Abstract
The petroleum production has been raised sharply over the past decades, whereas the petroleum exploitation has also caused serious environmental contamination. A pot experiment has been conducted to monitor the dynamic response of antioxidant defense system and the growth reaction of Amorpha fruticosa seedlings to soil petroleum contamination. The results show that (1) in 5 g kg−1 contaminated soil, A. fruticosa removes reactive oxygen species (ROS) by increasing the activities of antioxidant enzymes (glutathione reductase (GR), superoxide dismutase (SOD), catalase (CAT)), while in 10–15 g kg−1 long-term contaminated soil, A. fruticosa removes ROS by the cooperation of antioxidant enzymes and antioxidants (SOD, CAT, ascorbate peroxidase (APX), GR, ascorbic acid (AsA), glutathione (GSH), and proline (Pro)). In long-term 20 g kg−1 contaminated soil, the defense ability of APX and AsA decreases sharply, and A. fruticosa removes the ROS by the synergistic effect of antioxidant enzymes (SOD and CAT) and antioxidants (GSH and Pro). Only in 20 g kg−1 long-term petroleum contamination caused significant (P < 0.05) increase in H2O2 content in seedlings. (2) SOD, CAT, GR, GSH, and Pro exhibit increases in long-term severely contaminated soil, and these enzymes and antioxidants are the most important defender of A. fruticosa to ROS accumulation caused by petroleum contamination. (3) The growth of A. fruticosa seedlings is less affected in 5 g kg−1 petroleum-contaminated soil, while it significantly decreases in 10, 15, and 20 g kg−1 petroleum-contaminated soils (P < 0.05). (4) Considering comprehensively the response of antioxidant defense system and the growth reaction of seedlings to petroleum contamination, A. fruticosa could be utilized for phytoremediation in ≤15 g kg−1 contaminated soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With the expansion of population, the rapid development of industry and the urbanization, the demand for petroleum has increased sharply over the past decades, which has promoted the booming of petroleum industry. However, the oil spill in petroleum exploitation has also caused increasingly serious environmental pollution. Petroleum can cause the deterioration of soil properties, affect the growth of plants, and exhibit strong teratogenicity and carcinogenicity to animals and human beings (Ogboghodo et al. 2004; Wang et al. 2010; Rahn 2012; Ribeiro et al. 2013; Thavamani et al. 2012; Karamalidis et al. 2010). Hence, the remediation of soil petroleum contamination has become an urgent experimental case to be solved (Alkorta and Garbisu 2001).
Among the existing remediation technologies, phytoremediation has been seen as a promising approach due to its properties such as low cost, high biomass for extracting or transforming contaminants, less secondary contamination, and the ability of improving the eco-environment of contaminated regions. The selection of proper remediator plants has been well documented during the recent decades. Many crops, such as Zea mays, Sorghum bicolor, and Daucus carota (Li et al. 2006), and herbaceous plants, such as Mirabilis Jalapa, Carex stricta, Poa foliosa, Vetiveria zizanioides, Lolium perenne, Bassia scoparia, and Festuca spp., have been found to exhibit the potential of remediating the petroleum-contaminated soil (Peng et al. 2009; Euliss et al. 2008; Bramley-Alves et al. 2014; Brandt et al. 2006; Kirk et al. 2005; Liu et al. 2014; Moubasher et al. 2015). Furthermore, some researchers devoted to enhancing the remediating ability of plants by inoculating mycorrhizal fungi or endophyte (Hernandez-Ortega et al. 2012; Soleimani et al. 2010). However, because of the low biomass and shallow root system of crops or herbaceous species, they only exhibit a weak ability of extracting/transporting contaminants, and hence they are inadequate in remediation of the petroleum-contaminated soil in deep layer of long-term contaminated regions. As the exploitation continues, the crude oil in surface soil may reach the deep soil layer along with rainfall infiltration; thus it has become an urgent case to select tree remediator species with larger biomass, more favorable tolerance, and developed roots to remediate the petroleum contamination in deep layer soil.
Northern Shaanxi is an important petroleum production region in China. After several decades of petroleum exploitation, the soil here has shown an obvious trend of deep-layer petroleum contamination. Especially, the phytoremediation was conducted on the soil layer which covered on petroleum-contaminated soil in some long-term exploited oil fields. Hence, there are obvious limitations in using crops and herbaceous plants for phytoremediation in these regions, while the few investigated arbor tree remediator species, such as Rhizophora mangle (Moreira et al. 2011), Samanea saman (Bento et al. 2012), Populus deltoides × Populus nigra, and Salix nigra (Corseuil and Moreno 2001; Spriggs et al. 2005), cannot grow well in this arid region. Hence, a shrub species, Amorpha fruticosa, was studied in the present research. The performances of antioxidant system and growth indices of its seedlings were observed in a pot-planting experiment in petroleum-contaminated soil with different petroleum concentrations to assess the responses of A. fruticosa to soil petroleum contamination, aiming at providing scientific basis for the selection of plants for the phytoremediation of long-term, deep-layer petroleum-contaminated soil in arid area.
