Abstract
A pot experiment was conducted to monitor the dynamic response of photosynthesis of Amorpha fruticosa seedlings to different concentrations of petroleum-contaminated soils from April to September. The results showed that the photosynthetic rates, stomatal conductance and transpiration rate of seedlings significantly decreased in 5–20 g kg−1 petroleum-contaminated soil during the three given sampling period of July 31 (early), August 30 (mid-term) and September 29 (late). However, the intercellular CO2 concentration significantly increased in 10 g kg−1 contaminated soil, while declined in 20 g kg−1 contaminated soil during the early sampling period as well as in 20 g kg−1 contaminated soil during the late sampling period. The leaf relative water content of seedlings significantly increased in 20 g kg−1 contaminated soil during the early sampling period, while it dropped dramatically in 15–20 g kg−1 contaminated soil during the late sampling period. The contents of chlorophyll a, chlorophyll b and the total chlorophyll of seedlings showed a sharp decline during the three sampling periods in contaminated soil. Comprehensively, considering the negative effects of petroleum on the photosynthesis, growth performance and remediation effect on petroleum of A. fruticosa seedlings, this plant was tolerant of petroleum-contaminated soil and was potentially useful for the phytoremediation of petroleum-contaminated sites in northern Shaanxi, China.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, spilling of crude oil has severely damaged the eco-environment of petroleum production regions along with the rapid development of petroleum industry. Petroleum contaminants can hinder the uptakes of nutrient and water of plants (Alkorta and Garbisu 2001; Andrade et al. 2004; Shukry et al. 2013), cause their biomembrane injury by leading to accumulation of reactive oxygen species (Achuba 2014; Shukry et al. 2013), inhibit the photosynthesis and transpiration (Lin et al. 2002; Rahbar et al. 2012), and finally lead to the death of plants and the degradation of plant communities. In addition, petroleum contaminants and the heavy metals they carry can be bio-accumulated along with the food chain and finally threaten the human health (Park and Park 2011). Considering these, many researchers have devoted to remediate the petroleum-contaminated soil (Chien 2012; Khan et al. 2013; Silva et al. 2014; Venkidusamy et al. 2016).
Traditional physical and chemical methods used in remediation, such as organic solvents method, continuous irrigation and thermal oxidation, are costly and usually cause serious secondary contamination, while the low biomass of microorganism hinders the application of microbial remediation method in large-area petroleum-contaminated regions. Consequently, phytoremediation gradually becomes a preferred method in recent decades. Selecting suitable species is the basis for applying phytoremediation, thus many researchers have devoted themselves to screening crop or other herbaceous species which are suitable to be planted in petroleum-contaminated soil (Brandt et al. 2006; Ghazisaeedi et al. 2014; Pérez-Hernández et al. 2013; Wei and Pan 2010; Xu et al. 2013). Many species have been found to exhibit favorable abilities to remove petroleum contaminants, such as Lolium multiflorum, Zea mays, Medicago sativa, Sorghum vulgae (Kaimi et al. 2007), Brachiaria brizantha (Merkl et al. 2005), Poa foliosa (Bramley-Alves et al. 2014) and Mirabilis Jalapa (Peng et al. 2009). In addition, the co-bioremediation of plants and microbes were widely studied as well (Khan et al. 2013; Merkl et al. 2005). However, in some old petroleum production regions, the spilled petroleum during exploitation, storing and transportation has infiltrated the deep layer of soil, leading to severe contamination. Though crop and other herbaceous species exhibit favorable remediation effects on the petroleum contamination in the shallow layer of soil, their remediation effects on the deep-layer contamination are quite limited by the length of root system.
The north of Shaanxi is one of the most important oil fields in China. Due to the long-term extensive management, the petroleum pollutants were optionally poured out during petroleum production. What’s worse, the contaminated area was often covered by uncontaminated soil (Zhang 2013). The above-mentioned phenomena have caused severe contamination to deep soil layers in the region and threatened the fragile eco-environment there. Hence, woody plants with developed root system should be screened for the phytoremediation in that region to penetrate the uncontaminated soil layer, thus remediating the contaminated deep-layer soil. However, the existing researches about the woody remediation plants mainly focuses on the species which have strict requirements on soil moisture conditions (Bento et al. 2012; Moreira et al. 2011), while there are few studies concentrating on the selecting of woody remediation plants suitable for such arid or semi-arid petroleum-contaminated regions as northern Shaanxi.
Amorpha fruticosa is a commonly used shrub species for re-vegetation in northern Shaanxi, for its favorable adaptation to the drought and barren conditions of this region. Especially, because of its developed roots and large biomass, A. fruticosa might be able to remediate the deep-layer petroleum-contaminated soil (Zhang 2013). In addition, a latest report has inferred A. fruticosa could be utilized for phytoremediation in ≤15 g kg−1 petroleum-contaminated soil according to the antioxidant defense response and growth reaction of seedlings to petroleum-contaminated soil (Cui et al. 2016). Sequentially, in this study, a dynamic of the photosynthesis of A. fruticosa seedlings in petroleum-contaminated soils combined with plant water content and Chlorophyll content was mainly determined during February to August, 2011 in a pot cultivation experiment which was carried out in the nursery of Northwest A&F University, Yangling, China. In the end, the growth performance and remediation effect on petroleum of A. fruticosa seedlings in our previous report (Cui et al. 2016) were integrated to detect the tolerance of A. fruticosa to petroleum. The results might provide scientific basis for the screening of woody species suitable for arid or semi-arid petroleum-contaminated regions.
