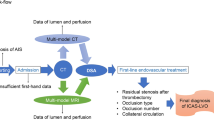
Abstract
Digital Subtraction Angiography (DSA) is the gold-standard imaging modality in acute cerebrovascular diagnosis. The role of DSA has become increasingly prominent since the incorporation of endovascular therapy in standards of care for acute ischemic stroke. It is used in the assessment of cerebral vessel patency; however, the therapeutic role of DSA from a prognostic standpoint merits further investigation. The current paper provides an update on current practice on diagnostic, therapeutic and prognostic use of DSA in acute cerebrovascular diseases and various indications and perspectives that may apply, or limit its use, in ongoing surveillance or prognosis. Pre-clinical and clinical studies on the aspects, including but not limited to the morphology of cerebrovasculature in acute ischaemic stroke, are required to delineate and inform its prognostic role.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Digital subtraction angiography (DSA) is the gold standard for diagnostic cerebrovascular assessment and is routinely used in the context of neurointerventional and vascular neurology [1, 2]. Besides providing anatomical information relevant for guiding endovascular therapies, DSA is used to assess injury to cervical and intracranial arteries and veins. It provides diagnostic information regarding intracranial arterial diseases, such as atherosclerosis, stenosis and occlusion, vessel dissection, Moyamoya disease [3, 4] and vasculitis, as well as aneurysms, arteriovenous malformations and blood shunting [5]. Its subtypes, intravenous-DSA (IV-DSA) and three-dimensional (3D)-DSA, are useful in assessing intracranial aneurysms. The burden of these cerebrovascular diseases is well established. In 2019, the World Health Organisation (WHO) estimated that stroke was the second-highest contributor to disability-adjusted life years (DALYs) in populations over 50 [6]. Furthermore, although rupture rates of cerebral aneurysms are low, aneurysmal complications are associated with high morbidity and mortality rates [7].
While the diagnostic and therapeutic utility is generally undisputed, DSA is not routinely applied in evaluating prognosis—despite growing evidence of its reliability and potential prognostic role [1]. Given that the DSA requires ionising radiation and is a relatively invasive procedure carrying procedural risks, it is suboptimal for routine vascular assessment and surveillance [3]. The role of DSA in patient selection, assessment of biomarkers such as collaterals and its association with angiographic and/or clinical outcomes are not well elucidated.
This article sought to explore the current and potential diagnostic, therapeutic and prognostic applications of DSA, and various technical considerations applicable to acute cerebrovascular conditions. Furthermore, the comparative clinical utility of DSA, computed tomography angiography (CTA) and magnetic resonance angiography (MRA) is also presented.
DSA as a diagnostic imaging modality
The diagnostic implications of DSA findings for cerebrovascular diseases are summarised in Table 1. Post-processing of DSA imaging involves the collection and subtraction of mask images from fluoroscopic contrast-enhanced images to remove the extravascular structures surrounding cerebral blood vessels [8].
DSA in intra-vessel occlusion diagnosis
DSA is the gold standard for diagnosing cerebrovascular occlusion in acute ischaemic stroke, regardless of location; however, CTA is most commonly used as the first line. Table 2 provides an overview of the applications of DSA in occlusive disease. Despite its superior spatial and temporal resolution, DSA is not considered a first-line imaging option in suspected intra-vessel occlusions. DSA is more useful in collateral assessment and delineating whether occlusion is partial or complete. Once ischaemic stroke patients undergo brain non-contrast computed tomography (NCCT) imaging to rule out haemorrhage, the preferred first-line imaging can presumably vary based on the location of intra-vessel occlusions. These first-line options are summarised in Table 2. The location of intra-vessel occlusions detected by DSA can be useful in determining the aetiology of disease when combined with clinical examination. For instance, isolated occlusions without the progressive clinical disease can suggest embolic causes. In incomplete vessel occlusions, slow antegrade flow distal to partial occlusions can be mistaken as retrograde collateral flow [9]. Subsequent four-dimensional (4D)-CTA assessment can be used to evaluate and quantify retrograde flow as well as evaluate venous drainage patterns [10]. Combined with DSA, this can reveal the severity of stenosis and the contribution of collaterals to perfusing ischaemic tissue [11].
DSA in acute ischaemic stroke: diagnosing stenosis
Imaging in acute ischaemic stroke (AIS) must be rapid and accessible. NCCT rules out haemorrhage, while CTA is utilised to evaluate intra- and extracranial vessels—being both rapid and readily available [9]. CTA alone can detect stenotic vessels in ischaemic stroke with a sensitivity of 96% and specificity of 89% when compared to the gold standard, DSA [12]. CT perfusion (CTP) imaging has also demonstrated utility in evaluating perfusion status and guiding reperfusion therapy [13], for patients arriving in a delayed fashion. In some instances, both (multi-detector CT) NCCT and CTA are bypassed, and patients presenting within 6 h of symptom onset with a moderate to severe stroke severity, and patients are taken directly to the angio-suite for a diagnostic workup/therapeutic procedure using latest generation angiographic system [14]. While transcranial Doppler (TCD) can rule out intracranial stenosis, it is unable to accurately determine the degree of vessel stenosis. Magnetic resonance angiography (MRA), especially 3 T MRA, can give an accurate indication of the degree of stenosis.
DSA provides comprehensive visualisation of large vessel occlusions and is useful in diagnosing the extent of stenosis. The North American Symptomatic Carotid Endarterectomy Trial (NASCET) stenosis calculation is applied to DSA findings to quantify stenosis of the internal carotid artery (ICA) and other intracranial arteries [15].
DSA in acute ischaemic stroke: imaging collaterals
The presence and grade of baseline collateral status have implications for clinical outcomes in AIS patients receiving reperfusion therapy [9]. Multivessel DSA is the standard criterion in assessment, structural and functional, of arterial collaterals in AIS patients [16]. Besides morphology, DSA can be used to determine the functional capacity of collaterals using parameters such as relative delay in filling time [17] and assess the capillary index score (CIS) as a screening tool for reperfusion [14]. The CIS is centred on the premise that the appearance of capillary blush on pre-treatment DSA indicates viable ischaemic tissue which could potentially benefit from successful and timely revascularisation [14]. Conversely, the absence of capillary blush is indicative of non-viable cerebral tissue bound to infarction irrespective of successful revascularization or time to endovascular thrombectomy (EVT). Capillary blush can be appreciated on DSA when all potential collaterals, venous phase images included, are opacified [14]. Given the simplicity of this score, future studies on its use in the selection of patients with AIS who are likely to benefit from futile recanalization are recommended.
However, owing to the time-critical nature of the reperfusion therapy [18, 19], the use of multivessel DSA is not practical and hence not commonly used in hyperacute settings. This along with poor correlation of DSA-based collateral flow with clinical outcome, CTA is considered the first-line imaging technique for collateral assessment given its easy availability, short acquisition time and low cost, and its established standards of care use in delineating vessel occlusion. Besides, collateral flow assessment on CTA and DSA are not interchangeable [20]. Furthermore, the advent of multi-phase CTA has allowed the assessment of the degree of collateral flow in a time-resolved manner [21]. We believe that the pragmatic approach to collateral assessment in hyperacute settings is more likely to involve CTA, preferably multi-phase CTA, as an alternative to DSA in routine clinical practice. Given the variations in collateral grading/assessment and post-processing methods in hyperacute settings, standardisation of collateral grading across centres is recommended [9].
During endovascular clot retrieval, pseudo-arterial-venous shunting or early venous filling may suggest irreversibly infarcted tissue and can be utilised to prognosticate during the procedure. Decisions on antiplatelet usage during a procedure could be made in conjunction with pre-procedural imaging.
