(III) in different concentration
It can be seen from Table 1 that the adsorbent has maximum and minimum adsorbing capacity (Q) for Cr(VI) and Cr(III), respectively, when pH is 2.5 (Li et al. 2014). In the subsequent experiments, pH = 2.5 was selected for the determination of Cr(VI).
Effect of adsorbent dosage
The influence of nano-Fe3O4@SiO2@DPC amount on the adsorption of Cr(VI) and Cr(III) in 5 mg L−1 initial concentration in the range of 0.05–0.12 g at pH 2.5 was investigated. The results are given in Fig. 4b. For Cr(VI), it was predictable that increasing Fe3O4@SiO2@DPC amount from 0.05 to 0.1 g would increase the active sites of DPC that coats the surface of nanomagnets. The decrease in Cr(VI) removal between 0.1 and 0.12 g could be associated with steric hindrance between Fe3O4@SiO2@DPC magnetic nanoparticles. For Cr(III), there was no significant difference in adsorption within the range of magnetic nanoparticles amount studied. Figure 4c shows the values of Q (mg g−1) in different concentration of Cr(VI) and Cr(III). As illustrated, according to selectivity effect of DPC for Cr(VI), Q has increased (1.17–4) with increasing in Cr(VI), but increasing in Q for Cr(III) was not significant.
Effect of sample volume, concentration, and time
In order to deal with real samples, the maximum applicable sample volume must be determined. In this regard, 25, 50 and 100 mL samples containing 5 mg L−1 of Cr(VI) and Cr(III) with 0.1 g of Fe3O4@SiO2@DPC at optimum pH 2.5 were prepared. The results depicted in Fig. 5a imply that maximum adsorption quantity of Cr(VI) and Cr(III) in 50 mL on the Fe3O4@SiO2@DPC was 94 and 8%, respectively. The adsorption of Cr(VI) with 25 and 100 mL was not affected. The interpretation is as follows: in the 25 mL volume of the sample, the collision of nano-Fe3O4@SiO2@DPC will not permit Cr ions to perch on its surface. On the contrary, in a 100-mL volume of sample, the adsorption was decreased with an increase in the volume of the sample due to wide space between Cr ions and nanomagnets. Therefore, 50 mL of samples was selected as an optimum volume for the speciation of Cr(VI) and Cr(III).
a Effect of sample volume in present 5 mg L−1 of metal concentration, Fe3O4@SiO2@DPC (0.1 g), pH = 2.5, extraction time 60 min, b effect of initial concentration of metal in present of Fe3O4@SiO2@DPC (0.1 g) and pH = 2.5, extraction time 60 min, sample volume 50 mL, c equilibration time for adsorption of metal ion (5 mg L−1) in the presence of Fe3O4@SiO2@DPC (0.1 g) at pH = 2.5
The influence of the initial concentration of Cr(VI) and Cr(III) on the selective speciation on the nanomagnetic Fe3O4@SiO2@DPC is an important aspect of this study. The effect of this parameter was studied in the range of 3–10 mg L−1 at pH 2.5 and 0.1 g of magnetic solid phase. The results are illustrated in Fig. 5b. It can be seen that the best initial concentration for adsorption of Cr(VI) (approximately 94%) is 5 mg L−1.
When the concentration of Cr(VI) is 3 mg L−1, Cr(VI) was profusely surrounded by water and attaching to the DPC of nanomagnets was difficult. On the other hand, aggregation and collision of Fe3O4@SiO2@DPC did not allow the adsorption of Cr(VI) on the Fe3O4@SiO2 modified with DPC. Furthermore, the degree of adsorption of Cr(VI) decreased with increased initial concentration in the range 5–10 mg L−1 as few active sites for adsorption of Cr(VI) were available.
For Cr(III), results of adsorption in the range of 3–10 mg L−1 initial concentration with 0.1 g Fe3O4@SiO2@DPC and pH 2.5 display a nonsignificant adsorption (7–16.2%). Hence, selective speciation of Cr(VI) and Cr(III) can be accomplished at any initial concentration.
Equilibration time for Cr(VI) and Cr(III) is shown in Fig. 5c. Apparently, Cr(VI) was rapidly adsorbed (about 80%) on the nano-Fe3O4@SiO2@DPC in the first 15 min, while Cr(III) demonstrated a low affinity to be adsorbed (about 2%) on the magnetic nanoparticles. The reason behind this is that in the first 15 min, DPC possessed adequate coordination capacity and selective adsorption for Cr(VI), but it was not the case for Cr(III). In the next 15–90 min, Cr(VI) adsorption increased gradually from 80 to 94%, whereas Cr(III) stands at 2–8%.