Materials and methods
Experimental field conditions
The experiment is carried out in the nursery of Northwest A&F University, which enjoys a continental monsoon climate of medium latitudes and is located at 454.5 m above sea level. The average annual sunshine hours are 2150. The average temperature is 12.9 °C, and the extreme maximum and minimum temperatures, respectively, are 42 and −19.4 °C. The temperature over 10 °C accumulates to 4185 °C, frost-free days reaches 221, and the average annual rainfall is 621.6 mm.
Materials
The A. fruticosa used in the experiment is 2-year-old seedlings purchased from nurseries in Huaziping town, Ansai County, Shaanxi Province, China. The petroleum is extracted from petroleum wells in Huaziping town. The soil is gathered from 0 to 20 cm contamination-free soil layer in sparsely populated waste grassland. The soil, whose pH value is 8.2, contains 8.45 g kg−1 organic matter, 0.42 g kg−1 full nitrogen, 5.5 mg kg−1 available phosphorous and 74.6 mg kg−1 available potassium.
Experiment design
The survey on the soil environment in the petroleum mining area in Huaziping town, Ansai County, shows that the petroleum concentration in petroleum mining area is unevenly distributed, generally within 5–20 g kg−1. Thus, 5 levels of petroleum concentrations are designed in the experiment: 0 g kg−1 (as the control), 5, 10, 15 and 20 g kg−1 (measured in dry weight of soil). The following specific steps are taken during the pot experiment:
Remove residual plant body and other impurities from the tested soil; air-dry the removed soil and pass it through a 4 mm sieve; determine the soil moisture content; put it into a plastic barrel (with the top diameter, bottom diameter and height of 31 cm × 23 cm × 27 cm), which each holds 12.94 kg (measured in dry weight of soil) contamination-free soil; add petroleum to the soil in a complete mixing way till it reaches the designed petroleum concentrations; water the mixture and let it stand when the soil and petroleum are evenly mixed with no organic solvent added; determine the field water capacities of the five petroleum concentrations of petroleum-contaminated soil, which decreases by 21.34, 21.19, 20.23, 19.19 and 19.07 %, respectively, with the rise of petroleum concentrations. Variance analysis shows there are no significant differences among these disposed soils.
Then, select seedlings with relatively consistent height and ground diameter as well as intact root system; plant seedlings into the above barrels in mid-April, 2011 and cut off their stems, with each treatment including 9 barrels, 3 seedlings within each; otherwise keep 3 barrels without planting seedlings, respectively, in every petroleum treatment. After that, put all the barrels in removable rain-proof shed for preventing natural rainfall and ensuring normal sunshine on clear days; water each treatment adequately to guarantee its survival and normal growth. At the end of June, the soil moisture will be kept at 75 % of soil field capacity by weighing method so that the treated A. fruticosa seedlings are all at optimum soil water condition. For this purpose, one barrel of seedlings for each treatment must be destroyed, and the average fresh weight of seedlings for each treatment will be measured. Thus, the effect of seedlings on weighing will be reduced to the minimum. The same work will be done at the ends of July and August. The whole experiments completes at the end of September.
Gas exchange
Measurements were taken on the young, fully expanded leaves (n = 9) which were exposed to full sunlight using a portable gas exchange system (Li-6400, Li-Cor Inc., Lincoln, NE, USA) at the end of July, August and September, respectively. All measurements were made between 0900 and 1130 h on sunny, cloudless days when natural light levels were saturated for photosynthesis (≥800 μmol m−2 s−1). The gas exchange parameters including net photosynthetic rate (A), intercellular CO2 concentration (Ci), stomatal conductance to water vapor (Gs) and transpiration rate (Tr) were all determined at a controlled CO2 concentration of 380 μmol mol−1, leaf temperature of 30 °C, photon flux density of 1200 μmol m−2 s−1 and an ambient relative humidity of 50 ± 10 %.
Leaf relative water content
At the same time in terms of the gas exchange measurements, the similar leaves mentioned above were collected in plastic bags and immediately transported to the laboratory for both determinations of leaf relative water content and chlorophyll content. Respectively, three replications were used, each composed of nine leaves from three seedlings within one barrel.
Leaf relative water content (LRWC) was evaluated with intact leaves. First, the fresh weight (FW) of the leaves was determined, after which the leaves were floated on distilled water in the dark in room temperature environment for 24 h. The leaves were then blotted dry with paper towels and reweighed to record turgid weight (TW). At last the leaves were oven-dried at 80 °C to a constant dry weight (DW). Leaf relative water content was calculated through the following formula (Barrs and Kozlowski 1968):
Chlorophyll content
The fresh leaves of each replication were cut into small pieces (ca.2 mm) and mixed uniformly. The 0.2 g was weighed and extracted with 25 ml of 80 % acetone in the dark at room temperature overnight. The supernatant obtained after centrifugation was used for determinations of chlorophyll a (Chl a), chlorophyll b (Chl b) and total chlorophyll (Chl a + b) contents with a Shimadzu UV-1700 spectrophotometer (Shimadzu Corp., Japan), through three wavelengths (663, 646 and 470 nm). Concentrations of pigments were estimated by the equations of Lichtenthaler (1987).
