Abstract
In this study, electric arc furnace dust, waste of steelmaking industry, was selected as heterogeneous Fenton catalysts for degradation of methylene blue solution. Co and Cu nanoparticles were added on EAF dust via impregnation method and characterized by ICP, XRF, XRD, BET, FESEM and HRTEM techniques. The Co/EAF catalyst displayed the best activity in removing high concentrated methylene blue solution (50 mg L−1) with initial pH, where decolorization was measured as response. Furthermore, response surface methodology with central composite design was applied to evaluate the effects of initial pH, catalyst dosage, the molar ratio of H2O2 to MB and their interactive effect. According to ANOVA results, quadratic model was suggested as a significant model. This statistical technique revealed that the low-cost and magnetic recyclable Co/EAF heterogeneous Fenton catalyst had suitable catalytic activity in different reaction conditions and able to remove methylene blue completely. Finally, we studied the catalytic activity of Co/EAF as the best catalyst, in dye removing from textile factory wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Treatment of wastewaters carrying recalcitrant contaminants has been major issues in the past decades. Recalcitrant compounds present high molecular weight and hydrophobic nature and are usually found in agricultural and industrial wastewaters. A great number of industrial activities are identified for generating recalcitrant wastewater including from printing, dyeing and textile industry effluents (Oller et al. 2011; Rahim Pouran et al. 2014). These contaminants include toxic synthetic aromatic compounds such as benzene, phenol, anilines or chlorophenols, among other, which are resistant to biodegradation. Those streams must be treated as inexpensively as possible in a safe and environmentally friendly manner (Rahim Pouran et al. 2014). For a complete degradation of these compounds, classical and conventional processes like biological treatments are not efficient and only degrade wastewater partially which produce contaminated intermediates and sludge. Therefore, the development of new and effective techniques is necessary (Martins et al. 2013). Advanced oxidation processes (AOPs) are considered a highly competitive water treatment technology for the removal of those organic pollutants not treatable by conventional techniques due to their high chemical stability and/or low biodegradability (Munoz et al. 2015). These processes operate at near ambient temperature and pressure involving the generation of hydroxyl radicals in sufficient quantity to allow oxidizing the organic pollutants to carbon dioxide, water and (if the pollutant contains heteroatoms) mineral acids (Munoz et al. 2015; Dias et al. 2016). Attending to the way of generating hydroxyl radicals, the AOPs are usually classified as chemical, electro-chemical, sono-chemical and photochemical processes. Among them, the chemical Fenton process is one of the most cost-effective AOP and has great potential for removing, degrading and mineralizing organic pollutants.
The conventional Fenton process is based on the generation of hydroxyl radicals from the decomposition of hydrogen peroxide in the presence of ferrous iron (Fe2+) at acidic conditions (Eq. (1)) yielding Fe3+. Additionally, these ferric ions react with hydrogen peroxide producing hydroperoxyl radicals and regenerating the catalyst, the ferrous ions (Eq. (2)). However, the process is much more complex and includes many other reactions, which could be classified in the three general groups of free-radical processes: initiation, propagation and termination reactions (Munoz et al. 2015; Dias et al. 2016).
Finally, the overall process of the oxidation of organic pollutants can be schematically described by the following paths:
Total reaction,
The Fenton process can be carried out under homogeneous and heterogeneous reactions. Homogeneous ones are commonly used but suffer some drawbacks like leaching iron catalyst into the solution, sludge generation, narrow pH range and unrecyclable catalysts (Rahim Pouran et al. 2015). In this context, the use of solid supported catalysts in the heterogeneous Fenton reaction is an excellent alternative (Munoz et al. 2015). Supported catalysts allows increasing the surface area of the metals species, diminishes the sintering of the active phase and improves the thermal and chemical stability of catalyst.