2 Materials and Methods
2.1 Introduction of Research Area
The pot cultivation experiment was carried out in the nurseries of Northwest A&F University, Yangling, China. The climate here is classified as warm semi-humid continental monsoon climate, with an altitude of 454.5 m, an average annual sunshine duration of 2150 h, an average annual precipitation of 635 mm, an average annual humidity of 70 %, an average annual temperature of 12.9 °C, an annual effective accumulative temperature (over 10 °C) of 4169.2 °C, and a frost-free period of 221 days.
2.2 Materials
The 1-year-old seedlings and crude oil used for experiments were purchased from Ansai County, northern Shaanxi, China. The cultivation media (soil) was collected from the surface (0–20 cm) of the uncontaminated wasted grassland of Ansai County, which contained 8.45 g kg−1 organic matters, 0.42 g kg−1 total nitrogen, 5.5 mg kg−1 available phosphorus, and 74.6 mg kg−1 available potassium, and its pH value was 7.8.
2.3 Experiment Design
In mid-February of 2011, the soil sample was air dried, screened with a 4-mm sieve, and divided into five parts, four of which were mixed with crude oil with the concentrations of 5, 10, 15, and 20 g kg−1 (the concentration was designed according to the actual level in the contaminated region of Ansai County). The contaminated soil was repeatedly stirred without any organic solvent, homogenized for 60 days, and then screened with a 4-mm sieve and used as cultivation media for A. fruticosa seedlings. The uncontaminated soil was used as the cultivation media of CK testing.
The prepared soil media were placed into cultivation pots in mid-April (each pot contained 12.94 kg uncontaminated dry soil), and each treatment had nine replications. Three A. fruticosa seedlings were planted in each pot, and the planted pots were placed in a removable rainshed to avoid natural precipitation. All seedlings were sufficiently watered to secure their survival and growth. At the end of June, the soil moisture of each pot was uniformly adjusted to 75 % of the field water-holding capacity according to the weight of the pots. To rule out the influence of petroleum on the water-holding capacity of contaminated soil, the actual soil moisture was determined, and no significant differences were found. In order to eliminate the error caused by seedling weight, the seedlings in one pot with the same treatment were pulled up and weighed to adjust the standard of weight (thereafter, the standard of weight was adjusted each 30 days according to this method to maintain the constant moisture during the period from July to the end of September).
At 9: 00 a.m., 31st of July, 30th of August, and 29th of September, the functional leaves from the middle part of the seedlings were collected and used for the determination of activities of antioxidant enzymes and contents of antioxidant and H2O2. On the 29th of September, all seedlings were fully harvested, and the length and quantity of first-level lateral roots were measured. Thereafter, the seedlings were dried at 80 °C for 12 h and then the dry weights of shoot and root of seedlings were determined. Simultaneously, the soil in pots was also sampled for the determination of the remnant petroleum concentration.
2.4 Determination of Indices
Superoxide dismutase (SOD) activity was determined according to the method of Beyer and Fridovich (1987) by taking 50 % of inhibiting nitro blue tetrazolium (NBT) light reaction as an enzyme activity unit. Catalase (CAT) activity was determined according to the method of Tanida (1996) by taking the OD240 value reduced by 0.1 as an enzyme activity unit. Ascorbate peroxidase (APX) activity was determined according to the method of Saruyama and Tanida (1995) by taking AsA consuming 1 μmol/min as an enzyme activity unit. (The calculation of AsA oxidation consumption is based on the extinction coefficient of 2.8 mmol/L · cm). Glutathione reductase (GR) activity was determined according to the method of Ding et al. (2005) by taking OD340 decreased by 0.01 per minute as an enzyme activity unit. Proline content was determined according to the method of Bradford (1976), and standard curve was drawn by using cattle’s blood serum (BSA). Ascorbic acid (AsA) content was determined according to the method of Tanaka et al. (1985). Reduced glutathione (GSH) content was determined according to the method of Ellman (1959). H2O2 content was determined as described by Mukherjes and Choudhuri (1983). The concentration of petroleum was determined by using gravimetric method suggested by Wang et al. (2015).