Statistical analysis
Statistical analysis was conducted by using the software Statistical Package for Social Sciences (SPSS 17.0 for Windows). Data were subjected to one-way analysis of variance (ANOVA) and Duncan’s multiple range test (p < 0.05). The normality assumption was tested with the Kolmogorov–Smirnov test and the equal variance assumption with the method of Bartlett. Pearson coefficients were calculated to assess the correlation among different variables. Origin 8.5 software was used for drawing.
Results and discussion
The changes in the gas exchange parameters of A. fruticosa seedlings
As shown in Fig. 1a, the net photosynthetic rate (A) of A. fruticosa seedlings under different petroleum concentrations in soil, compared with that in pollution-free soil, consistently decreases during the three sampling period. It should be noted that a dramatic fall takes place with the highest petroleum concentration (20 g kg−1), leading to a rate much lower than that with other petroleum concentrations.
Changes in A (a), Gs (b), Tr (c) and Ci (d) activities of Amorpha fruticosa seedlings during different sampling periods and at different petroleum concentrations. Bars represent mean ± SE (n = 9). Bars within the same date group followed by the different letters are significantly different at p < 0.05 according to Duncan’s test
As shown in Fig. 1b, on July 31, the early sampling period witnesses a slight decline in the stomatal conductance (Gs) of A. fruticosa seedlings with petroleum concentrations of 5 and 10 g kg−1 and a significant fall with those of 15 and 20 g kg−1, when the stomatal conductance under different petroleum concentrations is compared with that in the absence of petroleum. After that sampling period, however, the figure under various petroleum concentrations has a sharp decline in comparison with that free from petroleum pollution.
As shown in Fig. 1c, Transpiration rate (Tr) is closely related to stomatal conductance, and the decline of the latter is always accompanied by that of the former, in other words, the two make consonant changes, because stomatal conductance is the main mechanism of regulating transpiration (Cochard et al. 2002). It is worth noticing that the drop in the transpiration rate under the petroleum concentrations of 15 and 20 g·kg−1 on September 29, the late sampling period, compared with that in the absence of petroleum, is a bit extraordinary, considering the corresponding decline of stomatal conductance. It may indicate that water uptake from the soil by roots of A. fruticosa seedlings is restricted under both treatments at this stage. Under mild soil water limitation, A. fruticosa seedling can reduce its transpiration sharply to prevent further loss of water, which is an acclimation mechanism to drought (Li 1991).
The changes in intercellular CO2 concentration (Ci) and stomatal conductance are always used to determine whether the decline in photosynthetic rate is mainly attributed to stomatal limitation (the fall in stomatal conductance) or to non-stomatal limitation (the fall in the photosynthetic activity of mesophyll cells) (Farquhar and Sharkey 1982). As shown in Fig. 1d, on July 31, the early sampling period, compared with the figures in pollution-free soil, the stomatal conductance of A. fruticosa seedlings with the petroleum concentrations of 5 and 10 g kg−1 decreases slightly, while the intercellular CO2 concentration is on the rise and even reaches a significant level under the petroleum concentration of 10 g kg−1. Thus, a conclusion can be drawn that the decline in photosynthetic rate results from the decreasing photosynthetic activity of mesophyll cells, the stomatal conductance with the petroleum concentrations of 15 and 20 g kg−1 has a dramatic fall, and the intercellular CO2 concentration is also in decline and reaches a significant level under the petroleum concentration of 20 g kg−1, from which a conclusion can be drawn that the decline in photosynthetic rate is attributed to that in stomatal conductance. On August 30, the mid-term period, compared with the figures in the absence of petroleum, the net photosynthetic rate and the stomatal conductance with different petroleum concentrations have a sharp fall as with the intercellular CO2 concentration, which shows the influence of stomatal limitation. Compared with that in pollution-free soil, the intercellular CO2 concentration in petroleum-contaminated soil, however, does not reach a significant level, indicating that the photosynthetic activity of mesophyll cells suffers from a severe depression during this sampling period. On September 29, the late sampling period, the net photosynthetic rate and the stomatal conductance with different petroleum concentrations still decrease significantly compared with the figures in pollution-free soil; the intercellular CO2 concentration steps up with the increasing petroleum concentrations. Compared with the figures in the absence of petroleum, the intercellular CO2 concentration has a slight decline with the petroleum concentrations of 5, 10 and 15 g kg−1, while it increases dramatically with the petroleum concentrations of 20 g kg−1, indicating that the photosynthetic activity of mesophyll cells suffers from more and more serious inhibition with the aggravating petroleum pollution and becomes the main limitation of the photosynthesis during this sampling period.