Electric arc furnace (EAF) dusts are waste materials produced several tones a day by steel-making factories (Sofili 2004). These dusts include iron or iron oxides, zinc oxide, and another component based on the charged materials into arc furnace (Alizadeh and Momeni 2016). The scraps: a typical EAF dust usually contains 24% iron by weight. The downgrade of scrap can contain large amounts of zinc (as high as 44% ZnO). Steel-making factories have some problems with EAF dust management, handling and transportation, because of its environmental problems. Correct management of this waste material is a real challenge for the steel-making industries. Due to its abundance and low cost, the exploitation of this waste material has become very attractive (Sofili 2004; Alizadeh and Momeni 2016). Based on the chemical composition of this waste material, it was highly potential to be used as Fenton’s reaction catalyst. EAF dust contains high percentage of iron in magnetite phase that contains Fe2+ and Fe3+ both. Therefore, it can be remarkable that a waste, which can cause significant environmental pollution, is used in industrial wastewater decontamination by heterogeneous Fenton oxidation. In this regards, Meccozi et al. (2006) used the EAF dust and pure hematite without any modification or treatment as heterogeneous catalyst in order to remove pentachlorophenol (PCP) from solution, and the efficiency of PCP removal was around 50%. Although the catalytic activities of this type of waste materials are not very high, it is possible to increase their activity by some modification. Despite the advantages of heterogeneous Fenton reaction with regard to the conventional homogeneous Fenton process, its kinetic rate is lower. To overcome this problem, the use of additional transition metal as co-catalyst has been suggested (Munoz et al. 2015). Several researchers have proposed substituting transition metal into spinel structure of magnetite. Costa et al. (2006) found that the presence of Co or Mn increased the activity of magnetite, whereas Ni had inhibited H2O2 decomposition in the catalytic wet peroxide oxidation of methylene blue (MB). Similarly, Baldrian et al. (2006) realized that doping magnetite with Co, Cu or Mn led to effective oxidation of synthetic dyes, maintaining its activity upon successive runs. Their results show that the presences of Mn and Co are effective by two ways: (a) they promote the generation of hydroxyl radicals from decomposition of H2O2 and (b) they accelerate electron transfer giving rise to a more efficient regeneration of Fe(II). In the same way, Magalhães et al. (2007) demonstrated that the presence of small amounts of Cr in the magnetite structure led to a significant increase in activity in the oxidation of azo dyes with complete discoloration and high mineralization (Munoz et al. 2015).
The aim of this study is to synthesize an eco-friendly heterogeneous magnetic catalyst using EAF dust waste as catalyst support for Fenton’s reaction in order to degrade a typical dye, methylene blue. Copper and cobalt transition metals were chosen as co-catalysts to increase the catalytic activity of EAF dust materials. The advantages of EAF-based catalysts include recyclability, magnetic (easy to separate), active in wide pH range, cost effective and environmental friendly in two aspects by a waste.
The experimental work for this study was conducted in Materials and Energy Research Center (Alborz province, Iran) from September 2015 to November 2016.
Materials and methods
Material
Electric arc furnace dusts and slag were obtained from Iran National Steel Industrial Group (Iran, Khuzestan), while the ratio of scrap/direct reduced iron (DRI) was zero that means no scrap was charged to the furnace that time. Other chemicals (AR grade) were commercially available and used without further purification.
Characterization
The chemical composition of EAF dust was carried out by XRF equipment using ARL OPTIM’X 8410. To investigate the correct elements analysis, ICP-MS was established with Varian equipment. The XRD analyzes were established by Philips PW3710 to determine the crystalline phase, and founded phases were confirmed by X’pert software. FESEM and HRTEM were used to investigate surface morphology and particle size of EAF dust and synthesized catalyst. FESEM was performed with TESCAN Mira 3 FESEM. A HRTEM JEOL 2100F operating at an accelerating voltage of 200 kV was used to obtain transmission electronic microscope (TEM) image. To measure specific surface area, BET method was used with Gemini 2370. Decolorization and degradation of methylene blue was investigated by UV adsorption of methylene blue solution with UV–visible spectrophotometer (T80+, PG instrument ltd, England). COD (Chemical Oxygen Demand) analysis was carried by using Pallintest 8000 COD analyzer.