Eluent type and recovery
In order to elute Cr(VI) from the nano-Fe3O4@SiO2@DPC; HCl, HNO3 and H2SO4 in different concentrations were used. The results given imply that HNO3 was better than HCl and H2SO4 as (10 mL, 1 mol L−1) of HNO3 was sufficient for 96% recovery. As shown in Fig. 6, after 4 times elution of magnetic solid phase with the different eluents, Fe3O4@SiO2@DPC could remove up to 98% of Cr(VI) in the presence of HNO3, while in the presence of HCl and H2SO4, the adsorption ability decreased. The explanation is that since the protonation of the amine groups is carried out under strong acid conditions, the coordination interaction of DPC and Cr(VI) could be disrupted (Zhai et al. 2010). After washing the Fe3O4@SiO2@DPC with hydrochloric acid and sulfuric acid in the second time, the nanoparticles may have dissolved. In this interim, the groups of DPC were separated from the surface of magnetic nanoparticles and their efficiency to absorb Cr(VI) decreased.
Adsorption Isotherms of Cr(VI)
Figure 7 presents the variation of adsorption capacity as a function of contact time. The equilibrium could be reached after about 60 min in all cases. In terms of isotherms, the Langmuir adsorption isotherm assumes that the adsorbed layer is one molecule thick. The strength of the intermolecular attractive forces is believed to fall off rapidly with distance. Freundlich adsorption isotherm suggest that the Cr(VI) concentrations on the adsorbent will increase as long as there is an increase in the Cr(VI) concentration. Langmuir and Freundlich isotherms are represented by the following equations, respectively:
where Qm and KL are Langmuir constants related to adsorption efficiency and energy of adsorption, respectively. KF is the Freundlich capacity factor (mg g−1 (L mg−1)1/n) and (1/n) is the Freundlich intensity parameter (Darwish et al. 2016). The linear plot of Ce/Qe versus Ce suggests the applicability of Langmuir isotherm, whereas the linear plot of log Qe versus log Ce suggests the applicability of Freundlich isotherm.
The obtained data shown in Table 2 indicates that Cr(VI) adsorption fitted only by the Langmuir (R2 = 0.98) and not by the Freundlich isotherm (R2 = 0.92), suggesting the monolayer adsorption of Cr(VI) on Fe3O4@SiO2@DPC surface and the homogeneous nature of the adsorbent (Dada et al. 2012; Dai et al. 2012; Kara and Demirbel 2012).
The essential features of this isotherm may be expressed in terms of equilibrium parameter RL, which is a dimensionless constant referred to as separation factor or equilibrium parameter.
where C0 is the initial concentration of Cr(VI) and b is the constant related to the energy of adsorption (Langmuir Constant). RL value indicates the adsorption nature to be either unfavorable if RL > 1, linear if RL = 1, favorable if 0 < RL < 1 or irreversible if RL = 0 (Fan et al. 2017; Saadi et al. 2015). Here, Qm, b, RL and R2 values obtained are listed in Table 2.
Speciation of Cr(VI) and Cr(III)
In order to separate Cr(VI) from Cr(III), solutions containing different amounts of Cr(VI) and Cr(III) were prepared. Then, optimum conditions were applied to these solutions. The results given in Table 3 indicate that Fe3O4@SiO2@DPC is appropriate for the separation of Cr(VI) from Cr(III). The adsorption mechanism is based on the redox reaction of 1,5-diphenylcarbazide with Cr(VI) in acidic solution to form the Cr(III)-diphenylcarbazone complex. At this point, the ultimate goal of our study was to remove Cr(VI) not to prevent its reduction to Cr(III) on adsorbent surface. Relative selectivity factor, as an important parameter, was evaluated by the following equations (Beyki et al. 2014):
D is the distribution ratio (mL g−1) and α is the relative selectivity factor. The α value of Cr(VI)/Cr(III) was 2611, indicating that Cr(VI) could be determined even in the presence of Cr(III).
Evaluation of Fe3O4@SiO2@DPC in real sample
Fe3O4@SiO2@DPC magnetic nanoparticles were evaluated on chromium electroplating wastewater. To assure that all chromium in our sample is Cr(VI), oxidation procedure was performed as reported elsewhere (Narin et al. 2008); 0.5 mL of K2S2O8 (1%, W/V), one drop of AgNO3 (0.01%, W/V) and 0.5 mL concentrated H2SO4 were added to 50 mL of wastewater. Prior to oxidation, the sample was diluted to obtain 4 mg L−1 concentration. Subsequently, the pH was adjusted to 2.5 and 0.1 g of magnetic nanoparticle was added. The sample was taken after one hour of shaking. The result after N: 3 samples illustrated a 3.7 ± 0.01 mg L−1 adsorption of Cr(VI) from electroplating wastewater, i.e., the composite is highly effective for real matrixes.