Numerous studies have shown that photosynthetic rates in plants would decrease due to oil exposure (Caudle and Maricle 2014; Lin and Mendelssohn 1996; Naidoo et al. 2010; Pajević et al. 2009; Pezeshki and de Laune 1993; Redondo-Gomez et al. 2014). But there was a variety of changes in other gas exchange parameters. Pajević et al. (2009) found an inhibition of Gs (stomatal conductance) in willow genotypes under condition of soil polluted with 1 % diesel fuel and explained that the depression of photosynthesis was sever at the stomatal level. Caudle and Maricle (2014) also found that, in many species, the stomatal conductance was reduced, while the internal CO2 concentration (Ci) was increased by oil, indicating that non-stomatal limitations was caused by the toxic effects of oil on photosynthesis. Additionally, some studies have shown there were no differences in leaf stomatal conductance (Naidoo et al. 2010; Redondo-Gomez et al. 2014) and transpiration (Naidoo et al. 2010) in some species between oiled treatments and the untreated controls, also a following increase of Ci with oiling indicated the limitation to net photosynthetic rate could be accounted for by non-stomatal limitations from light absorption by oil and/ or from toxity of oil (Naidoo et al. 2010; Redondo-Gomez et al. 2014). However, the reduced transpiration on mangroves has been found once with dispersed oil in soil and is suggested to be a consequence of damage of conducting tissues of fine roots (Getter et al. 1985). In this study, all of the above changes in gas exchange parameters have been observed dynamically in A. fruticosa seedlings under different petroleum concentrations in soil from 5 to 20 g kg−1. The significant reduction in net photosynthetic rate in A. fruticosa seedlings not only resulted from non-stomatal limitation by petroleum but also stomatal limitation. Both of them occurred intertwined and showed one of the more dominant positions with the different intensities of petroleum contamination stress and the alteration of stress time. In less polluted soil under the petroleum concentrations of 5 and 10 g kg−1, the inhibition to photosynthetic rate is attributed to non-stomatal limitation at first and then partly affected by stomatal limitation, but on the whole, it is non-stomatal limitation that plays the leading role. Under higher petroleum concentrations of 15 and 20 g kg−1, however, the fall in photosynthetic rate is firstly influenced by stomatal limitation and later by non-stomatal limitation, and the latter gradually dominates.
In terms of the inhibition to photosynthesis of plants growing in oil-contaminated soil, numerous studies attributed mainly the induced toxicities to living cells by oil (Lin and Mendelssohn 1996; Naidoo et al. 2010; Redondo-Gomez et al. 2014), because the toxic components in the oil could be taken by the plant via the roots, stem and accumulate in the leaves, which may alter the integrity and permeability of plant membranes leading to disturbance of both carbon metabolism in the leaves and ion and water uptake in the roots (Naidoo et al. 2010; Reis 1996; Suprayogi and Murray 1999). Furthermore, it can not be neglected that the hydrophobic nature of petroleum hydrocarbons which prevents water from spreading inhomogeneous in the contaminated soil, resulting in a water deficiency (Baker 1970; Merkl et al. 2004; Pezeshki et al. 2000).
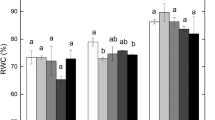
The changes in the leaf relative water content of A. fruticosa seedlings
As shown in Fig. 2, compared with the LRWC in the absence of petroleum contamination, the figures under the petroleum concentrations of 5 and 10 g kg−1 have no significant changes during all three sampling periods, but show a decreasing trend during the late sampling period; the figure under the petroleum concentrations of 15 g kg−1 does not show a dramatic change during the early and mid-term sampling periods, but falls significantly by 9.21 %; though the figure under the petroleum concentrations of 20 g kg−1, showing no significant change during the mid-term sampling period, rises markedly by 11.20 % during the early sampling period and falls by 17.53 % during the later sampling period, leading to a leaf relative water content much lower than that under other petroleum concentrations.
In this experiment, the slight decreases without statistical significance (p > 0.05) were observed in the water holding capacity of treated soils with petroleum. This can be attributed to the hydrophobic properties of petroleum which has altered water infiltration and humidity of the soil (Merkl et al. 2004, 2005). In the meantime, this result has also allowed the possible additional effect of soil moisture to be ruled out at the beginning of the experiment by the same adequate water supply strategy. Therefore, the results of the LRWC directly reflected the effect of petroleum contamination in soil on the water status of A. fruticosa seedlings. It can be seen that in less polluted soil under the petroleum concentrations of 5 and 10 g kg−1, the internal water, not badly influenced by petroleum contamination, is as adequate as that in the pollution-free soil. This further accounts for the decrease in photosynthetic rates of seedlings at 5 and 10 g kg−1 as a result of deleterious effects caused by the petroleum contamination in soil, while the seedlings showed stomatal limitation and (or) non-stomatal limitation. In seriously polluted soil under the petroleum concentrations of 15 and 20 g kg−1, because of the toxicity of petroleum, the photosynthetic rate has a sharp fall during the early and mid-term sampling periods and the fall reaches a significant level under the petroleum concentrations of 20 g kg−1. What cannot be neglected is the increasing seedling moisture during the early sampling period, the cause of which is that the water taken by roots outweighs the transpiration through leaf stoma due to the severely depressed stomatal conductance and transpiration. It should be noted that the LRWC under the petroleum concentrations of 15 and 20 g kg−1 during the late sampling period, compared with the average LRWC (79.3 %) of the all sampling periods in the absence of petroleum, decreases, respectively, by 7.44 and 15.89 % (to 73.41 and 66.68 %). It showed a deterioration of leaf water status. Ramos et al. (2009) also observed that plant individuals in contact with higher petroleum concentration (50 and 75 g kg−1) contained lower humidity content in their composition in comparison with those in contact with lower concentration (25 g kg−1) or in the absence of petroleum. The results further confirmed water taken from the soil by roots of A. fruticosa seedlings was restricted during this period, which had been reflected by substantially reduced transpiration in the previous paragraph. It is well known that a decrease of photosynthesis was often observed under mild or moderate water limitation (Chaves et al. 2003, 2009). Therefore, the water limitation here could exacerbate the adverse effects of petroleum contamination on the photosynthesis function of A. fruticosa seedlings and become a part of stomatal limitation.