DSA in Moyamoya disease
Moyamoya disease, a chronic progressive stenotic-occlusive cerebrovascular disease of the terminal internal carotid artery and its proximal branches, is estimated to have the highest prevalence in East Asia, e.g. affecting 10.6 per 100,000 in Japan and 9.1 per 100,000 in Korea [22]. DSA is the gold standard for the diagnosis and staging of Moyamoya disease [4]. Clinically, a six-vessel analysis on DSA involving bilateral ICA, bilateral ECA to assess the quality and size of the donor vessel for surgical planning, and bilateral vertebral arteries is used to determine disease progression. The latest guidelines from Japan regarding Moyamoya disease recommend DSA staging, achieved using Suzuki’s system, as the gold standard, looking for forking, absence and minimisation of the ICA [23]. Time of flight (TOF)-MRA findings may also be used in the definitive staging of the disease. The staging of Moyamoya disease using DSA findings is defined in Supplementary Information (SI Table 1). For MRA-identified Moyamoya disease, DSA should still be considered, as it has increased diagnostic sensitivity compared with MRA—including the ability to better differentiate vasculitis—and offers information regarding transdural collaterals, which is useful for pre-operative planning. The extent of leptomeningeal, callosal and pial collaterals can indicate the rate of progression of the disease [24]. Intracranial aneurysms can develop in Moyamoya vessels, with an estimated 14% prevalence in patients with Moyamoya disease. Such aneurysms are also diagnosed using DSA, although MRA can also be used where DSA is unavailable [25]. The Matsushima Grading Scale specifically grades post-operative angiographic images to assess the post-operative middle cerebral artery (MCA) hemispheric perfusion after bypass surgery for Moyamoya disease [26].
DSA in cerebral vasculitis
DSA’s role in diagnosing cerebral vasculitis varies on the disease profile [27]. Alternating patterns of stenosis, occlusion and dilatation of arteries are often described on DSA of vasculitic vessels [5]. Importantly, it detects circumferential and eccentric vessel irregularities, which is essential in differentiating atherosclerotic versus vasculitic vessel wall changes [28]. In large vessel angiitis, such as Takayasu’s arteritis, DSA is the gold standard for the demonstration of vessel stenoses or aneurysms. It is also useful in medium-vessel disease [28]. Whilst MRI with and without contrast is the imaging technique of choice to evaluate and monitor cerebral involvement; ultrasound Doppler (colour duplex), CTA and MRI with MRA are commonly used as a screening tool and/or in the diagnosis of clinically indicated vasculitis [27]. Brain biopsy is the current gold standard for CNS vasculitis diagnosis; however, its diagnostic yield is limited by the segmental nature of CNS vasculitis [29]. This limitation, coupled with DSA’s invasive nature, means that MR imaging is the most commonly used modality for investigating suspected cerebral vasculitis [28].
Besides, identification of vasculopathy such as posterior reversible encephalopathy syndrome (PRES) and reversible cerebral vasoconstriction syndrome (RCVS), especially in young patients, poses a clinical challenge, given their overlapping presumed etiologies and predisposing factors [30]. Interpretation of DSA findings in PRES and RCVS is complicated as the diagnostic validation depends on the resolution of angiographic findings within 8–12 weeks [31].
DSA in screening for vasospasm
Arterial vasospasm post-sub-arachnoid haemorrhage (SAH) occurs in up to 70% of patients and is associated with poor outcome despite otherwise successful ruptured intracranial aneurysm treatment [32]. Vasospasm is associated with higher rates of secondary cerebral infarction, neurological deterioration and death [33]. Among other factors, arterial vasospasm can predict the progression of the disease and thus DSA is a useful screening tool. Vasospasm is visualised as stenosis in the week following SAH, with DSA preferred in patients where balloon angioplasty is being considered [34]. TCD is also routinely used to monitor the progression, with obvious advantages of bedside accessibility and the absence of repeated radiation exposure [35]. CTA (or multi-slice/multi-detector CTA in particular [36]) and CTP have demonstrated comparative accuracy to that of DSA [37]. The sensitivity, specificity and accuracy of CTA in detecting the absence of spasm or mild and moderate cerebral vasospasm in proximal arterial locations is high [37]. However, mild and moderate spasm in distal locations is less accurately detected on CTA. CTA can overestimate the degree of cerebral vasospasm because it tends to underestimate the diameter of large cerebral arteries. When compared to DSA, CTA is less accurate in estimating the degree of vasospasm and assessing distal artery vasospasm. TCD or CTA and CTP can be used as a screening method for suspected symptomatic vasospasm, thereby reducing the need for DSA. However, DSA must be considered in clinically indicated patients with negative TCD or CTA findings, especially that DSA will have, in addition to its diagnostic value, therapeutic implications with intra-arterial pharmacological therapy or balloon angioplasty.
In the early days after SAH, cerebral autoregulation is compromised [38]. Several studies have indicated that impairment of dynamic cerebral autoregulation is linked to vasospasm and delayed cerebral ischaemia (DCI) after SAH [39]. Therefore, monitoring of cerebral autoregulation, in the early days after SAH, could aid to identify patients at increased risk of developing secondary complications [38]. Further studies are warranted to validate the prognostic and therapeutic use of cerebral autoregulation in managing SAH patients.
Transcranial Doppler is commonly used to monitor the progression over time of cerebral vasospasm after subarachnoid haemorrhage but given its poor sensitivity to identifying patients at high risk of clinical deterioration or infarction from DCI, its diagnostic or prognostic utility to DCI, is debatable [40]. CTP has also shown value in predicting DCI in SAH patients [41]. Early CTP [relative cerebral blood flow (rCBF)] can aid in the prediction of DCI in SAH patients. Late CTP [rCBF and mean transit time (MTT)] in SAH patients is strongly predictive of angiographic vasospasm and DCI. Whilst transcranial Doppler is potentially useful in screening middle cerebral artery vasospasm, it is relatively less effective in the evaluation of other intracranial arteries. Indeed, CTA and ultrasound have value in screening; however, due to lower diagnostic sensitivity and specificity for cerebral vasospasm than DSA, their utility as a surrogate or predictor of DCI remain suboptimal [34].
DSA in vessel anomalies: intracranial aneurysms
Although intracranial aneurysms can be diagnosed using less invasive imaging, such as TOF-MRA, intra-arterial DSA remains the gold standard for visualising unruptured intracranial aneurysms (UIAs) and can be used to monitor aneurysmal progression [42]. Morphology identified on DSA, including the perpendicular height and size ratio of an aneurysm, can increase rupture risks and is hence important from diagnostic and prognostic standpoint [43, 44]. Various geometrical parameters including the size ratio (SR) (perpendicular height or largest diameter/neck diameter or diameter of parent vessel), aspect ratio (AR) (the maximum dimension of the dome/width of the neck of an aneurysm), the non-sphericity index (NI), the undulation index (UI) and the vessel aneurysm inclination angle (AA) may have a role in risk prediction of aneurysm rupture [44]. The SR can predict the risk of rupture of small (< 5 mm) unruptured intracranial aneurysms (UIAs) [45]. The neck-to-dome ratio (dome width/neck width) is also an essential element in making treatment decisions for aneurysms which may include coiling, stenting, intravascular devices or combined procedures [46]. DSA could also be useful in the evaluation of precise sizing of the neck and maximum size for devices, wall lobulations, daughter lobes or nipples, and relationship to essential parent arteries emerging at the base or from aneurysm.