Catalyst synthesis
After prewashing the EAF dust, the magnetite particles were separated from some impurities by magnet. Copper and cobalt metals were settled on the surface of dust particles by impregnation method. For the preparation of Cu/EAF catalyst, 950 mg of EAF dust was added to 80 ml distilled water, stirred about 10 min and then sonicated for 20 min. After that, 196 mg of CuSO4 (copper sulfate) dissolved in 20 ml water was added drop-wise into EAF dust suspension, while suspension was stirring about 1 h. The nanoparticles deposited on the surface of EAF dust were drying at 110 °C over night. The remained solid was named Cu/EAF. The cobalt nanoparticles were settled by similar methods, using cobalt sulfate as Co-precursor and labeled Co/EAF.
Fenton catalytic activity test
Fenton’s reaction was carried out in a glass vessel. Methylene blue solution was prepared with concentration of 50 mg L−1. The initial pH was adjusted on 7 by adding NaOH or HCl 0.1 mol L−1 solutions. Then, 50 mg of catalyst was added to 50 ml of methylene blue solution. The reaction was initiated while 1 ml H2O2 was added. The Vessel was shaken with 180 rpm for 3 h. Cu/EAF, Co/EAF, EAF dust and EAF slag were tested. The reaction mixture was centrifuged while the reaction was finished, and then decolorization and degradation of methylene blue was investigated by UV–visible spectrophotometer with the max absorbance of 664 nm (Chen et al. 2014; Rahim Pouran et al. 2015). Prior to measure absorbance of methylene blue, a calibration curve was obtained with standard solutions. Color intensity was measured in terms of space units (SU) by measuring color absorbance in visible 400–700 nm wavelengths with UV–visible spectrophotometer and calculating the area below curves (Chinwetkitvanich 2000). COD (chemical oxygen demand) was determined according to the Standard Methods (Eaton et al. 1998).
Experimental design
The response surface methodology (RSM) was used to design experiments to obtain an optimal response. To evaluate the effects of initial pH (pH), catalyst dosage (Cat), and the molar ratio of H2O2 to methylene blue (HMR) on the degree of decolorization of methylene blue by the Fenton oxidation process, a central composite design was employed using Design-Expert software 7.1.5 (Stat-Ease, Inc., Minneapolis, MN). The variables were simultaneously changed in a central composite constrained design. For three variables and two levels (low and high, or + 1 and − 1), 20 experiments were performed including six central points in order to examine the statistical consistency of the mathematical model. The effect of pH, catalyst dosage and the molar ratio of H2O2 to methylene blue were assessed in the ranges of 4.62–9.38, 0.7–1.3 g L−1, and 0.0028–0.0052, respectively. The ranges of the variables were chosen according to the values currently reported (Liang et al. 2012; Rahim Pouran et al. 2015).
Results
Catalyst characterization
XRF analysis result of the EAF waste material is shown in Table 1. The result showed that the EAF dusts contain about 40% of iron oxide.
Transmission electron microscopy (TEM) was used to investigate surface morphology and particle sizes of the catalysts (Fig. 1). EAF dusts are mainly formed by spherical particles with particle sizes between 20 and 300 nm. The incorporation of transition metals Co and Cu leads to more amorphous and porous materials. The difference in the degree of crystallinity and the particle size between Co or Cu and EAF is also appreciated. They are not homogeneous materials; Fig. 1c–f shows areas with crystalline highly well defined, but also areas with more amorphous materials.
Figure 2a–d shows the EAF dust and synthesized catalysts have different microstructures particles. Based on the 2a and 2b FESEM images, EAF dust have spherical shape with different sizes. Meanwhile, the shape of Co/EAF (2c) and Cu/EAF (2d) catalysts is modified and an increase in the porosity is observed.
Moreover, the energy-dispersive X-ray mapping results indicated that both Co and Cu were uniformly distributed in Co/EAF and Cu/EAF, respectively (Fig. 3). A well localized of Co and Cu over EAF dust can have a significant effect on their catalytic activity in Fenton reaction.