2.5 Statistical Analysis
One-way analysis of variance was performed using SPSS 19.0 software. Duncan’s test was used to determine the differences among treatment means at a significance level of P < 0.05. Sigma Plot 12.5 software was employed for drafting.
3 Results
3.1 Responses of Antioxidant System of A. fruticosa to Petroleum Contamination
3.1.1 Antioxidant Enzyme Activities
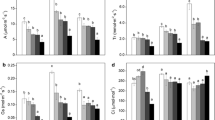
The activities of antioxidant enzymes of A. fruticosa seedlings generally exhibited significant increases during the late stage of growth. Specifically, the SOD activity of A. fruticosa seedlings did not show significant alterations in Jul. and Aug. (Fig. 1a), while it exhibited a significant increase in Sep. (P < 0.05) in 5 g kg−1 petroleum-contaminated soil. In 10 g kg−1 petroleum-contaminated soil, the SOD activity exhibited significant (P < 0.05) increases in Aug. and Sep., while in 15 and 20 g kg−1 petroleum-contaminated soil, the SOD activity increased steadily and significantly during the whole period (P < 0.05). In 5 g kg−1 petroleum-contaminated soil, the CAT activity of seedlings significantly increased only in Sep., while it significantly increased during Jul. to Sep. in 10 and 15 g kg−1 petroleum-contaminated soil. However, in 20 g kg−1 petroleum-contaminated soil, the CAT did not exhibit significant increase in Jul., while it only showed significant increase in Aug. and Sep. (Fig. 1b). For APX, its activity exhibited no significant alteration during all sampling stages in 5 and 20 g kg−1 petroleum-contaminated soil. In 10 g kg−1 petroleum-contaminated soil, the APX activity exhibited significant increase in Aug. and Sep., while it only showed significant increase in Sep. in 15 g kg−1 petroleum-contaminated soil (Fig. 1c). For GR, its activity generally exhibited significant increase in petroleum-contaminated soil during all sampling stages except in Aug. in the 5 and 20 g kg−1 contaminated soil (Fig. 1d).
Changes in SOD (a), CAT (b), APX (c), and GR (d) activities of A. fruticosa seedlings at different sampling times and with different petroleum concentrations. The petroleum concentration is presented as  0,
0,  5,
5,  10,
10,  15, and
15, and  20 g kg−1. Bars represent means ± SE (n = 5). Bars within the same date group followed by the different letters are significantly different at P < 0.05 according to Duncan’s test
20 g kg−1. Bars represent means ± SE (n = 5). Bars within the same date group followed by the different letters are significantly different at P < 0.05 according to Duncan’s test
3.1.2 Antioxidant Contents
The alterations of antioxidants in A. fruticosa seedlings were similar with those of antioxidant enzymes. The content of AsA, GSH, and proline (Pro) in the 5 g kg−1 contaminated soil did not exhibit significant differences compared with those in the CK soil. In the 10 and 15 g kg−1 contaminated soil, the content of AsA generally showed significant increases in all sampling stages, except in Aug. in the 15 g kg−1 contaminated soil. In the 20 g kg−1 contaminated soil, the content of AsA exhibited a significant increase in Jul., while in Aug. and Sep., it did not show statistical differences compared with that in CK (Fig. 2a). In 10, 15, and 20 g kg−1 contaminated soil, the GSH content exhibited significant increase in all sampling stages except in Jul. in 15 and 20 g kg−1 contaminated soil (Fig. 2b). In 10 g kg−1 contaminated soil, Pro content did not show significant alteration compared with that in CK soil in Jul., while it significantly increased in Aug. and Sep. In 15 and 20 g kg−1 contaminated soil, Pro content exhibited significant increase during all sampling stages, and the average increment reached to 102 and 272 %, respectively (Fig. 2c).
Changes in AsA (a), GSH (b), Pro (c), and H2O2 (d) content of A. fruticosa seedlings at different sampling times and with different petroleum concentrations. The petroleum concentration is presented as  0,
0,  5,
5,  10,
10,  15, and
15, and  20 g kg−1. Bars represent means ± SE (n = 5). Bars within the same date group followed by the different letters are significantly different at P < 0.05 according to Duncan’s test
20 g kg−1. Bars represent means ± SE (n = 5). Bars within the same date group followed by the different letters are significantly different at P < 0.05 according to Duncan’s test
3.1.3 H2O2 Contents
Compared with those planted in CK soil, the H2O2 content of A. fruticosa seedlings did not exhibit statistical differences in all sampling stages in 5, 10, and 15 g kg−1 contaminated soil. In 20 g kg−1 contaminated soil, the H2O2 content exhibited no significant increases in Jul. and Aug., whereas it significantly increased by 26.43 % in Sep. (Fig. 2d).