MRA is favoured in non-acute settings while CTA with high numbers of detector rows have also shown improving sensitivity in detecting unruptured aneurysms [47]. Despite this, traditional DSA continues to have a superior spatial resolution. Furthermore, 3D-DSA has enhanced diagnostic power [48], detecting unruptured intracranial aneurysms that were deemed false negatives by DSA; particularly aneurysms ≤ 3 mm [49]. This rotational mode of DSA holds potential for application in other cervicocranial diseases where detailed anatomy is poignant for diagnosis and management. Unruptured giant aneurysms are a particular challenge, not only due to rupture risk, but also mass effect [50]. The angiogram may show only a small portion fill, suggesting thrombosis in the remaining giant aneurysm. This information is crucial to develop the right strategic plan.
DSA in vessel anomalies: arterial dissections
Although conventional DSA remains the gold standard for diagnosis of craniocervical dissections [51, 52], non-invasive radiology, including ultrasound, CTA or MRA, is commonly used for diagnosis [52]. Diagnostic features on DSA include the vessel wall irregularities, pseudoaneurysm formation and ‘string and pearl’ sign—an angiographic trademark of ICA dissections [53]. However, DSA may be suboptimal in delineating the ICA dissection imaging features such as the arterial wall’s thickness and content [54]. DSA also has a role in identifying fusiform dilatations caused by subadventitial dissections [55]. Some acute-phase findings such as double lumens and intimal flaps are better visualised on MRA [56].
A shortfall of lone MRA use in arterial dissections is its poor success in distinguishing intraluminal from intramural thrombus [52]. The addition of T1-MRI and/or fat-suppressed images can improve this differentiation and tissue characterisation [57, 58]. Despite its diminishing role in diagnosis, and in cases where MRA is the first-line imaging, DSA can still be used to assess collaterals to assist treatment planning.
DSA in vessel anomalies: pseudoaneurysms
Abnormal dilations of a cerebral artery caused by an outpouching of the adventitial layer are known as pseudoaneurysms. DSA can differentiate pseudoaneurysms from their true counterparts by delineating the absence of an aneurysmal neck in saccular enlargements, showing filling and emptying delays and revealing minimal flow within outpouchings [59]. Whilst there is little evidence to support the use of CTA in detecting these lesions [60], it is often considered the first line. It can be useful in the assessment of thick slab maximum intensity projection (MIP) reconstructions.
DSA in vessel anomalies: AVMs
Arteriovenous malformations (AVMs) are vascular defects in which feeding arteries connect directly to draining veins with an interposed nidus, and where the usual capillary systems separating the two are absent. This causes shunting of high-flow blood through the AVM nidus, resulting in hyperplastic changes in draining veins [61]. CTA can be performed to detect AVMs, however, owing to poor anatomical delineation and low sensitivity for AVMs and small aneurysms, its utility in AVMs is limited. DSA is the gold standard for evaluating cerebral AVMs, with its high resolution and high frame rate providing a more accurate haemodynamic evaluation of the lesions [62]. The 4D-DSA is a promising technique that is increasingly proving crucial in the pre-operative planning and staging of complex embolization sessions [63]. Furthermore, recent developments in 4D-DSA have allowed provision for data on both velocity and geometry required for blood flow quantification.
Disease grading is performed using the Spetzler–Martin AVM grading scale, which considers AVM size, location and pattern of drainage (SI Table 2), and is calculated using DSA findings [64]. As well as determining the grade, AVMs can spontaneously haemorrhage, with their risk of the haemorrhagic presentation related to their radiologic features. The features visible on DSA imaging are summarised in Table 3. DSA could also be used in the assessment of the supply of AVM, en passage vs direct supply to the nidus. En passage supply may make embolization difficult or impossible.
As well as predicting haemorrhagic transformation, DSA imaging can be used to establish aneurysms proximal to or within the nidus of AVMs. MRA is limited in its ability to delineate these lesions [65]. When combined with MRI, especially the gradient echo or susceptibility-weighted MRI, it can demonstrate the relationship between the AVM and haemosiderin deposition around the brain [66]. The deposition of haemosiderin along the leptomeninges can lead to superficial siderosis especially in patients with recurrent occult or extensive subarachnoid haemorrhages [66]. Emerging 4D-MRA offers spatial resolution similar to DSA [62].
Haemodynamic information obtained through DSA is important for detecting arteriovenous shunting, which is visualised as opacification of draining veins during arterial phase DSA [67]. This shunting is relevant in understanding disease progression [68]. More recently the Modified Lawton Score (combined with the traditional Spetzler–Martin Score) has shed further light on risk stratification for AVMs (especially Grade 3 AVMs) [69]. An AVM with a “compact” nidus on an angiogram poses a more acceptable surgical risk than a “diffuse” nidus AVM [69].
DSA in vessel anomalies: arteriovenous fistulas
Dural AVFs (dAVFs) occur when blood shunts from a dural artery to a dural vein/sinus, while a carotid-cavernous fistula (CCF) occurs when there is shunting from the ICA or ECA to the cavernous sinus. Six-vessel DSA is the gold standard in diagnosing dAVF, indicating arterial feeders and patterns of venous drainage to exclude the presence of a nidus [70]. Dural AVFs are graded based on the venous drainage morphology using Merland–Cognard and the Borden classification systems [71]. Cognard classification considers the location of the fistula, presence of cortical venous drainage, presence of venous ectasia and direction of blood flow through the fistula [72]. Unlike the Cognard classification, Borden classification involves the grouping of dAVF lesions into three types based on the site of venous drainage and the presence or absence of cortical venous drainage [73].
While CTA and MRA may be used to distinguish changes in cortical veins and delineate pial vessels, their temporal resolution is not comparable to DSA, making DSA superior in imaging flow patterns [74]. dAVFs that occur along the spinal cord, spinal dAVFs, require precise localisation and grading for treatment. MRA and MRI can show swelling at the level of the lesion, but do not provide the detailed visualisations afforded by DSA. Thus, DSA is considered the gold standard for spinal dAVF diagnosis.
CCFs are definitively diagnosed using DSA and the Barrow classification. Barrow classification is determined based on feeding arteries and drainage patterns, with the flow rates obtained on DSA also relevant for EVT planning [74].
DSA in diagnosing subclavian steal syndrome
Subclavian steal syndrome (SSS) describes the phenomenon of retrograde flow in the vertebral artery to compensate for increased demand for blood flow during activity. This is a consequence of a stenosed or occluded subclavian artery and can occur unilaterally or bilaterally. Although the retrograde flow can be detected using duplex ultrasound, the anatomy of the vertebral arteries and the underlying cause cannot be distinguished [75]. CTA and MRA may also be used as minimally invasive options for planning interventions when DSA is contraindicated [76].
Therapeutic role of DSA
In conjunction with endovascular therapies in AIS patients
In conjunction with endovascular therapies in AIS patients, DSA enables characterisation of the site and accessibility of the occlusion in AIS patients in hyperacute settings. DSA also provides a post-intervention evaluation of the recanalisation status; expressed by Thrombolysis in Cerebral Infarction (TICI) or modified TICI (mTICI) scores [77]. Modified TICI is the gold standard for evaluating cerebral recanalization pre- and post-mechanical thrombectomy [78]. While recanalisation is an important indicator of the success of endovascular therapy, there is evidence to suggest that reperfusion is equally important [79]. DSA perfusion imaging, although not routinely utilised, demonstrates potential in assessing post-intervention reperfusion rates, and thus predicting complications such as haemorrhagic transformation [80].
Routinely used to guide ascending catheters with the hydrophilic wire, DSA prevents iatrogenic vessel injury and dissection during EVT [2]. DSA can also be used to confirm clot retrieval and improve treatment efficacy in stent retriever thrombectomy. Successful stent retrieval of a clot requires adequate dwell time for the clot to integrate with the stent before a pull is attempted [81]. Current operator dwell times are based on a minutes-long standard and do not assess the efficacy of clot integration [81]. A case study of four patients noted that DSA-detected blood flows through the stent immediately before a pull was associated with failed pulls [82]. Furthermore, the addition of radio-opaque indicators and wires to the stent retrievers can allow for more accurate visualisation of stent location during treatment [83].