Conclusion
Fe3O4@SiO2@DPC, as modified magnetic nanoparticles, was successfully synthesized. MSPE combined with FAAS for the selective speciation of Cr(VI) and Cr(III) in water media was developed. MSPE nanoparticles were characterized by XRD, FESEM and FTIR. Results have shown that Fe3O4@SiO2@DPC nanoparticles have many advantages: (i) good nature for speciation of Cr(VI) and Cr(III) and (ii) four times recyclability with HNO3. Furthermore, Langmuir isotherm fitted well with the data which indicates that the adsorption of Cr(VI) is a type of monolayer sorption. In conclusion, we believe that Fe3O4@SiO2@DPC developed in this work may hold a promising ability for speciation of Cr(VI) and Cr(III). It can be used efficiently for this purpose in a variety of fields like tannery wastewater treatment, electroplating.
References
Becker JS (2005) Trace and ultratrace analysis in liquids by atomic spectrometry TrAC. Trends Anal Chem 24:243–254
Beyki MH, Bayat M, Miri S, Shemirani F, Alijani H (2014) Synthesis, characterization, and silver adsorption property of magnetic cellulose xanthate from acidic solution: prepared by one step and biogenic approach. Ind Eng Chem Res 53:14904–14912
Brown RJ, Milton MJ (2005) Analytical techniques for trace element analysis: an overview TrAC. Trends Anal Chem 24:266–274
Bulut V, Duran C, Tufekci M, Elci L, Soylak M (2007) Speciation of Cr(III) and Cr(VI) after column solid phase extraction on Amberlite XAD-2010. J Hazard Mater 143:112–117
Cohen MD, Kargacin B, Klein CB, Costa M (1993) Mechanisms of chromium carcinogenicity and toxicity CRC. Crit Rev Toxicol 23:255–281
Cui C, He M, Chen B, Hu B (2014) Chitosan modified magnetic nanoparticles based solid phase extraction combined with ICP-OES for the speciation of Cr(III) and Cr(VI). Anal Methods 6:8577–8583
Dada A, Olalekan A, Olatunya A, Dada O (2012) Langmuir, Freundlich, Temkin and Dubinin-Radushkevich isotherms studies of equilibrium sorption of Zn2 + unto phosphoric acid modified rice husk. J Appl Chem 3:38–45
Dai J, Ren F, Tao C (2012) Adsorption of Cr(VI) and speciation of Cr(VI) and Cr(III) in aqueous solutions using chemically modified chitosan Int J Env Res. Public Health 9:1757–1770
Darwish M, Mohammadi A, Assi N (2016) Integration of nickel doping with loading on graphene for enhanced adsorptive and catalytic properties of CdS nanoparticles towards visible light degradation of some antibiotics. J Hazard Mater 320:304–314
Dayan A, Paine A (2001) Mechanisms of chromium toxicity, carcinogenicity and allergenicity: review of the literature from 1985 to 2000. Hum Exp Toxicol 20:439–451
Fan Z-J et al (2010) Facile synthesis of graphene nanosheets via Fe reduction of exfoliated graphite oxide. ACS Nano 5:191–198
Fan S et al (2017) Facile synthesis of tea waste/Fe 3 O 4 nanoparticle composite for hexavalent chromium removal from aqueous solution. RSC Adv 7:7576–7590
Gabriel K, Salifoglou A (2005) A chromium (iii)-citrate complex from aqueous solutions. J Agroaliment Process Technol 11:57–60
Ghembaza H, Zerga A, Saïm R (2012) Effects of thickness and chemical quality of SiO2 barrier on POCl3 diffusion during the formation of emitter. Energy Procedia 18:733–740
Janssen L, Koene L (2002) The role of electrochemistry and electrochemical technology in environmental protection. Chem Eng J 85:137–146
Kadirvelu K, Faur-Brasquet C, Cloirec PL (2000) Removal of Cu (II), Pb(II), and Ni (II) by adsorption onto activated carbon cloths. Langmuir 16:8404–8409
Kara A, Demirbel E (2012) Kinetic, isotherm and thermodynamic analysis on adsorption of Cr(VI) ions from aqueous solutions by synthesis and characterization of magnetic-poly (divinylbenzene-vinylimidazole) microbeads. Water Air Soil Pollut 223:2387–2403
Li Y, Gao B, Wu T, Sun D, Li X, Wang B, Lu F (2009) Hexavalent chromium removal from aqueous solution by adsorption on aluminum magnesium mixed hydroxide. Water Res 43:3067–3075