The changes in the leaf chlorophyll content of A. fruticosa seedlings
As shown in Fig. 3, compared with figures in the absence of petroleum contamination, Chl a, Chl b and the tatal Chl content of A. fruticosa seedlings has a sharp fall in all three sampling periods. The consonant changes the three make lead to a slight change of Chl a/b, but the figure decreases dramatically under the petroleum concentration of 20 g kg−1 during the mid-term and late sampling periods. The petroleum concentration of 5 g kg−1 witnesses the slightest drop in the total Chl content all the time, keeping falling by 10 % during the mid-term and later sampling periods. The figure, however, rises to 26 % with longer pollution time. The petroleum concentrations of 10, 15 and 20 g kg−1 see a fall of 36 % at last. The figure first appears during the mid-term sampling period under the severest petroleum concentrations of 20 g kg−1, rising from 28 % during the early sampling period. Under the petroleum concentrations of 10, 15 g kg−1, however, the total Chl content has a fall of 25 % during the early sampling period, which rises to 27 % during the mid-term sampling period and to 36 % at last. It can be seen that the changes in the leaf Chl content of A. fruticosa seedlings are affected by both the intensities of petroleum contamination stress and the alteration of stress time.
Changes in Chl content of Amorpha fruticosa seedlings in different sampling periods and at different petroleum concentrations. The bars are divided into two parts, the lower part of which is the content of Chl a, while the upper part is the content of Chl b. The letters within the bars represent the multiple comparative results. The whole bars represent the total Chl content and the top letters represent its multiple comparative results. Bars represent mean ± SE (n = 3). Bars within the same date group followed by the different letters differ a lot at p < 0.05 according to Duncan’s test
Decreases in plant Chl contents due to petroleum-contaminated soil have already been reported and attributed to the direct toxic effects which petroleum exerted on plants (Achuba 2006; Chaineau et al. 1997; Li et al. 2008) and also to the deficiencies of nutrients (mainly available nitrogen) resulted from alterations of chemical properties of the soil by petroleum (Balasubramaniyam and Harvey 2014; Bento et al. 2012; Chaineau et al. 1997), however, not mentioned the respective effects on Chl a and b. Redondo-Gomez et al. (2014) found a reduction in Chl content of Spartina argentinensis tillers at 2 and 3 % diesel concentrations in soil, including a reduction in Chl b content and an unaffected Chl a content, which was suggested to be a toxic effect of enhanced tiller Mn concentrations in the presence of diesel fuel. Another research showed the decreased total Chl, Chl a, Chl b concentrations and Chl a/Chl b ratios in four evergreen plant species around the refinery, in comparison with the control sites, maybe arise from higher ambient SO2 gas concentrations and accumulation of metals in the leaves (Deniz and Duzenli 2007). Moreover, Wang et al. (2005) observed an equivalent negative effect on the Chl a and b content of rice leaves when the Chl content decreased significantly with enhanced naphthalene (one component of crude oil) stress, resulting in no significant difference in the ratio of Chl a to Chl b. In present study, the continuous accumulations of toxic hydrocarbons and metals in the A. fruticosa seedlings and the lack of nitrogen available for plant in soil may be the reasons why the Chl contents in leaves of seedlings growing in almost all levels of petroleum-contaminated soil decreased constantly instead of the seedlings grown in uncontaminated soil. Furthermore, the enhanced contamination intensity aggravated and accelerated this process, especially at the highest petroleum concentration (20 g kg−1). The excessive toxic matter accumulations and nutrients deficiency also exerted a greater impact on Chl a than Chl b, resulting in a decrease in the ratio of Chl a to Chl b. Chlorophyll is an important material base of both light energy absorption and transformation in photosynthesis. The decrease in chlorophyll content could lead to a decline in photosynthetic functioning (Redondo-Gomez et al. 2014) and become an important part of non-stomatal limitation.
The changes in the growth of A. fruticosa seedlings and petroleum degradation rates in pot soils
In consideration of the importance of growth performance of seedlings and strong link with photosynthesis, the shoot and root dry weights of A. fruticosa seedlings have to be taken from our previous report (Cui et al. 2016) to obtain a full discussion. As shown in Table 1, the shoot and root dry weights of seedlings showed no statistical difference in 5 g kg−1 petroleum concentration compared with CK, but significant decreases in 10–20 g kg−1 concentration (Cui et al. 2016). The total dry weights showed same changing trend. Moreover, the higher the petroleum concentration, the greater the decrease. But the shoot/root ratio with petroleum contamination always being slightly lower than that without contamination, the difference between them is not significant (p > 0.05).