In aneurysm
DSA has demonstrated value in diagnosis and surveillance of aneurysm, large-vessel cerebral vasospasm and DCI in subarachnoid haemorrhage patients who survive ruptured aneurysm bleed, in addition to guiding treatment such as endovascular procedures including balloon angioplasty for the symptomatic vasospasm treatment [84]. Although ongoing neurological monitoring and imaging (using transcranial ultrasound Doppler and CTA with or without perfusion) are commonly used for detection and management of DCI patients, DSA remains the reference standard [84]. Endovascular therapy such as coiling has better outcomes than surgical clipping [85,86,87]. The landmark International Subarachnoid Aneurysm Trial (ISAT) demonstrated, in patients with ruptured intracranial aneurysm, a higher survival rate at 1 year with endovascular coiling than neurosurgical clipping [85]. These findings were also found to be consistent in the long-term follow-up studies [86, 87]. However, long-term risks of further bleeding, albeit low with either treatment, were more frequent with endovascular coiling.
In EVT, DSA is routinely used to guide endovascular treatment [88]. DSA is also considered the reference standard in the post-coiling follow-up assessment of aneurysms for the first 12 months [89], irrespective of the endovascular device used (e.g. flow diversion devices, coils, stents or flow restrictor devices), classifying progression through the Raymond–Roy Occlusion Classification (RROC) scale [90]. An increasing number of operators are now using modified RROC (MRRC) as this has better predictive accuracy with aneurysm occlusion than RROC (MRRC IIIa vs IIIb) [91]. For follow-up of treated aneurysms, DSA is a reasonable option as the most sensitive imaging (Class IIa; Level of Evidence C) [92]. DSA can be used in assessing suspected recurrence in cases where metallic artefact may make non-invasive imaging less effective. Moreover, DSA is also useful in the follow-up diagnosis and monitoring of vasospasm and DCI [41]. Despite this, CTA or MRA are generally preferred for follow-up of the treated aneurysm due to their less invasive nature; while when deciding on therapy, DSA is deemed as necessary (Class IIa; Level of Evidence C) [90]. Besides, for UIAs, CTA and MRA are useful for detection and follow-up (Class I; Level of Evidence B) [92].
In vasculitis
Although primary treatment of vasculitis involves corticosteroids, invasive treatment during non-acute phases is indicated in some cases. Symptomatic steno-occlusive lesions may be recanalised using DSA-guided endovascular angioplasty techniques, including ballooning [93].
Role of DSA in prognosis
DSA findings can have prognostic implications for a variety of intracranial vascular diseases. These findings are summarised in Table 4. There is evidence to suggest that DSA imaging may also be of prognostic value in AIS patients. In ICA occlusive stroke patients, involvement in distal cavernous and terminus portions of the vessel increased the risk of poor 90-day modified Rankin Score (mRS) [94]. A comparison of M1 versus more distal strokes, defined as M2, M3, anterior cerebral artery (ACA) and posterior cerebral artery (PCA) strokes, found that alteplase-treated M1 strokes were associated with higher median National Institutes of Health Stroke Scale (NIHSS) scores and poorer long-term mRS [95]. Distal occlusions were associated with significantly higher recanalisation rates and lower rates of haemorrhagic transformation in comparison to M1 occlusion strokes, treated with alteplase. One smaller study of 80 patients, 39 of which had experienced lacunar strokes, reported higher rates of normal DSA in patients aged less than 50 [96]. Determining the prognostic implication of occlusion site based on DSA findings is restricted; with most literature dichotomising proximal versus distal occlusions, limiting the ability to associate occlusion site with stroke outcomes. The predominant majority of studies on occlusion site in AIS use non-DSA imaging modalities, which reduces our ability to quantify the effectiveness of DSA in assessing prognosis.
The presence of collateral blood supply to ischaemic regions is a prognostic measure with a larger volume of supportive evidence [9]. Reperfusion using thrombolysis with intravenous tissue-type plasminogen activator (IV-tPA) and EVT has been shown to significantly improve clinical outcome in several studies. Collateral circulation is also believed to support favourable reperfusion outcomes when vessel occlusion occurs. While recognised as the gold standard for evaluating cerebral collaterals, most of the research associating collaterals with clinical and angiographic outcomes feature non-invasive imaging markers. Jansen et al. found no significant association between DSA collateral score and clinical outcomes [97]. This may, however, be a consequence of their limited DSA imaging, with patients only undergoing EVT adjacent single-vessel DSA—limiting collateral assessment to collateral supply from the anterior circulation. There is some evidence of collateral importance in posterior circulation occlusions, with the ENDOSTROKE study revealing that higher ASITN/SIR grades were associated with better rates of recanalization [98]. The same ENDOSTROKE study did not find a significant improvement in clinical outcomes based on collateral grade. Recent research by Liu et al. found that the presence of lenticulostriate arteries on DSA in MCA strokes increased the odds of a good clinical outcome [99]. Lenticulostriate arteries connect the MCA and PCA through perforator collaterals and may contribute to improved recovery of the ischaemic penumbra. Filling time delays of collaterals on both DSA and 4D-CTA are correlated with morphological appearance—for example, greater reperfusion delays were observed in patients with lower collateral scores [17]. Further research into combined DSA and perfusion imaging is essential in understanding the true reperfusion effect of the collaterals identified on DSA. Furthermore, the lack of routinely assessed long-term clinical outcomes limits our ability to understand the effect of DSA-determined collaterals on stroke recurrence and prognosis.
Assessment of recanalization on post-intervention DSA may also be of prognostic value. Good angiographic recanalization post-thrombectomy was significantly associated with favourable clinical outcomes on adjustment for baseline characteristics such as age, comorbidities, baseline stroke severity and site of occlusion [78]. Finally, a sub-study of a large Dutch trial found that CTA-confirmed carotid artery stenosis (CAS) of more than or equal to 50% in AIS patients was associated with higher mortality and morbidity when compared to non-stenosed participants [100]. There is a lack of current research directly exploring the relationship between CAS found on DSA and associated clinical outcomes in AIS patients. However, it can be extrapolated that CAS observed on DSA would provide similar prognostic reliability, consistent with a high correlation between DSA and CTA for CAS estimation [101].
Technical considerations in DSA
Considerations prior to DSA include a neurological exam to establish the patient’s baseline, a peripheral artery examination and blood analysis to check for coagulopathies and ensuring sufficient or normal kidney function (serum creatinine, electrolytes and urea) [2]. Kidney function limits the amount of contrast that can be safely injected during imaging. Clinical history should also investigate any prior history of reactions to iodinated contrast. Relative contraindications include renal insufficiency and hypersensitivity to iodinated contrast media. For these situations, macrocyclic gadolinium-based agents can be considered. Most guidelines suggest fasting 6 h prior to DSA; however, one study indicated that this does not significantly affect peri-procedure sensations such as nausea and vomiting [102]. Patients undergoing cerebral DSA may be asked to pause anticoagulants and diabetic medication prior to the procedure to minimise bleeding risk; however, this needs to be considered on a case-to-case basis depending on the institutional protocol as DSA is performed on anticoagulation at most centres [2]. Many stroke patients with large vessel occlusion on novel oral anticoagulants (NOACs) regimen, do not have the option for reversal, are contraindicated for IV-tPA, and thus only suited to EVT in this setting. Finally, although DSA can be performed during pregnancy; however, in general, any endovascular work is best avoided in pregnancy due to radiation exposure; however, radiation can be minimised in neuro-DSA by maximising ALARA (as low as reasonably achievable) techniques. Use of lead shielding should be avoided as this could lead to increased internal scatter and may potentially increase the radiation dose to the foetus. Pre- and in-procedure IV hydration could be considered in high-risk cases. Vascular access in DSA is obtained from the femoral or radial artery, on delineating its location on the femoral/radial head X-ray. The standard of practice for safe common femoral artery direct puncture for all endovascular work is puncture under direct visualisation using ultrasound imaging [103]. This ensures that the exact anatomy is punctured and pre-empties any underlying problems such as anatomical variation or significant atherosclerotic disease which may impact haemostasis post-procedure.