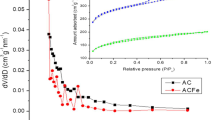
Since the specific surface area of the catalyst can have an effect on its activity, the BET surface area of EAF dust, Cu/EAF and Co/EAF was measured, and 8.4, 13.7 and 15.8 m2 g−1 were obtained, respectively. These results show that the BET surface area of the synthesized catalysts increased during the nanoparticles impregnation method. Also, all of the catalysts were analyzed with ICP-Mass to examine the percentage of loaded metal. The results show that Co/EAF catalyst has 4.6 wt% of Co and Cu/EAF has 4.5 wt% of Cu.
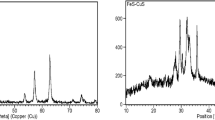
All of the samples were characterized by XRD technique to identify different phases. XRD patterns are displayed in Fig. 4. The main characteristic peaks of magnetite were observed in the recorded data (Sofili 2004). Based on the stoichiometric ratio of iron in Fe3O4 and iron percentage of EAF dust obtained by XRF, magnetite percentage in EAF dust could be computed around 40%. There are some differences between XRD patterns of EAF dust and supported catalysts. The main differences are the peaks at 2θ = 41.8º and 44º in the XRD pattern of EAF dust, attributed to oxides such as hematite (Fe2O3) and magnetite (Fe3O4) phases, which disappear completely in XRD patterns of synthesized catalysts. On the other hand, all of the synthesized catalysts had new peaks at 2θ = 28º, 30 º, 43º which can be attributed to the other forms of iron such as iron oxide-hydroxide phases. Due to the EAF dust includes CaO component, the formation process of iron oxide-hydroxide species during synthesis of catalyst was assumed. This fact can be described as follows:
Iron oxide-hydroxides have been reported as a hopeful material for Fenton-like reactions, because of its channels structure (Zhang et al. 2016). Also, due to the fact that impregnation method has been used to deposit cobalt and copper nanoparticles on the surface of the EAF dust, it is expected that these nanoparticles will be in the oxide state on the surface of catalyst (Bukhtiyarova et al. 2010). In XRD pattern of Co/EAF, the small reflections at 2θ of 30.7 º, 37º, and 74º were assignable to the Co3O4 (JCPDS number of cart: 74-2102), and the others at 2θ = 37º, 42.5º and 62.3º were due to the CoO species (JCPDS number of cart: 78-0431) (Chenga et al. 2017). The XRD pattern of the Cu/EAF, in addition of magnetite characteristic peaks, exhibited some small peaks at 2θ = 36º, 42.2º and 61.8º which were assigned to the reflection lines of copper oxides specially Cu2O (JCPDS No. 78-2076) nanoparticles (Li et al. 2017).
Fenton’s reaction influence on decolorization of methylene blue
After each reaction, methylene blue concentrations were calculated from calibration curve, and results for all catalysts are shown in Table 2.
In Fig. 5, it is observed an evident difference of decolorization due to the Fenton’s degradation. The results showed that Co/EAF and Cu/EAF catalysts degraded the methylene blue by Fenton oxidation with high percentage.
The efficiency and activity of the catalysts decrease in the subsequent way: Co/EAF > Cu/EAF > EAF dust. This priority of activity of Co/EAF and Cu/EAF catalysts indicates that the cobalt or copper nanoparticles increased the catalytic activity of the counterpart EAF dust in the Fenton degradation of methylene blue.
The first step in the heterogeneous reaction is the formation of hydrogen peroxide-iron complex on the surface of catalyst, leading to hydroxyl radicals in reaction solution. The addition of Co or Cu nanoparticles on the surface of catalysts can improve decolorization by the formation of radical species during the reaction with hydrogen peroxide in a cyclic electron transfer process at the parallel way with the iron oxides of the catalyst support. In the case of Co/EAF catalyst, the reaction between peroxo-cobalt complex and the methylene blue to generate organic radicals on the surface of catalyst was suggested (Eqs. 9–11) (Bokare and Choi 2014):
According to the literature, the heterogeneous peroxo-cobalt complex exhibited high reactivity over a wide pH range even in alkaline conditions. As a result, the existence of cobalt ions is a reason for greater activity of Co/EAF than the presence of only iron ion pairs in the counterpart EAF dust. On the other hand, it is well known that Cu2+ ions catalyze the Fenton reaction similar to the iron ion pairs by acyclic electron transfer process, leading to the formation of hydroxyl radicals (Eqs 12, 13):
But it is necessary to mention that the oxidation of Cu+ to Cu2+ by molecular oxygen in acidic and near-neutral solutions reduces the role of copper ions in comparison with Fe2+ and Co2+. The reaction of the iron and cobalt ions with hydrogen peroxide is nearly independent of oxygen concentration, especially in acidic solutions (Bokare and Choi 2014). This fact can be a reason for higher activity of Co/EAF catalyst than Cu/EAF catalyst in Fenton degradation of methylene blue solution. Therefore, Co/EAF nanocatalyst was chosen as best catalyst to optimize the Fenton reaction conditions.