3.1.4 Responses of Growth Indices of A. fruticosa to Petroleum Contamination
Petroleum contamination considerably lowered the biomass accumulation of A. fruticosa seedlings. In 5 g kg−1 contaminated soil, the shoot/root dry weight of seedlings showed no statistical differences compared with those growing in CK soil. Along with the increase of soil petroleum concentration (10–20 g kg−1), the shoot dry weight of seedlings significantly decreased by 7.34, 55.36, and 65.16 %, respectively, and the root dry weight of seedlings significantly decreased by 38.26, 54.81, and 65.34%, respectively (Table 1). In 5–10 g kg−1 contaminated soil, the length and quantity of first-level lateral roots of seedlings exhibited no statistical differences compared with those in CK soil, while in severely contaminated soil, root development of seedlings was considerably inhibited. Specifically, the length of first-level lateral roots significantly decreased by 37.75 and 63.98 % in 15 and 20 g kg−1 contaminated soil, respectively, while the quantity of first-level lateral roots significantly decreased by 25.47 and 34.66%, respectively (Table 1).
3.1.5 Decreases of Petroleum Concentration in A. fruticosa-Planted Soil
After 5.5-month experiment, a significant natural degradation rate of petroleum contaminants (30.73 % average) was observed in unplanted soil, while the natural degradation rates of different contaminated soils exhibited no statistical differences (Fig. 3a). A. fruticosa exhibited favorable phytoremediation effects on contaminated soil, and the degradation rates of petroleum in 5–20 g kg−1 petroleum-contaminated soil were 75.68, 75.99, 88.54, and 88.10 % (Fig. 3b), respectively, which were 2.46, 2.87, 2.68, and 3.12 times of the corresponding natural degradation rates.
4 Discussion and Conclusion
4.1 Responses of Antioxidant System of A. fruticosa Seedlings to Petroleum Contamination
Under normal conditions, the production and scavenging of ROS in plants are tightly controlled in a homeostatic way by antioxidant defense system (including antioxidant enzymes and nonenzymatic antioxidants), and ROS are kept at compatible low levels exhibiting no harm to cells. When suffering various environmental stresses, plants will overproduce ROS, and at the meantime, it also actively regulates the antioxidant defense system to keep the equilibrium of production and scavenging; but, if the production of ROS exceeds its scavenging capacity, the equilibrium will be disturbed and ROS will accumulate in large amount, causing oxidative damage to plant cells and further affecting plant growth, even withering plants. Previous studies have indicated that petroleum and its products can significantly inhibit the activities of antioxidant enzymes such as SOD and CAT of Vigna unguiculata, Z. mays, and L. perenne (Achuba 2014; Zhang et al. 2012). In contrast, our study indicates that in 5 g kg−1 contaminated soil, only the GR activity of A. fruticosa seedlings significantly increased in Jul. and Aug., while the other components of antioxidant system did not exhibit obvious responses, which demonstrates that A. fruticosa has favorable tolerance to 5 g kg−1 petroleum contamination. The accumulation of ROS in A. fruticosa seedlings is negligible and subthreshold (Fig. 2d). Under this petroleum concentration, only the long-term contamination could cause significant increases in SOD, CAT, and GR activities, which demonstrates that long-term 5 g kg−1 petroleum contamination does show stresses to A. fruticosa seedlings, but it only stimulates the activities of a part of antioxidant enzymes, and these responses of seedlings are strong enough to remove the superfluous ROS (Fig. 2d). However, along with the increase in petroleum concentration in soil (10–15 g kg−1), the antioxidant enzyme activities and antioxidants contents (CAT, GR, AsA, GSH) showed rapid increases in a short-term, which conforms with the findings of Xun et al. (2015), Gong et al. (2011), and Rong (2010). These indicate that in 10–15 g kg−1 contaminated soil, the ROS stress caused by petroleum increases sharply, but the antioxidant system of A. fruticosa seedlings still could be rapidly activated. Along with the extension of contaminated duration, almost all of the enzymes and antioxidants of A. fruticosa seedlings showed significant increases in activities and contents in 10 and 15 g kg−1 contaminated soil at the late sampling stages, which indicates that the components of antioxidant system perform favorable synergistic action (Asada 1999; Han et al. 2008), and the Halliwell-AsAda cycle is effectively reactivated to maintain an efficient removal of ROS (Fig. 2d). However, in 20 g kg−1 contaminated soil, the operation of antioxidant system of A. fruticosa seedlings was significantly hindered. Although the activities of SOD and GR and the contents of AsA, GSH, and Pro still exhibited significant increases in Jul. and Aug. relative to those in CK testing, and the content of H2O2 did not show significant increases as well, so, the results demonstrate that the entire cooperation of antioxidant system is disturbed (APX activity did not show a simultaneous increase with other enzymes and antioxidants) in 20 g kg−1 contaminated soil, and thus, the Halliwell-AsAda cycle is hindered. Correspondingly, at the later sampling stage, significant H2O2 accumulation was observed in A. fruticosa seedlings. Hence, considering the ability of antioxidant system, A. fruticosa is only more tolerant to petroleum contamination whose concentration is below 15 g kg−1.