The transradial approach is becoming increasingly popular and adopted at some centres while performing neuroendovascular procedures given the obvious limitations of the femoral approach [104]. The transfemoral approach is associated with an increased risk of vascular/bleeding complications, pseudoaneurysms and femoral nerve injury in comparison to the transradial cerebral interventions [105]. Moreover, the transfemoral approach has also been associated with increased complications in obese patients on antiplatelet or anticoagulant regimen [106]. With transradial approach, without the use of a closure device, neuroangiography procedures can be performed with better haemostatic control offering patients on anticoagulation therapy, or those with coagulopathies, a safe alternative to transfemoral access. In interventional cardiology, the demonstrated benefits of transradial approach compared to transfemoral access include lower rates of haematoma formation, bleeding and vascular complications, decreased morbidity and mortality, early ambulation, longer fluoroscopic time, less amount of contrast used and lower costs with coronary interventions including angiography and catheterization [105, 107]. However, overall access and fluoroscopy time were greater in transradial than the transfemoral approach [107]. A meta-analysis on comparison between right versus left radial artery approach for coronary angiography and angioplasty procedures demonstrated significantly lower fluoroscopic time and contrast use favouring left radial in comparison to right radial approach [108]. However, the reduced fluoroscopic time for access to coronaries is not applicable to cerebral DSA and thus is of limited use. Further studies on the efficacy and benefits of left radial access over right radial approach, and that of transradial approach over femoral, in performing neuroendovascular interventions are warranted.
Lidocaine used as a local anaesthetic may be risky in obese patients where the femoral artery may be difficult to locate and the risk of injection to the femoral nerve. However, lidocaine is not necessarily risk in obese patients for subcutaneous injection to reduce the pain of initial access. Benzodiazepines should accompany anaesthetics to allow minimal sedation so as not to mask potential neurological deterioration caused by the procedure. During the procedure, catheters ascending with the hydrophilic wire, guided by initial angiography images, can prevent iatrogenic vessel injury and dissection. Double flushing with saline and anticoagulant should be performed once the catheter separates from the wire to remove air bubbles and clots from within the catheter [2]. In neuro-DSA, a continuous saline drip via three-way tap can also be used (this avoids clot formation and blood-stagnation). It also aids embolisation by preventing coils from drying out and being stuck in the delivery catheters. The amount of contrast used varies based on the procedure, with patients with normal kidney function generally able to withstand 400–800 mL of contrast without side effects. Contrast can be injected by hand or machine after the patient is instructed to stay still and not breathe or swallow. The amount and rate of contrast injection vary depending on the vessel being injected. Standard rate and volume settings are provided in SI Table 3. While the aortic arch may not directly be relevant in cerebrovascular disease, aortograms may indicate a patient’s level of atherosclerotic disease—which is a useful prognostic and diagnostic indicator. Furthermore, this may also inform other barriers to vascular access including extensive arteriosclerosis/atherosclerosis changes, tortuosity, common vascular variants and local anatomy of the target vessel [109]. When intracranial aneurysms require a 4-vessel angiography, it should include bilateral ICA and vertebral arteries (VAs) injections without definite need to visualise ECAs; while Moyamoya diagnosis relies on a 6-vessel angiogram (bilateral ICA, bilateral ECA and bilateral vertebral arteries) [2]. Microcatheter and ECA injections may be utilised for cases where malformations in specific sites or external vessels are suspected. For example, dural AVFs require the assessment of the ECA and its branches, as well as the pharyngeal and occipital arteries. Furthermore, microcatheter injections within feeding arteries may help define the extent of AVFs in non-haemorrhagic settings [2]. However, with good-quality CTA and a definite source of SAH, 4-vessel angiography may not be always necessary.
X-ray images are typically taken at a rate of 2–5 frames per second (fps); however, this can be increased to 8–20 fps for the analysis of high-flow lesions including AVMs [1]. To minimise radiation exposure, the frame rate is often varied through arterial and venous phases, with the latter requiring a smaller number of fps. Assessment of the arterial versus venous phase is essential in diagnosing AVMs, wherein persistent abnormal opacification of veins during the arterial phase indicates shunting. The latter phase is useful in delineating the dominance of draining veins and planning treatment for these lesions. The venous phase can also reveal thromboembolism, which appears as persistent opacification of arteries during the expected venous phase. The total imaging sequence from the arterial-venous phase can take between 10 and 12 s. Biplanar angiography is the standard practice in DSA, as it enables the capture of orthogonal views with a single injection. This reduces radiation exposure and length of the procedure [2]. After DSA is complete, closure of the puncture site to prevent hematoma formation, through the use of, either, manual compression or an endovascular closure system (though relatively rare, leg elevation could be considered) and IV fluids should be implemented until the patient can walk [2].
The traditional method of closure or haemostasis has been manual compression over the arterial puncture spot. Systemic anticoagulation, advanced age, vascular disease and the use of an indwelling sheath over a prolonged period are all considered to increase the risk of complications [110]. While sufficient haemostasis is normally accomplished using this procedure, the time required to maintain haemostasis is comparatively long, requiring extended bed rest following compression which leads to increased patient pain, prolonged hospital stays and increased healthcare costs [111]. Over the last years, various percutaneous vascular closure devices (e.g. AngioSeal, VasoSeal and Perclose) have been used to facilitate prompt closure of the arteriotomy site, irrespective of the coagulation status, and early ambulation and discharge [112]. However, the evidence to support preferential use of closure devices over manual compression remains controversial.
With regards to post-procedural care, immobilisation of patient for 4–6 h, in supine position, is recommended. Possibility of haematomas and femoral pseudoaneurysm at puncture site, as a usual post-procedural complication, requires frequent monitoring.
Comparative role of DSA relative to CTA and MRA
DSA provides superior spatial resolution, with a resolution of 0.3 mm2 reported in the literature [113]. The resolutions achieved by MRA, and CTA are not comparable (Table 5), justifying the standing of DSA as the gold standard of cerebrovasculature visualisation. For the major intracranial arteries, with luminal diameters ranging from 1.7 to 4.3 mm, this introduces between 5 and 12% error [114]. The implication is that DSA provides higher diagnostic accuracy than its non-invasive counterparts and can more clearly rule out differential diagnoses. Diagnostically superior to common imaging modes, DSA can be combined with high-resolution CT maximum intensity projection (MIP) to provide detailed and accurate angiographic findings.
DSA also remains superior to these modalities (CTA, MRA or CTA-MIP) in temporal resolution. Time-resolved imaging, referred to as 4D imaging, can increase the frames captured by non-invasive imaging modalities. The frame rate and length 4D-MRA means that each image is a collection of information across multiple time-points, with some newer sequences offering up to 2 fps without loss of spatial resolution [115]. 4D-CTA is produced through computation of timing variant CTA and has higher diagnostic power than traditional CTA for several intracranial diseases including collaterals and thrombus estimation [116]. However, it is not standard practice given its limited availability and has been associated with high radiation exposure. A shortfall of current DSA technology is its inability to visualise or appraise vessel wall characteristics, including thickening, atherosclerotic plaque burden and inflammation; although this is a limitation shared by other neuroradiological modalities [1]. In AIS patients, atherosclerotic changes are highly prevalent, with a study of 339 fatal stroke autopsies finding that 62.2% of patients with fatal AIS had intracranial atherosclerotic plaques [117].