Li L, Duan H, Wang X, Luo C (2014) Adsorption property of Cr (vi) on magnetic mesoporous titanium dioxide–graphene oxide core–shell microspheres. New J Chem 38:6008–6016
Ma W, Cai R, Lin Z (1998) Studies on adsorption properties of Chromium (VI) on the nanometer-size TiO2 powders surfaces using on-line flow-injection analysis. Chem J Chin Univ Chin 19:1566–1569
Marczenko Z, Balcerzak M, Kloczko E (2000) Separation, preconcentration and spectrophotometry in inorganic analysis. Elsevier Science, New York
Marques M, Salvador A, Morales-Rubio A, De la Guardia M (2000) Chromium speciation in liquid matrices: a survey of the literature. Fresenius J Anal Chem 367:601–613
Mattia GD et al (2004) Impairment of cell and plasma redox state in subjects professionally exposed to chromium. Am J Ind Med 46:120–125
Narin I, Kars A, Soylak M (2008) A novel solid phase extraction procedure on Amberlite XAD-1180 for speciation of Cr(III), Cr(VI) and total chromium in environmental and pharmaceutical samples. J Hazard Mater 150:453–458
Ozaki H, Sharma K, Saktaywin W (2002) Performance of an ultra-low-pressure reverse osmosis membrane (ULPROM) for separating heavy metal: effects of interference parameters. Desalination 144:287–294
Pyrzynska K (2010) Carbon nanostructures for separation, preconcentration and speciation of metal ions TrAC. Trends Anal Chem 29:718–727
Saadi R, Saadi Z, Fazaeli R, Fard NE (2015) Monolayer and multilayer adsorption isotherm models for sorption from aqueous media. Korean J Chem Eng 32:787–799. https://doi.org/10.1007/s11814-015-0053-7
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Int 31:739–753
Simpson NJ (2000) Solid-phase extraction: principles, techniques, and applications. CRC Press, Boca Raton
Singh S, Barick K, Bahadur D (2011) Surface engineered magnetic nanoparticles for removal of toxic metal ions and bacterial pathogens. J Hazard Mater 192:1539–1547
Vilensky MY, Berkowitz B, Warshawsky A (2002) In situ remediation of groundwater contaminated by heavy-and transition-metal ions by selective ion-exchange methods. Environ Sci Technol 36:1851–1855
Wang J, Jia B, Guo L, Lin Q (2005) The determination of chromium in feeds by flame atomic absorption spectrophotometry. Guang pu xue yu guang pu fen xi = Guang pu 25:1142
Weckhuysen BM, Wachs IE, Schoonheydt RA (1996) Surface chemistry and spectroscopy of chromium in inorganic oxides. Chem Rev 96:3327–3350
WooáLee J, BináKim S (2011) Enhanced Cr(VI) removal using iron nanoparticle decorated graphene. Nanoscale 3:3583–3585
Xing Y, Chen X, Wang D (2007) Electrically regenerated ion exchange for removal and recovery of Cr(VI) from wastewater. Environ Sci Technol 41:1439–1443
Yao H, Ding Q, Zhou H, Zhao Z, Liu G, Wang G (2016) An adsorption–reduction synergistic effect of mesoporous Fe/SiO2–NH2 hollow spheres for the removal of Cr (vi) ions. RSC Adv 6:27039–27046
Zhai Y, Se Duan, He Q, Yang X, Han Q (2010) Solid phase extraction and preconcentration of trace mercury(II) from aqueous solution using magnetic nanoparticles doped with 1,5-diphenylcarbazide. Microchim Acta 169:353–360. https://doi.org/10.1007/s00604-010-0363-8
Acknowledgement
The authors wish to thank Science and Research Branch, Islamic Azad University for the Instrumental support of this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Q. Aguilar-Virgen.
Rights and permissions
About this article
Cite this article
Assi, N., Aberoomand Azar, P., Saber Tehrani, M. et al. Selective solid-phase extraction using 1,5-diphenylcarbazide-modified magnetic nanoparticles for speciation of Cr(VI) and Cr(III) in aqueous solutions. Int. J. Environ. Sci. Technol. 16, 4739–4748 (2019). https://doi.org/10.1007/s13762-018-1868-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-018-1868-7