Interestingly, the antioxidant system of A. fruticosa exhibited variable response approaches to petroleum contamination with the increase in petroleum concentration, and the different enzymes and antioxidants exhibited different response trends as well. In 5 g kg−1 contaminated soil, A. fruticosa combated with ROS only by increasing the activities of antioxidant enzymes, while in lasting severely contaminated soil, A. fruticosa effectively removed ROS by the cooperation of antioxidant enzymes and antioxidants (Halliwell-AsAda cycle). However, in long-term 20 g kg−1 contaminated soil, the defense ability of APX and AsA decreased sharply, and the Halliwell-AsAda cycle could not be effectively reactivated, but A. fruticosa could still remove the ROS by the synergistic effect of antioxidant enzymes (SOD and CAT) and antioxidants (GSH and Pro). Generally, according to the response characteristics of enzymes and antioxidants to the concentration and duration of petroleum contamination, SOD, CAT, GR, GSH, and Pro exhibit increases in long-term severely contaminated soil, and these enzymes and antioxidants are the most important defender of A. fruticosa to ROS accumulation caused by petroleum contamination. Among them, GR is the most sensitive, rapidly activated component. The defense effects of APX and AsA are negligible in 20 g kg−1 contaminated soil. These might be caused by the difference in the characteristics of different enzymes and antioxidants.
In addition, as for the activities of SOD, CAT, GR, and APX and the content of AsA, no obvious concentration-response relations have been observed during all sampling stages. Generally, the increase of their activities and content occurred in 10–15 g kg−1 contaminated conditions, which might be attributed to the fact that the contamination degree exceeds the margins of tolerance of A. fruticosa seedlings and thus hinders the increase in enzyme activities and the synthesis of antioxidants. However, at the same sampling stage, the contents of GSH and Pro usually increase with the increase of petroleum concentration (especially in Aug. and Sep.), which indicates that these antioxidants exhibit more important effects in the antioxidation of A. fruticosa on long-term petroleum contamination.
4.2 Responses of Seedling Growth of A. fruticosa to Petroleum Contamination and Its Remediation Effects on Contaminated Soil
Petroleum contamination usually affects plant growth seriously. For instance, Kirk et al. (2002) indicated that petroleum contamination significantly inhibited the development of roots of Schizachyrium scoparium and Coronilla varia. Bento et al. (2012) stated that the growth of Acacia holosericea and Acacia mangium in severely petroleum-contaminated soil was remarkably hindered. The investigation of Tang et al. (2011) demonstrated that the root elongation of Triticum aestivum and Z. mays was inhibited in contaminated soil. In addition, significant lower biomass accumulations of plants were reported by del C Rivera-Cruz et al. (2006) and Ramos et al. (2009).
Similarly, our results reveal that although 5 g kg−1 petroleum-contaminated soil does not statistically inhibit the growth of A. fruticosa seedlings, the growth of A. fruticosa is significantly inhibited in 10–20 g kg−1 contaminated soil, and the development of first-level lateral roots of seedlings is remarkably hindered as well. These might be attributed to the alterations in soil physical and chemical properties caused by petroleum contamination. Andrade et al. (2004) indicated that petroleum contamination might significantly decrease the porosity of soil, affect the air and water permeability of soil, and hinder the water absorption and respiration of roots, thus forcing roots to increase their diameter and decrease their length (Kechavarzi et al. 2007). In addition, petroleum contamination can inhibit the nitrification and dephosphorylation and thus decrease the availability of N and P in soil (Ogboghodo et al. 2004; Wang et al. 2010). Furthermore, the components with high molecular weight in petroleum can cover the roots of plants, hindering their respiration and nutrient uptakes (Yang 1987). Besides, Yang (1987) indicated that the components with lower molecular weight can easily enter the tissue of plant for their lipid solubility and cause the increase in ROS contents in tissues and affect the growth of plants. Shukry et al. (2013) indicated that petroleum seriously injures the biomembrane (increase the MDA content). Our results also demonstrate that in 20 g kg−1 long-term contaminated soil, the H2O2 content in seedlings significantly increases, and meanwhile, the growth of seedlings is significantly inhibited, which conforms with the abovementioned findings. Because the increase in ROS can cause injures of chloroplastid, decrease the contents of photosynthetic pigments and the efficiency of photo-energy conversion, and it considerably inhibits the transpiration simultaneously (Yang 1987; Lin et al. 2002), these phenomena, of course, would hinder the growth and development of plants and lead to decreases of their biomass.