With both higher spatial and temporal resolution, DSA remains diagnostically superior to CTA and MRA for the assessment of cerebrovascular structures. Its intra-arterial nature also provides opportunities for endovascular therapies [1]. Despite these advantages, DSA is not considered first-line imaging due to several limitations. Higher training demands and consequently poorer accessibility limit the implementation of DSA as standard practice [2]. It is complicated pre-procedural preparation increases time to imaging as well as time to procedure when it is combined with therapeutics. The invasiveness and complications of the procedure also limit DSA’s application. The greatest contraindication to DSA in patients is poor renal function. Contrast-induced nephropathy (CIN) features an acute decline in renal function post-DSA. However, a recent study from 2002 comparing CIN in CTA and DSA across 80 patients found both had similar risks of a transient decrease in kidney function [118]. Moreover, use of newer contrasts, such as second-generation low/iso-osmolar contrast media (LOCM), intravenous administration route (relative to intra-arterial administration which may be linked to higher CIN), intravenous hydration with normal saline or sodium bicarbonate, and better pre-procedural preparation are renal protection strategies, to reduce CIN, which may be considered in those at risk [119].
Contrast reactions as severe as anaphylaxis and respiratory distress may be predisposed in patients with a history of allergy to contrast and other atopic conditions such as asthma. Invasive vascular access introduces the potential for severe complications. Complications of transfemoral access vary from haematomas and vascular spasm to pseudoaneurysm and retroperitoneal haemorrhage [104]. Older studies report local complications with rates as high as 4% [120], whereas recent studies, reported no cases of local infection and groin haematoma in only 1.55% of participants [121]. Arterial damage is generally not severe, with a requirement for intervention in approximately 0.2% cases [1]. Perhaps one of the most concerning complications is the production of catheter-associated emboli, which can induce cerebral ischaemia and neurological adverse effects [122]. It is estimated that DSA carries a rate of transient neurological complications of 1.3–2.6% [115]. This rate stands at 0.1–0.5% for permanent complications, and lower still in some studies [121]. The rate of neurological adverse effects varies and is a consequence of both patient- and operator-dependent factors [121].
Discussion and conclusion
DSA remains the gold standard in the diagnosis of AIS as well as other cerebrovascular conditions, such as intracranial aneurysms, Moyamoya disease, cerebral vasculitis and diseases, featuring abnormal arteriovenous anatomy. It provides detailed information regarding the location and degree of stenosis and occlusion in arteries and is used in conjunction with non-invasive modalities to investigate differentials and diagnose cerebrovascular disease [115]. Although it is not commonly a first-line investigation, it provides unmatched spatial resolution and anatomical detail [115]. The superior spatial and temporal resolution of DSA makes it the imaging of choice for guiding EVT procedures [2]. In EVT, DSA not only guides but also enhances the efficacy of treatment delivery by estimating the success of clot retrievals [83].
Besides diagnostic and therapeutic applications, evidence of DSA’s role in predicting clinical and angiographic outcomes continues to grow. For other intracranial diseases, imaging parameters can prove important in determining risks of aneurysm rupture, rates of complication and responses to medication [28, 43, 123]. In AIS, assessing occlusion site, collateral supply and revascularisation have all shown promise in providing prognostic information. Additional DSA findings including CAS may further inform our understanding of patient outcomes [100]. Only a limited number of studies exist on these areas of interest.
With a lack of adequate research regarding prognostic utility, a large prospective study of imaging markers and meta-analysis of the current uses of DSA in prognosis is desirable. Furthermore, association with long-term outcomes including recurrence rates should be investigated in AIS patients undertaking DSA imaging to better predict future adverse or ischaemic events.
In conclusion, DSA findings are not only diagnostically and therapeutically valuable but may also provide prognostic information in various cerebrovascular disease settings. Despite limitations to its use, it remains the gold standard in assessing cerebrovascular anatomy and guiding endovascular treatment. Further research is required if there is to be a routine application of this technique in surveillance or gauging clinical outcomes.
Data availability
The original contributions presented in the study are included in the article and Supplementary Material/s, further inquiries can be directed to the corresponding author.
References
Vranic JE, Mossa-Basha M (2020) Vessel based imaging techniques. Springer International Publishing, Cham
Harrigan MR, Deveikis JP (2009) Handbook of cerebrovascular disease and neurointerventional technique. Springer International Publishing, Cham
Watchmaker JM, Frederick BD, Fusco MR, Davis LT, Juttukonda MR, Lants SK et al (2019) Clinical use of cerebrovascular compliance imaging to evaluate revascularization in patients with moyamoya. Neurosurgery 84(1):261–271. https://doi.org/10.1093/neuros/nyx635
Hung SC, Liang ML, Lin CF, Lin CJ, Guo WY, Chang FC et al (2014) New grading of moyamoya disease using color-coded parametric quantitative digital subtraction angiography. J Chin Med Assoc 77(8):437–442. https://doi.org/10.1016/j.jcma.2014.05.007
Lee NJ, Chung MS, Jung SC, Kim HS, Choi CG, Kim SJ et al (2016) Comparison of high-resolution MR imaging and digital subtraction angiography for the characterization and diagnosis of intracranial artery disease. AJNR Am J Neuroradiol 37(12):2245–2250. https://doi.org/10.3174/ajnr.A4950
Lanas F, Seron P (2021) Facing the stroke burden worldwide. Lancet Glob Health 9(3):e235–e236. https://doi.org/10.1016/S2214-109X(20)30520-9
D’Souza S (2015) Aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol 27(3):222–240. https://doi.org/10.1097/ANA.0000000000000130
Gyano M, Gog I, Orias VI, Ruzsa Z, Nemes B, Csobay-Novak C et al (2019) Kinetic imaging in lower extremity arteriography: comparison to digital subtraction angiography. Radiology 290(1):246–253. https://doi.org/10.1148/radiol.2018172927
Ravindran AV, Killingsworth MC, Bhaskar S (2021) Cerebral collaterals in acute ischaemia: Implications for acute ischaemic stroke patients receiving reperfusion therapy. Eur J Neurosci 53(4):1238–1261. https://doi.org/10.1111/ejn.14955
Bhaskar S, Bivard A, Parsons M, Nilsson M, Attia JR, Stanwell P et al (2017) Delay of late-venous phase cortical vein filling in acute ischemic stroke patients: Associations with collateral status. J Cereb Blood Flow Metab 37(2):671–682. https://doi.org/10.1177/0271678x16637611