The reductions in plant biomass by the presence of petroleum in soil have been reported in many documents (de Farias et al. 2009; Inckot et al. 2011; Merkl et al. 2004, 2005; Xun et al. 2015; Peng et al. 2009; Redondo-Gomez et al. 2014; Adam and Duncan 2003). Such an inhibition of plant growth should be a consequence of the stress imposed by petroleum presence (Huang et al. 2005; Merkl et al. 2005; Ramos et al. 2009). While evaluating species for its stress tolerance, the inhibition in plant growth should be as low as possible. The A. fruticosa seedling could be well tolerant of petroleum-contaminated soil at the 5 g kg−1 level as the inhibition effect of the petroleum concentration on the overall growth of A. fruticosa seedling was insignificant compared with the control. An obvious inhibition to the overall growth of A. fruticosa seedling occurred as the petroleum concentration in soil was up to 10 g kg−1 level and was aggravated again at the 15 g kg−1 level. However, a good endurance to petroleum contaminants has to be paid to A. fruticosa seedling since the differences of the plant growth performance at 10 and 15 g kg−1 levels were both insignificant compared with the 5 g kg−1 level. With an extremely high level (20 g kg−1) of petroleum contamination, A. fruticosa seedling was still alive. Nevertheless, a decrease of 65.28 % in total biomass compared to the control indicated a serious deterioration in the overall development and health of the plant. Due to the difference of growth inhibition effects of petroleum-contaminated soil on plant’s shoot and root, there often showed a change in the ratio of shoot to root, the drop of which indicated an improved allocation of carbon from the shoot to the root, leading to a better development in the root compared with the shoot (Inckot et al. 2011; Merkl et al. 2005). It is quite important for phytoremediation since it shows the capacity of the plant to respond to water and nutrient deficiency and a maintaining for stimulation of degrading microorganisms (Merkl et al. 2005). Also, it may be a strategy so that the roots are able to reach uncontaminated areas (Kechavarzi et al. 2007). In this study, there showed a slight decreasing tendency in the ratios of shoot to root of A. fruticosa seedlings growing in different concentrations of petroleum-contaminated soil compared with the ratio of those growing in uncontaminated soil. This could suggest a certain potential in this regard for A. fruticosa seedling, probably a further decline in plant’s ratio of shoot to root requires enough time of exposure to the petroleum contaminant.
Several studies have observed the reduction in biomass of plant in the presence of oil was closely related to the decrease in plant photosynthesis (Lin and Mendelssohn 1996; Pezeshki and de Laune 1993; Redondo-Gomez et al. 2014). Redondo-Gomez et al. (2014) pointed out that an increasing diesel concentration would lead to an aggravating negative effect on the growth of S. argentinensis, which could be the consequence of continuously reduced photosynthesis. Lin and Mendelssohn (1996) also indicated the reduction in biomass of S. patens was probably due to a substantial reduction in photosynthesis during the period of vigorous growth (in August), and simultaneously a trend of lower biomass and photosynthetic rate with higher oil dosage. Nevertheless, a reduction in the photosynthetic rate of S. alterniflora being delayed to late October would hardly affect the biomass. In this study, the analysis of A. fruticosa seedling showed an extremely significant positive correlation (0.977 or 0.976, p = 0.004) between total biomass and photosynthetic rate either on July 31 or August 30 even (0.880, p = 0.049) on September 29. Furthermore, there existed an extremely significant negative correlation (−0.982, p = 0.003) between total biomass and petroleum concentration in the soil. So, it can be inferred that the reductions in the biomass of A. fruticosa seedling exposed to petroleum-contaminated soil should be attributed to the decrease in photosynthetic rate especially during the vigorous growth period. Around August, a weak advantage in photosynthetic rate at 5 g kg−1 petroleum concentration relative to 10 and 15 g kg−1 probably gave rise to its biomass with no significant difference in comparison with the control of both 10 and 15 g kg−1 petroleum concentrations.
In view of the prospect as a phytoremediation tree, remediation effect of A. fruticosa seedlings on petroleum has also to be taken from our previous report (Cui et al. 2016) to obtain a more comprehensive conclusion. As shown in Table 2, planting A. fruticosa seedlings could significantly decrease the petroleum concentration in soil up to two to three times higher than those in natural conditions (Cui et al. 2016). The favorable remediation ability of A. fruticosa to petroleum-contaminated soil has been demonstrated.
Conclusion
In this study, petroleum contamination in soil resulted in negative effects on the growth of A. fruticosa seedlings, which should be attributed to the reduced photosynthetic rate especially during the vigorous growth period. Moreover, the reduction in photosynthetic rate of A. fruticosa seedlings could be caused by the intertwined stomatal and non-stomatal limitations which mainly resulted from the toxicities of petroleum to plant. Despite the negative impact of the presence of petroleum, the plants survived and even up to the 15 g kg−1 contaminated soil level still could remain the considerable biomasses after 165 days. In the meantime, the plants could significantly enhance the degradation rates of petroleum in soil. For these reasons, we suggest that this plant is tolerant of petroleum-contaminated soil and is potentially useful for the phytoremediation of petroleum-contaminated sites as a native superior afforestation tree species in northern Shaanxi, China. However, a long-term study will be needed in the future and it is quite necessary to test the Rhizosphere microbial communities for increasing petroleum degradation about the plant. The results can thus help to further assess plant species for phytoremediation.