Our results also demonstrate that planting A. fruticosa seedlings could significantly decrease the petroleum concentration in soil up to one to two times higher than that in natural conditions, indicating the favorable remediating ability of A. fruticosa to petroleum-contaminated soil. However, in >15 g kg−1 contaminated soil, the growth and root development of A. fruticosa would be unacceptably inhibited. Additionally, considering the ROS removal ability of antioxidant system in contaminated soil, we suggest that A. fruticosa be utilized for phytoremediation in ≤15 g kg−1 contaminated soil.
References
Achuba, F. I. (2014). Petroleum products in soil mediated oxidative stress in cowpea (Vigna unguiculata) and maize (Zea mays) seedlings. Open Journal of Soil Science, 4(12), 417.
Alkorta, I., & Garbisu, C. (2001). Phytoremediation of organic contaminants in soils. Bioresource Technology, 79(3), 273–276. doi:10.1016/S0960-8524(01)00016-5.
Andrade, M. L., Covelo, E. F., Vega, F. A., & Marcet, P. (2004). Effect of the Prestige oil spill on salt marsh soils on the Coast of Galicia (Northwestern Spain). Journal of Environmental Quality, 33(6), 2103–2110.
Asada, K. (1999). The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annual Review of Plant Biology, 50(1), 601–639.
Bento, R. A., Saggin-Júnior, O. J., Pitard, R. M., Straliotto, R., da Silva, E. M. R., Tavares, S. R. D. L., et al. (2012). Selection of leguminous trees associated with symbiont microorganisms for phytoremediation of petroleum-contaminated soil. Water, Air, & Soil Pollution, 223(9), 5659–5671. doi:10.1007/s11270-012-1305-3.
Beyer, W. F., & Fridovich, I. (1987). Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Analytical Biochemistry, 161(2), 559–566.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1), 248–254.
Bramley-Alves, J., Wasley, J., King, C. K., Powell, S., & Robinson, S. A. (2014). Phytoremediation of hydrocarbon contaminants in subantarctic soils: an effective management option. Journal of Environment Management, 142, 60–69. doi:10.1016/j.jenvman.2014.04.019.
Brandt, R., Merkl, N., Schultze-Kraft, R., Infante, C., & Broll, G. (2006). Potential of vetiver (Vetiveria zizanioides (L.) Nash) for phytoremediation of petroleum hydrocarbon-contaminated soils in Venezuela. International Journal of Phytoremediation, 8(4), 273–284. doi:10.1080/15226510600992808.
Corseuil, H. X., & Moreno, F. N. (2001). Phytoremediation potential of willow trees for aquifers contaminated with ethanol-blended gasoline. Water Research, 35(12), 3013–3017.
del C Rivera-Cruz, M., Trujillo-Narcía, A., Ferrera-Cerrato, R., Rodríguez-Vázquez, R., Volke-Haller, V., Sánchez-García, P., et al. (2006). Fitorremediación de suelos con Benzo (a) Pireno mediante microorganismos autóctonos y pasto alemán [Echinochloa polystachya (HBK) Hitchc.]. Universidad y Ciencia, 22(1), 1–12.
Ding, H., Zhu, W., Yang, S., & Yang, X. (2005). Dynamic changes in antioxidative systems in roots of tomato (Lycopersicom esculentum Mill.) seeding under zinc stress and recovery. Chinese Journal of Applied & Environmental Biology, 11(5), 531–535.
Ellman, G. L. (1959). Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics, 82(1), 70–77.
Euliss, K., Ho, C. H., Schwab, A. P., Rock, S., & Banks, A. K. (2008). Greenhouse and field assessment of phytoremediation for petroleum contaminants in a riparian zone. Bioresource Technology, 99(6), 1961–1971. doi:10.1016/j.biortech.2007.03.055.
Gong, X., Rong, L., Yang, L., Liu, Z., & Fang, Y. (2011). Effect and eco-toxicity of maize growth in petroleum contaminated soil. Environmental Science and Technology, 34(10), 71–75.
Han, G., Dang, Q., & Zhao, Z. (2008). Response of antioxidation protection system of Hedysarum scoparium to drought stress. Acta Botanica Boreali-Occidentalia Sinica, 28(5), 1007–1013.