Establishment of RS model
After examination of different transformation and models and considering different statistical criteria, a quadratic polynomial was chosen as RS model. Analysis of variance (ANOVA) showed that all the coefficients of model equation are significant. Therefore, the established RS model for the degree of decolorization of methylene blue was as follows:
ANOVA indicated that this equation was significant (P < 0.05). Based on this, the above equation was used to estimate the predicted values of the degree of decolorization of methylene blue under different conditions. The predicted results of 20 combinations for model establishment are shown in Table 3. Almost all factors and their quadratic terms were entered significantly in the RS model, and the effects of these factors on the strain seem to be important and significant.
As illustrated in Fig. 6, the regression of the observed and predicted degree of decolorization of methylene blue also supports the accuracy of our RS model. To determine which of the variables has influenced significantly on the degree of decolorization of methylene blue, a statistical analysis was performed using correlation indices. It was observed that the ratio of H2O2 concentration to methylene blue concentration (HMR) was the most decisive factor for the degree of decolorization of methylene blue, with a statistically significant correlation (P < 0.0001). This finding is in agreement with previous studies (Li et al. 2015).
As RS model shows HMR has linear and quadratic effects (both are significant). Its positive linear effect is clear; the more H2O2 concentration, the more decolorization will result. Negative quadratic effect could be explained by scavenging effect of excess H2O2 in the solution. The excess H2O2 can react with OH· resulting HOO· and H2O that inhibit further OH· production by the Fenton reaction (Liang et al. 2012; Chen et al. 2014).
Initial pH was the next statistically important factor. This factor has always been regarded as an important factor in the Fenton reaction. A close look at the model equation shows that three terms contain pH effects. The linear and quadratic effects of pH indicated negative effects on the degree of decolorization of methylene blue. The negative sign means that acidic situation would increase the reaction rate and the reaction efficiency. In most studies, acidic conditions have been introduced favorable to the Fenton reaction (Wang et al. 2014). But it should be considered that acidic pH will lead to the leaching of Fe3+ and increase the chance of homogenous reaction, which is not desirable (Chen et al. 2014). Indeed unlike the homogenous reaction, heterogeneous reaction could take place in a wide range of pH. Interestingly, the small coefficients of linear and quadratic effect of pH in our model are in quit agreement with the fact that we adjusted the reaction conditions in favor of heterogeneous reaction and also indicates the prepared Co/EAF catalyst can overcome the problem of a narrow pH range of homogeneous Fenton reaction.
The reusability of catalyst
The stability and reusability are two main properties of heterogeneous catalyst, especially for industrial catalysts. There is no catalyst recyclability for homogeneous Fenton process. As mentioned before, all of the synthesized catalysts were magnetic and easy to separate from the solution. The reusability of Co/EAF was investigated during the same situation. Figure 7 shows that decolorization of methylene blue did not changed seriously during 10 times repetition and MB removal was not less than 90% after 10th run.
The activity of Co/EAF catalyst in dye removing from textile factory wastewater
Taking into account the high activity of this catalyst in removing high concentrated methylene blue solution, it was decided to test it on an industrial sample. Textile wastewater was provided by Golrang Textile Factory from Eshtehard Industrial Zone (Alborz Province, Iran) with 550 mg L−1 COD and color level of 130 SU. The catalyst showed high activity, and the solution was decolorized completely as shown in Fig. 8. The overall color removal was 95% in terms of SU. Seventy-seven percentage COD removal was obtained which is considerable in removing COD and means high mineralization.