References
Achuba F (2006) The effect of sublethal concentrations of crude oil on the growth and metabolism of Cowpea (Vigna unguiculata) seedlings. Environmentalist 26:17–20
Achuba FI (2014) Petroleum products in soil mediated oxidative stress in cowpea (Vigna unguiculata) and maize (Zea mays) seedlings. Open J Soil Sci 4:417
Adam G, Duncan H (2003) The effect of diesel fuel on common vetch (Vicia sativa L.) plants. Environ Geochem Health 25:123–130
Alkorta I, Garbisu C (2001) Phytoremediation of organic contaminants in soils. Bioresour Technol 79:273–276. doi:10.1016/S0960-8524(01)00016-5
Andrade ML, Covelo EF, Vega FA, Marcet P (2004) Effect of the Prestige oil spill on salt marsh soils on the coast of Galicia (northwestern Spain). J Environ Qual 33:2103–2110
Baker J (1970) The effects of oils on plants. Environ Pollut 1:27–44
Balasubramaniyam A, Harvey PJ (2014) Scanning electron microscopic investigations of root structural modifications arising from growth in crude oil-contaminated sand. Environ Sci Pollut Res 21:12651–12661
Barrs H, Kozlowski T (1968) Determination of water deficits in plant tissues. Water Deficits Plant Growth 1:235–368
Bento RA, Saggin-Júnior OJ, Pitard RM, Straliotto R, da Silva EMR, de Lucena Tavares SR, de Landa FHTG, Martins LF, Volpon AGT (2012) Selection of Leguminous trees associated with symbiont microorganisms for phytoremediation of petroleum-contaminated soil. Water Air Soil Pollut 223:5659–5671. doi:10.1007/s11270-012-1305-3
Bramley-Alves J, Wasley J, King CK, Powell S, Robinson SA (2014) Phytoremediation of hydrocarbon contaminants in subantarctic soils: an effective management option. J Environ Manag 142:60–69. doi:10.1016/j.jenvman.2014.04.019
Brandt R, Merkl N, Schultze-Kraft R, Infante C, Broll G (2006) Potential of vetiver (Vetiveria zizanioides (L.) Nash) for phytoremediation of petroleum hydrocarbon-contaminated soils in Venezuela. Int J Phytoremediat 8:273–284. doi:10.1080/15226510600992808
Caudle KL, Maricle BR (2014) Physiological relationship between oil tolerance and flooding tolerance in marsh plants. Environ Exp Bot 107:7–14. doi:10.1016/j.envexpbot.2014.05.003
Chaineau C, Morel J, Oudot J (1997) Phytotoxicity and plant uptake of fuel oil hydrocarbons. J Environ Qual 26:1478–1483
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought—from genes to the whole plant. Funct Plant Biol 30:239–264
Chaves M, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
Chien YC (2012) Field study of in situ remediation of petroleum hydrocarbon contaminated soil on site using microwave energy. J Hazard Mater 199:457–461
Cochard H, Coll L, Le Roux X, Améglio T (2002) Unraveling the effects of plant hydraulics on stomatal closure during water stress in walnut. Plant Physiol 128:282–290
Cui BX, Zhang XX, Han G, Li KR (2016) Antioxidant defense response and growth reaction of Amorpha fruticosa seedlings in petroleum-contaminated soil. Water Air Soil Pollut 227:1–10
de Farias V, Maranho LT, de Vasconcelos EC, da Silva Carvalho Filho MA, Lacerda LG, Azevedo JAM, Pandey A, Soccol CR (2009) Phytodegradation potential of Erythrina crista-galli L., Fabaceae, in petroleum-contaminated soil. Appl Biochem Biotechnol 157:10–22
Deniz M, Duzenli S (2007) The effect of refinery pollution on non-enzymatic foliar defense mechanisms in four evergreen plant species in Turkey. Acta Physiol Plant 29:71–79
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33:317–345
Getter CD, Ballou TG, Koons CB (1985) Effects of dispersed oil on mangroves synthesis of a seven-year study. Mar Pollut Bull 16:318–324
Ghazisaeedi F, Zamani MM, Ghadbeigi S, Mortazavi SH, Fallahpour M, Ghazisaidi H, Zamani M, Bakhtiarian A, Sima NAK, Amini M (2014) Bioremediation of the crude oil contamination of soil by the indigenous, herbaceous plant salicorniaeuropea in Iran. Thrita 3(2):e17409
Huang XD, El-Alawi Y, Gurska J, Glick BR, Greenberg BM (2005) A multi-process phytoremediation system for decontamination of persistent total petroleum hydrocarbons (TPHs) from soils. Microchem J 81:139–147
Inckot RC, de Oliveira Santos G, De Souza LA, Bona C (2011) Germination and development of Mimosa pilulifera in petroleum-contaminated soil and bioremediated soil. Flora 206:261–266
Kaimi E, Mukaidani T, Tamaki M (2007) Screening of twelve plant species for phytoremediation of petroleum hydrocarbon-contaminated soil. Plant Prod Sci 10:211–218
Kechavarzi C, Pettersson K, Leeds-Harrison P, Ritchie L, Ledin S (2007) Root establishment of Perennial ryegrass (L. perenne) in diesel contaminated subsurface soil layers. Environ Pollut 145:68–74
Khan S, Afzal M, Iqbal S, Khan QM (2013) Plant–bacteria partnerships for the remediation of hydrocarbon contaminated soils. Chemosphere 90:1317–1332
Li J (1991) Studies on drought tolerance of some main tree species used in afforestation in Taihang Montain Region (V)——transpiration and stomatal response. J Beijing For Univ S2:251–265
Li C, Wang W, Cao Y, Tian H (2008) Eco-toxicity of petroleum-contaminated soil on the growth of soybean. J Northwest A&F Univ 36(1):116–120
Lichtenthaler HK (1987) Chlorophyls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzimol 148(34):350–382