Hernandez-Ortega, H. A., Alarcon, A., Ferrera-Cerrato, R., Zavaleta-Mancera, H. A., Lopez-Delgado, H. A., & Mendoza-Lopez, M. R. (2012). Arbuscular mycorrhizal fungi on growth, nutrient status, and total antioxidant activity of Melilotus albus during phytoremediation of a diesel-contaminated substrate. Journal of Environment Management, 95(Suppl), S319–324. doi:10.1016/j.jenvman.2011.02.015.
Karamalidis, A., Evangelou, A., Karabika, E., Koukkou, A., Drainas, C., & Voudrias, E. (2010). Laboratory scale bioremediation of petroleum-contaminated soil by indigenous microorganisms and added Pseudomonasaeruginosa strain Spet. Bioresource Technology, 101(16), 6545–6552.
Kechavarzi, C., Pettersson, K., Leeds-Harrison, P., Ritchie, L., & Ledin, S. (2007). Root establishment of Perennial ryegrass (L. perenne) in diesel contaminated subsurface soil layers. Environmental Pollution, 145(1), 68–74.
Kirk, J. L., Klirnomos, J. N., Lee, H., & Trevors, J. T. (2002). Phytotoxicity assay to assess plant species for phytoremediation of petroleum-contaminated soil. Bioremediation Journal, 6(1), 57–63.
Kirk, J. L., Klironomos, J. N., Lee, H., & Trevors, J. T. (2005). The effects of perennial ryegrass and alfalfa on microbial abundance and diversity in petroleum contaminated soil. Environmental Pollution, 133(3), 455–465. doi:10.1016/j.envpol.2004.06.002.
Li, F., Yao, J., & Wang, Q. (2006). Screening of plants for phytoremediation of petroleum-contaminated soils. Chinese Agricultural Science Bulletin, 22(9), 429–431.
Lin, Q., Mendelssohn, I. A., Suidan, M. T., Lee, K., & Venosa, A. D. (2002). The dose-response relationship between No. 2 fuel oil and the growth of the salt marsh grass. Spartina alterniflora. Marine Pollution Bulletin, 44(9), 897–902.
Liu, R., Xiao, N., Wei, S. H., Zhao, L. X., & An, J. (2014). Rhizosphere effects of PAH-contaminated soil phytoremediation using a special plant named Fire Phoenix. Science of the Total Environment, 473, 350–358. doi:10.1016/j.scitotenv.2013.12.027.
Moreira, I. T. A., Oliveira, O. M. C., Triguis, J. A., dos Santos, A. M. P., Queiroz, A. F. S., Martins, C. M. S., et al. (2011). Phytoremediation using Rizophora mangle L. in mangrove sediments contaminated by persistent total petroleum hydrocarbons (TPH’s). Microchemical Journal, 99(2), 376–382. doi:10.1016/j.microc.2011.06.011.
Moubasher, H. A., Hegazy, A. K., Mohamed, N. H., Moustafa, Y. M., Kabiel, H. F., & Hamad, A. A. (2015). Phytoremediation of soils polluted with crude petroleum oil using Bassia scoparia and its associated rhizosphere microorganisms. International Biodeterioration & Biodegradation, 98, 113–120. doi:10.1016/j.ibiod.2014.11.019.
Mukherjes, S., & Choudhuri, M. (1983). Implication of water stress induced change in the lever of endogenous ascorbic acid under hydrogen peroxide in vigna seedings. Physiology. Plant, 58(2), 166–170.
Ogboghodo, I., Iruaga, E., Osemwota, I., & Chokor, J. (2004). An assessment of the effects of crude oil pollution on soil properties, germination and growth of maize (Zea mays) using two crude types–Forcados light and Escravos light. Environmental Monitoring and Assessment, 96(1-3), 143–152.
Peng, S., Zhou, Q., Cai, Z., & Zhang, Z. (2009). Phytoremediation of petroleum contaminated soils by Mirabilis Jalapa L. in a greenhouse plot experiment. Journal of Hazardous Materials, 168(2-3), 1490–1496. doi:10.1016/j.jhazmat.2009.03.036.
Rahn, J. H. (2012). A test method for the evaluation of soil microbial health in a petroleum hydrocarbon contaminated boreal forest soil. Dissertation, The University of Guelph.
Ramos, D. T., Maranho, L. T., Godoi, A. F. L., da Silva Carvalho Filho, M. A., Lacerda, L. G., & de Vasconcelos, E. C. (2009). Petroleum hydrocarbons rhizodegradation by Sebastiania commersoniana (BAILL.) LB SM. & Downs. Water, Air, & Soil Pollution: Focus, 9(3-4), 293–302.