The data in Table 4 compare the results obtained over Co/EAF and other catalysts used as heterogeneous Fenton catalysts in dye removal.
As shown in Table 4, most of the Fenton reactions reported in the previous studies were carried out at the acidic pH that will direct to the leaching of Fe3+, which is not favorable. On the other hand, the ratio of amount of catalysts to dye is at least 10 times higher than this study. For example, recently Nasuha et al. (2017) used EAF slag (another waste materials in steel-making factory) as catalyst for removal of methylene blue in acidic pH (~ 3) and catalyst to methylene blue ratio equal to 20. These results confirmed that the lower amount of modified EAF dust with cobalt nanoparticles had higher catalytic activity in natural pH for removing dye in comparison with other reported catalysts. In fact, the catalyst used in the present study might be one of the best catalysts in respect of advanced oxidation of dye contaminates.
Conclusion
In this work, we have reported the use of EAF-based materials as low-cost, magnetic recyclable and very efficient catalysts for the degradation of methylene blue by heterogeneous Fenton oxidation in aqueous solution under mild reaction conditions. The EAF dust, a waste material of steel-making factory, was used as raw material for synthesis of Co/EAF and Cu/EAF catalysts by impregnation method. According to our results, the EAF dust presents higher activity than EAF slag. However, the addition of cobalt nanoparticles on the surface of this waste material increased significantly the Fenton’s reaction rate. Furthermore, response surface methodology was used to optimize reaction parameter. Its results demonstrated that pH did not have very strong effect, unlike the hydrogen peroxide amount had significant positive linear effect on degradation of methylene blue. Moreover, the Co/EAF catalyst exhibited the highest catalytic activity even after ten cycles, and its dosage had positive effect on the rate of reaction. This catalyst would provide a promising path way in recycle of industrial waste material as raw material for removing pollutant from environment.
References
Alizadeh M, Momeni M (2016) The effect of the scrap/DRI ratio on the specification of the EAF dust and its influence on mechanical properties of the concrete treated by its dust. Constr Build Mater 112:1041–1045
Amorim CC, Leão MMD, Moreira RFPM et al (2013) Performance of blast furnace waste for azo dye degradation through photo-fenton-like processes. Chem Eng J 224:59–66
Baldrian P, Merhautová V, Gabriel J et al (2006) Decolorization of synthetic dyes by hydrogen peroxide with heterogeneous catalysis by mixed iron oxides. Appl Catal B Environ 66:258–264
Bokare AD, Choi W (2014) Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J Hazard Mater 275:121–135
Bukhtiyarova GA, Yakushkin SS, Shuvaeva MA, Bayukov OA (2010) State of iron in nanoparticles prepared by impregnation of silica gel and aluminum oxide with FeSO4 solutions. Phys Solid State 52:826–837
Chen Y, Li N, Zhang Y, Zhang L (2014) Novel low-cost Fenton-like layered Fe-titanate catalyst: preparation, characterization and application for degradation of organic colorants. J Colloid Interface Sci 422:9–15
Chenga M, Duan S, Fan H et al (2017) Core@shell CoO@Co3O4 nanocrystals assembling mesoporous microspheres for high performance asymmetric supercapacitors. Chem Eng J 327:100–108
Chinwetkitvanich S (2000) Anaerobic decolorization of reactive dyebath effluents by a two-stage UASB system with tapioca as a co-substrate. Water Res 34:2223–2232
Costa R, Lelis M, Oliveira L et al (2006) Novel active heterogeneous Fenton system based on Fe3 − xMxO4 (Fe Co, Mn, Ni): the role of M2 + species on the reactivity towards H2O2 reactions. J Hazard Mater 129:171–178