Lin Q, Mendelssohn IA (1996) A comparative investigation of the effects of south Louisiana crude oil on the vegetation of fresh, brackish and salt marshes. Mar Pollut Bull 32:202–209
Lin Q, Mendelssohn IA, Suidan MT, Lee K, Venosa AD (2002) The dose-response relationship between No. 2 fuel oil and the growth of the salt marsh grass, Spartina alterniflora. Mar Pollut Bull 44:897–902
Merkl N, Schultze-Kraft R, Infante C (2004) Phytoremediation in the tropics—the effect of crude oil on the growth of tropical plants. Biorem J 8:177–184
Merkl N, Schultze-Kraft R, Infante C (2005) Assessment of tropical grasses and legumes for phytoremediation of petroleum-contaminated soils. Water Air Soil Pollut 165:195–209. doi:10.1007/s11270-005-4979-y
Moreira ITA et al (2011) Phytoremediation using Rizophora mangle L. in mangrove sediments contaminated by persistent total petroleum hydrocarbons (TPH’s). Microchem J 99:376–382. doi:10.1016/j.microc.2011.06.011
Naidoo G, Naidoo Y, Achar P (2010) Responses of the mangroves Avicennia marina and Bruguiera gymnorrhiza to oil contamination. Flora 205:357–362
Pajević S, Borišev M, Nikolić N, Krstić B, Pilipović A, Orlović S (2009) Phytoremediation capacity of poplar (Populus spp.) and willow (Salix spp.) clonesin relation to photosynthesis. Arch Biol Sci 61:239–247
Park IS, Park JW (2011) Determination of a risk management primer at petroleum-contaminated sites: developing new human health risk assessment strategy. J Hazard Mater 185:1374–1380. doi:10.1016/j.jhazmat.2010.10.058
Peng S, Zhou Q, Cai Z, Zhang Z (2009) Phytoremediation of petroleum contaminated soils by Mirabilis Jalapa L. in a greenhouse plot experiment. J Hazard Mater 168:1490–1496. doi:10.1016/j.jhazmat.2009.03.036
Pérez-Hernández I, Ochoa-Gaona S, Schroeder RA, Rivera-Cruz M, Geissen V (2013) Tolerance of four tropical tree species to heavy petroleum contamination. Water Air Soil Pollut 224:1–13
Pezeshki S, De Laune R (1993) Effect of crude oil on gas exchange functions of Juncus roemerianus and Spartina alterniflora. Water Air Soil Pollut 68:461–468
Pezeshki S, Hester M, Lin Q, Nyman J (2000) The effects of oil spill and clean-up on dominant US Gulf coast marsh macrophytes: a review. Environ Pollut 108:129–139
Rahbar FG, Kiarostami K, Shirdam R (2012) Effects of petroleum hydrocarbons on growth, photosynthetic pigments and carbohydrate levels of sunflower. J Food Agric Environ 10:773–776
Ramos DT, Maranho LT, Godoi AFL, da Silva Carvalho Filho MA, Lacerda LG, de Vasconcelos EC (2009) Petroleum hydrocarbons rhizodegradation by Sebastiania commersoniana (BAILL.) LB SM. & DOWNS. Water Air Soil Pollut Focus 9:293–302
Redondo-Gomez S, Petenello MC, Feldman SR (2014) Growth, nutrient status, and photosynthetic response to diesel-contaminated soil of a cordgrass, Spartina argentinensis. Mar Pollut Bull 79:34–38. doi:10.1016/j.marpolbul.2014.01.009
Reis DJC (1996) Environmental control in petroleum engineering. Gulf Professional Publishing, Houston
Shukry W, Al-Hawas G, Al-Moaikal R, El-Bendary M (2013) Effect of petroleum crude oil on mineral nutrient elements, soil properties and bacterial biomass of the rhizosphere of jojoba. Br J Environ Clim Change 3:103–118
Silva RdCF, Almeida DG, Rufino RD, Luna JM, Santos VA, Sarubbo LA (2014) Applications of biosurfactants in the petroleum industry and the remediation of oil spills. Int J Mol Sci 15:12523–12542
Suprayogi B, Murray F (1999) A field experiment of the physical and chemical effects of two oils on mangroves. Environ Exp Bot 42:221–229
Venkidusamy K, Megharaj M, Marzorati M, Lockington R, Naidu R (2016) Enhanced removal of petroleum hydrocarbons using a bioelectrochemical remediation system with pre-cultured anodes. Sci Total Environ 539:61–69
Wang Z, Luo J, Gao H, Wan D, Ge C, Luo S, Yang J (2005) Effects of 1, 2, 4-trichlorobenzene and naphthalene stress on photosynthetic characteristics of rice at heading period. Sci Agric Sin 38(6):1113–1119
Wei S, Pan S (2010) Phytoremediation for soils contaminated by phenanthrene and pyrene with multiple plant species. J Soils Sed 10:886–894
Xu H, Xie WJ, Lu ZH (2013) Petroleum contaminated soil remediation using six wild plant species in the Yellow River Delta. In: Applied mechanics and materials. Trans Tech Publ, Switzerland, pp 598–601
Xun F, Xie B, Liu S, Guo C (2015) Effect of plant growth-promoting bacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) inoculation on oats in saline-alkali soil contaminated by petroleum to enhance phytoremediation. Environ Sci Pollut Res 22:598–608
Zhang LJ (2013) The selection of tree and grass species to tolerrate oil contamination in the Loess Plateau of Northern Shaanxi. Dissertation, Northwest A&F University
Acknowledgments
This work was supported by “the National Forestry Industry Research Special Funds for Public Welfare Projects (201104002-4)” and “the Fundamental Research Funds for Northwest A&F University (QN2011162)”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, G., Cui, B.X., Zhang, X.X. et al. The effects of petroleum-contaminated soil on photosynthesis of Amorpha fruticosa seedlings. Int. J. Environ. Sci. Technol. 13, 2383–2392 (2016). https://doi.org/10.1007/s13762-016-1071-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-016-1071-7