Frolich AM, Psychogios MN, Klotz E, Schramm R, Knauth M, Schramm P (2012) Antegrade flow across incomplete vessel occlusions can be distinguished from retrograde collateral flow using 4-dimensional computed tomographic angiography. Stroke 43(11):2974–2979. https://doi.org/10.1161/STROKEAHA.112.668889
Lee SJ, Liu B, Rane N, Mitchell P, Dowling R, Yan B (2021) Correlation between CT angiography and digital subtraction angiography in acute ischemic strokes. Clin Neurol Neurosurg 200:106399. https://doi.org/10.1016/j.clineuro.2020.106399
Katyal A, Bhaskar S (2021) CTP-guided reperfusion therapy in acute ischemic stroke: a meta-analysis. Acta Neurol Scand 143(4):355–366. https://doi.org/10.1111/ane.13374
Psychogios M-N, Maier IL, Tsogkas I, Hesse AC, Brehm A, Behme D et al (2019) One-stop management of 230 consecutive acute stroke patients: report of procedural times and clinical outcome. J Clin Med 8(12):2185. https://doi.org/10.3390/jcm8122185
Saba L, Sanfilippo R, Montisci R, Mallarini G (2010) Assessment of intracranial arterial stenosis with multidetector row CT angiography: a postprocessing techniques comparison. AJNR Am J Neuroradiol 31(5):874–879. https://doi.org/10.3174/ajnr.A1976
Malhotra K, Liebeskind DS (2020) Collaterals in ischemic stroke. Brain Hemorrhages 1(1):6–12. https://doi.org/10.1016/j.hest.2019.12.003
Muehlen I, Kloska SP, Golitz P, Holter P, Breuer L, Ditt H et al (2019) Noninvasive collateral flow velocity imaging in acute ischemic stroke: intraindividual comparison of 4D-CT Angiography with digital subtraction angiography. Rofo 191(9):827–835. https://doi.org/10.1055/a-0825-6660
Chowdhury SZ, Baskar PS, Bhaskar S (2021) Effect of prehospital workflow optimization on treatment delays and clinical outcomes in acute ischemic stroke: a systematic review and meta-analysis. Acad Emerg Med. https://doi.org/10.1111/acem.14204
Santana Baskar P, Cordato D, Wardman D, Bhaskar S (2021) In-hospital acute stroke workflow in acute stroke—systems-based approaches. Acta Neurol Scand 143(2):111–120. https://doi.org/10.1111/ane.13343
Jansen IGH, Berkhemer OA, Yoo AJ, Vos JA, Lycklama à Nijeholt GJ, Sprengers MES et al (2016) Comparison of CTA- and DSA-based collateral flow assessment in patients with anterior circulation stroke. Am J Neuroradiol. https://doi.org/10.3174/ajnr.A4878
Menon BK, d’Esterre CD, Qazi EM, Almekhlafi M, Hahn L, Demchuk AM et al (2015) Multiphase CT angiography: a new tool for the imaging triage of patients with acute ischemic stroke. Radiology 275(2):510–520. https://doi.org/10.1148/radiol.15142256
Kim JS (2016) Moyamoya Disease: epidemiology, clinical features, and diagnosis. J Stroke 18(1):2–11. https://doi.org/10.5853/jos.2015.01627
Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis, Health Labour Sciences Research Grant for Research on Measures for Intractable Diseases (2012) Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol Med Chir 52(5):245–266. https://doi.org/10.2176/nmc.52.245
Rosi A, Riordan CP, Smith ER, Scott RM, Orbach DB (2019) Clinical status and evolution in moyamoya: which angiographic findings correlate? Brain Commun. https://doi.org/10.1093/braincomms/fcz029
Kim JH, Kwon TH, Kim JH, Chong K, Yoon W (2018) Intracranial aneurysms in adult moyamoya disease. World Neurosurg 109:e175–e182. https://doi.org/10.1016/j.wneu.2017.09.127
Matsushima Y, Inaba Y (1984) Moyamoya disease in children and its surgical treatment. Introduction of a new surgical procedure and its follow-up angiograms. Childs Brain 11(3):155–170. https://doi.org/10.1159/000120172
Berlit P (2010) Diagnosis and treatment of cerebral vasculitis. Ther Adv Neurol Disord 3(1):29–42. https://doi.org/10.1177/1756285609347123
Abdel Razek AA, Alvarez H, Bagg S, Refaat S, Castillo M (2014) Imaging spectrum of CNS vasculitis. Radiographics 34(4):873–894. https://doi.org/10.1148/rg.344135028
Spiotta AM, Turner RD, Chaudry MI, Turk AS (2019) Management of cerebrovascular disorders: a comprehensive, multidisciplinary approach. Springer, Cham
Chen H, Xu Z, Yuan Y (2020) Posterior reversible encephalopathy syndrome and reversible cerebral vasoconstriction syndrome associated spinal subdural hematoma: a case report. Medicine. https://doi.org/10.1097/MD.0000000000021522
Singhal AB, Topcuoglu MA, Fok JW, Kursun O, Nogueira RG, Frosch MP et al (2016) Reversible cerebral vasoconstriction syndromes and primary angiitis of the central nervous system: clinical, imaging, and angiographic comparison. Ann Neurol 79(6):882–894. https://doi.org/10.1002/ana.24652
Pluta RM, Hansen-Schwartz J, Dreier J, Vajkoczy P, Macdonald RL, Nishizawa S et al (2009) Cerebral vasospasm following subarachnoid hemorrhage: time for a new world of thought. Neurol Res 31(2):151–158. https://doi.org/10.1179/174313209X393564
Vergouwen MD, Ilodigwe D, Macdonald RL (2011) Cerebral infarction after subarachnoid hemorrhage contributes to poor outcome by vasospasm-dependent and -independent effects. Stroke 42(4):924–929. https://doi.org/10.1161/STROKEAHA.110.597914
Arias EJ, Vajapey S, Reynolds MR, Chicoine MR, Rich KM, Dacey RG Jr et al (2015) Utility of screening for cerebral vasospasm using digital subtraction angiography. Stroke 46(11):3137–3141. https://doi.org/10.1161/STROKEAHA.115.010081
Samagh N, Bhagat H, Jangra K (2019) Monitoring cerebral vasospasm: How much can we rely on transcranial Doppler. J Anaesthesiol Clin Pharmacol 35(1):12–18. https://doi.org/10.4103/joacp.JOACP_192_17
Yoon DY, Choi CS, Kim KH, Cho BM (2006) Multidetector-row CT angiography of cerebral vasospasm after aneurysmal subarachnoid hemorrhage: comparison of volume-rendered images and digital subtraction angiography. AJNR Am J Neuroradiol 27(2):370–377
Joo SP, Kim TS, Kim YS, Moon KS, Lee JK, Kim JH et al (2006) Clinical utility of multislice computed tomographic angiography for detection of cerebral vasospasm in acute subarachnoid hemorrhage. Minim Invasive Neurosurg 49(5):286–290. https://doi.org/10.1055/s-2006-954826
Otite F, Mink S, Tan CO, Puri A, Zamani AA, Mehregan A et al (2014) Impaired cerebral autoregulation is associated with vasospasm and delayed cerebral ischemia in subarachnoid hemorrhage. Stroke 45(3):677–682. https://doi.org/10.1161/STROKEAHA.113.002630
Budohoski KP, Czosnyka M, Smielewski P, Varsos GV, Kasprowicz M, Brady KM et al (2016) Monitoring cerebral autoregulation after subarachnoid hemorrhage. Acta Neurochir Suppl 122:199–203. https://doi.org/10.1007/978-3-319-22533-3_40
Carrera E, Schmidt JM, Oddo M, Fernandez L, Claassen J, Seder D et al (2009) Transcranial Doppler for predicting delayed cerebral ischemia after subarachnoid hemorrhage. Neurosurgery 65(2):316–323. https://doi.org/10.1227/01.Neu.0000349209.69973.88 (Discussion 23-4)
Washington CW, Zipfel GJ, The Participants in the International Multi-disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage (2011) Detection and monitoring of vasospasm and delayed cerebral ischemia: a review and assessment of the literature. Neurocrit Care. https://doi.org/10.1007/s12028-011-9594-8