Dias FF, Oliveira AAS, Arcanjo AP et al (2016) Residue-based iron catalyst for the degradation of textile dye via heterogeneous photo-Fenton. Appl Catal B Environ 186:136–142
Eaton AD, Clesceri LS, Greenberg AE et al (1998) Standard methods for the examination of water and wastewater. American Public Health Association, Washington, DC
Li H, Li Y, Xiang L et al (2015) Heterogeneous photo-Fenton decolorization of Orange II over Al-pillared Fe-smectite: response surface approach, degradation pathway, and toxicity evaluation. J Hazard Mater 287:32–41
Li Z, Dai K, Zhang J, Liang C et al (2017) Facile synthesis of novel octahedral Cu2O/Ag3PO4 composite with enhanced visible light photocatalysis. Mater Lett 206:48–51
Liang X, Zhong Y, He H et al (2012) The application of chromium substituted magnetite as heterogeneous Fenton catalyst for the degradation of aqueous cationic and anionic dyes. Chem Eng J 191:177–184
Magalhães F, Pereira MCC, Botrel SECEC et al (2007) Cr-containing magnetites Fe3-xCrxO4: the role of Cr3 + and Fe2 + on the stability and reactivity towards H2O2 reactions. Appl Catal A Gen 332:115–123
Martins RC, Henriques LR, Quinta-Ferreira RM (2013) Catalytic activity of low cost materials for pollutants abatement by Fenton’s process. Chem Eng Sci 100:225–233
Mecozzi R, Di Palma L, Pilone D, Cerboni L (2006) Use of EAF dust as heterogeneous catalyst in Fenton oxidation of PCP contaminated wastewaters. J Hazard Mater 137:886–892
Munoz M, de Pedro ZM, Casas JA, Rodriguez JJ (2015) Preparation of magnetite-based catalysts and their application in heterogeneous Fenton oxidation—a review. Appl Catal B Environ 176–177:249–265
Nasuha N, Ismail S, Hameed BH (2017) Activated electric arc furnace slag as an effective and reusable Fenton-like catalyst for the photodegradation of methylene blue and acid blue 29. J Environ Manag 196:323–329
Oller I, Malato S, Sánchez-Pérez JA (2011) Combination of advanced oxidation processes and biological treatments for wastewater decontamination—a review. Sci Total Environ 409:4141–4166
Pataquiva-Mateus AY, Zea HR, Ramirez JH (2017) Degradation of Orange II by Fenton reaction using ilmenite as catalyst. Environ Sci Pollut Res 24:6187–6194
Rahim Pouran S, Abdul Raman AA, Wan Daud WMA (2014) Review on the application of modified iron oxides as heterogeneous catalysts in Fenton reactions. J Clean Prod 64:24–35
Rahim Pouran S, Abdul Aziz AR, Wan Daud WMA, Embong Z (2015) Niobium substituted magnetite as a strong heterogeneous Fenton catalyst for wastewater treatment. Appl Surf Sci 351:175–187
Sofili T (2004) Characterization of steel mill electric-arc furnace dust. J Hazard Mater 109:59–70
Wang Q, Tian S, Ning P (2014) Degradation mechanism of methylene blue in a heterogeneous Fenton-like reaction catalyzed by ferrocene. Ind Eng Chem Res 53:643–649
Zhang X, Bai B, Li Puma G et al (2016) Novel sea buckthorn biocarbon SBC@β-FeOOH composites: efficient removal of doxycycline in aqueous solution in a fixed-bed through synergistic adsorption and heterogeneous Fenton-like reaction. Chem Eng J 284:698–707
Zheng J, Gao Z, He H et al (2016) Efficient degradation of Acid Orange 7 in aqueous solution by iron ore tailing Fenton-like process. Chemosphere 150:40–48
Acknowledgements
We would like to thank the Research Council of Material and Energy research Center for supporting this work by Science Foundation (771394069).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: M. Abbaspour.
Rights and permissions
About this article
Cite this article
Maleki Rizi, M.H., Aghabarari, B., Alizadeh, M. et al. The role of cobalt and copper nanoparticles on performance of magnetite-rich waste material in Fenton reaction. Int. J. Environ. Sci. Technol. 16, 373–382 (2019). https://doi.org/10.1007/s13762-017-1579-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-017-1579-5