Ribeiro, H., Mucha, A. P., Almeida, C. M., & Bordalo, A. A. (2013). Bacterial community response to petroleum contamination and nutrient addition in sediments from a temperate salt marsh. Science of the Total Environment, 458–460, 568–576. doi:10.1016/j.scitotenv.2013.04.015.
Rong, L. (2010). Study on the effects of eco-toxicity of petroleum-contaminated soil on plants growth. Dissertation, Nanchang University.
Saruyama, H., & Tanida, M. (1995). Effect of chilling on activated oxygen-scavenging enzymes in low temperature-sensitive and-tolerant cultivars of rice (Oryza sativa L.). Plant Science, 109(2), 105–113.
Shukry, W., Al-Hawas, G., Al-Moaikal, R., & El-Bendary, M. (2013). Effect of petroleum crude oil on mineral nutrient elements, soil properties and bacterial biomass of the rhizosphere of jojoba. British Journal of Environment and Climate Change, 3(1), 103–118.
Soleimani, M., Afyuni, M., Hajabbasi, M. A., Nourbakhsh, F., Sabzalian, M. R., & Christensen, J. H. (2010). Phytoremediation of an aged petroleum contaminated soil using endophyte infected and non-infected grasses. Chemosphere, 81(9), 1084–1090. doi:10.1016/j.chemosphere.2010.09.034.
Spriggs, T., Banks, M. K., & Schwab, P. (2005). Phytoremediation of polycyclic aromatic hydrocarbons in manufactured gas plant–impacted soil. Journal of Environmental Quality, 34(5), 1755–1762.
Tanaka, K., Suda, Y., Kondo, N., & Nakano, Y. (1985). Ozone tolerance and the ascorbate-dependent hydrogen peroxide decomposing system in chloroplasts. Plant & Cell Physiology, 26(1425), 3.
Tang, J., Wang, M., Wang, F., Sun, Q., & Zhou, Q. (2011). Eco-toxicity of petroleum hydrocarbon contaminated soil. Journal of Environmental Sciences, 23(5), 845–851.
Tanida, M. (1996). Catalase activity of rice seed embryo and its relation to germination rate at a low temperature. Breeding Science, 46(1), 23–27.
Thavamani, P., Malik, S., Beer, M., Megharaj, M., & Naidu, R. (2012). Microbial activity and diversity in long-term mixed contaminated soils with respect to polyaromatic hydrocarbons and heavy metals. Journal of Environmental Management, 99, 10–17.
Wang, X., Feng, J., & Zhao, J. (2010). Effects of crude oil residuals on soil chemical properties in oil sites, Momoge Wetland, China. Environmental Monitoring and Assessment, 161(1-4), 271–280.
Wang, R., Wang, M., Niu, X., & Tang, C. (2015). Determination of total petroleum hydrocarbons content in soil by ultrasonic-soxhlet extraction-gravimetric analysis. Chinese Journal of Analytical Chemistry, 38(3), 417–420.
Xun, F., Xie, B., Liu, S., & Guo, C. (2015). Effect of plant growth-promoting bacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) inoculation on oats in saline-alkali soil contaminated by petroleum to enhance phytoremediation. Environmental Science and Pollution Research, 22(1), 598–608.
Yang, C. (1987). Analysis technology for the petroleum contaminants in environment. Beijing: China Environmental Science Press.
Zhang, X. S., Kang, Y. J., & Xu, D. J. (2012). Physiological response of Lolium perenne to petroleum pollution and removal efficiency in petroleum-polluted soil. Advanced Materials Research, 518, 2665–2669. Trans Tech Publ.
Acknowledgments
The present research project is supported by “the Fundamental Research Funds for Northwest A&F University (QN2011162)” and “the National Forestry Industry Research Special Funds for Public Welfare Projects (201104002-4).”
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, B., Zhang, X., Han, G. et al. Antioxidant Defense Response and Growth Reaction of Amorpha fruticosa Seedlings in Petroleum-Contaminated Soil. Water Air Soil Pollut 227, 121 (2016). https://doi.org/10.1007/s11270-016-2821-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-2821-3






 ) in different petroleum contaminated soils with (a) and without (b) planting A. fruticosa seedlings. Bars represent means ± SE (n = 5). Bars within the same date group followed by the different letters are significantly different at P < 0.05 according to Duncan’s test
) in different petroleum contaminated soils with (a) and without (b) planting A. fruticosa seedlings. Bars represent means ± SE (n = 5). Bars within the same date group followed by the different letters are significantly different at P < 0.05 according to Duncan’s test