Stafa A, Leonardi M (2008) Role of neuroradiology in evaluating cerebral aneurysms. Interv Neuroradiol 14(Suppl 1):23–37. https://doi.org/10.1177/15910199080140S106
Mocco J, Brown RD Jr, Torner JC, Capuano AW, Fargen KM, Raghavan ML et al (2018) Aneurysm morphology and prediction of rupture: an international study of unruptured intracranial aneurysms analysis. Neurosurgery 82(4):491–496. https://doi.org/10.1093/neuros/nyx226
Zanaty M, Chalouhi N, Tjoumakaris SI, Fernando Gonzalez L, Rosenwasser RH, Jabbour PM (2014) Aneurysm geometry in predicting the risk of rupture. A review of the literature. Neurol Res 36(4):308–313. https://doi.org/10.1179/1743132814Y.0000000327
Kashiwazaki D, Kuroda S (2013) Size ratio can highly predict rupture risk in intracranial small (<5 mm) aneurysms. Stroke 44(8):2169–2173. https://doi.org/10.1161/strokeaha.113.001138
De Vries J, Boogaarts HD, Sørensen L, Holtmannspoetter M, Benndorf G, Turowski B et al (2021) eCLIPs bifurcation remodeling system for treatment of wide neck bifurcation aneurysms with extremely low dome-to-neck and aspect ratios: a multicenter experience. J NeuroIntervent Surg 13(5):438. https://doi.org/10.1136/neurintsurg-2020-016354
Hacein-Bey L, Provenzale JM (2011) Current imaging assessment and treatment of intracranial aneurysms. AJR Am J Roentgenol 196(1):32–44. https://doi.org/10.2214/AJR.10.5329
Ciescinski J, Serafin Z, Strzesniewski P, Lasek W, Beuth W (2012) DSA volumetric 3D reconstructions of intracranial aneurysms: a pictorial essay. Pol J Radiol 77(2):47–53. https://doi.org/10.12659/pjr.882970
van Rooij WJ, Sprengers ME, de Gast AN, Peluso JP, Sluzewski M (2008) 3D rotational angiography: the new gold standard in the detection of additional intracranial aneurysms. AJNR Am J Neuroradiol 29(5):976–979. https://doi.org/10.3174/ajnr.A0964
Badea R, Olaru O, Ribigan A, Ciobotaru A, Dorobat B (2019) Unruptured giant intracerebral aneurysms: serious trouble requiring serious treatment—case report and literature review. Maedica (Bucur) 14(4):422–427. https://doi.org/10.26574/maedica.2019.14.4.422
Sorimachi T, Suzuki K, Sasaki O, Koike T, Fujii Y (2010) Three-dimensional digital subtraction angiography in evaluation of vertebro-basilar artery dissections: comparison with 2D DSA. J Neuroimaging 20(3):228–233. https://doi.org/10.1111/j.1552-6569.2009.00445.x
Mehdi E, Aralasmak A, Toprak H, Yildiz S, Kurtcan S, Kolukisa M et al (2018) Craniocervical dissections: radiologic findings, pitfalls, mimicking diseases: a pictorial review. Curr Med Imaging Rev 14(2):207–222. https://doi.org/10.2174/1573405613666170403102235
Hermes J, Lakshmanan R, Watkins L, Davagnanam I (2017) Teaching neuroimages: intracranial arterial dissection. Neurology 88(12):e111–e112. https://doi.org/10.1212/WNL.0000000000003733
Rodallec MH, Marteau V, Gerber S, Desmottes L, Zins M (2008) Craniocervical arterial dissection: spectrum of imaging findings and differential diagnosis. Radiographics 28(6):1711–1728. https://doi.org/10.1148/rg.286085512
Onofrj V, Cortes M, Tampieri D (2016) The insidious appearance of the dissecting aneurysm: imaging findings and related pathophysiology. A report of two cases. Interv Neuroradiol 22(6):638–642. https://doi.org/10.1177/1591019916659265
Kanoto M, Hosoya T (2016) Diagnosis of intracranial artery dissection. Neurol Med Chir (Tokyo) 56(9):524–533. https://doi.org/10.2176/nmc.ra.2015-0294
Debette S, Compter A, Labeyrie MA, Uyttenboogaart M, Metso TM, Majersik JJ et al (2015) Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection. Lancet Neurol 14(6):640–654. https://doi.org/10.1016/S1474-4422(15)00009-5
Delfaut EM, Beltran J, Johnson G, Rousseau J, Marchandise X, Cotten A (1999) Fat suppression in MR imaging: techniques and pitfalls. Radiographics 19(2):373–382. https://doi.org/10.1148/radiographics.19.2.g99mr03373
Brzozowski K, Frankowska E, Piasecki P, Ziecina P, Zukowski P, Boguslawska-Walecka R (2011) The use of routine imaging data in diagnosis of cerebral pseudoaneurysm prior to angiography. Eur J Radiol 80(3):e401–e409. https://doi.org/10.1016/j.ejrad.2010.12.019
Sarioglu O, Capar AE, Belet U (2019) Interventional treatment options in pseudoaneurysms: different techniques in different localizations. Pol J Radiol 84:e319–e327. https://doi.org/10.5114/pjr.2019.88021
Ajiboye N, Chalouhi N, Starke RM, Zanaty M, Bell R (2014) Cerebral arteriovenous malformations: evaluation and management. Sci World J 2014:649036. https://doi.org/10.1155/2014/649036
Tanweer O, Harrison G, Rozman P, Riina HA (2019) Arteriovenous malformations: surgical indications and technique. In: Spiotta AM, Turner RD, Chaudry MI, Turk AS (eds) Management of cerebrovascular disorders. Springer, Cham, pp 291–308
Ruedinger KL, Schafer S, Speidel MA, Strother CM (2021) 4D-DSA: development and current neurovascular applications. AJNR Am J Neuroradiol 42(2):214–220. https://doi.org/10.3174/ajnr.A6860
Spetzler RF, Martin NA (1986) A proposed grading system for arteriovenous malformations. J Neurosurg 65(4):476–483. https://doi.org/10.3171/jns.1986.65.4.0476
Warren DJ, Hoggard N, Walton L, Radatz MW, Kemeny AA, Forster DM et al (2007) Cerebral arteriovenous malformations: comparison of novel magnetic resonance angiographic techniques and conventional catheter angiography. Neurosurgery 61(1 Suppl):187–196. https://doi.org/10.1227/01.neu.0000279215.07763.a1 (Discussion 96-7)
Kumar N (2010) Neuroimaging in superficial siderosis: an in-depth look. AJNR Am J Neuroradiol 31(1):5–14. https://doi.org/10.3174/ajnr.A1628
Illies T, Forkert ND, Ries T, Regelsberger J, Fiehler J (2013) Classification of cerebral arteriovenous malformations and intranidal flow patterns by color-encoded 4D-hybrid-MRA. AJNR Am J Neuroradiol 34(1):46–53. https://doi.org/10.3174/ajnr.A3204
Rammos SK, Gardenghi B, Bortolotti C, Cloft HJ, Lanzino G (2016) Aneurysms associated with brain arteriovenous malformations. AJNR Am J Neuroradiol 37(11):1966–1971. https://doi.org/10.3174/ajnr.A4869
Lawton MT, Kim H, McCulloch CE, Mikhak B, Young WL (2010) A supplementary grading scale for selecting patients with brain arteriovenous malformations for surgery. Neurosurgery 66(4):702–713. https://doi.org/10.1227/01.Neu.0000367555.16733.E1 (Discussion 13)
Farb RI, Agid R, Willinsky RA, Johnstone DM, Terbrugge KG (2009) Cranial dural arteriovenous fistula: diagnosis and classification with time-resolved MR angiography at 3T. AJNR Am J Neuroradiol 30(8):1546–1551. https://doi.org/10.3174/ajnr.A1646
Gomez J, Amin AG, Gregg L, Gailloud P (2012) Classification schemes of cranial dural arteriovenous fistulas. Neurosurg Clin N Am 23(1):55–62. https://doi.org/10.1016/j.nec.2011.09.003
Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A et al (1995) Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology 194(3):671–680. https://doi.org/10.1148/radiology.194.3.7862961
Borden JA, Wu JK, Shucart WA (1995) A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg 82(2):166–179. https://doi.org/10.3171/jns.1995.82.2.0166
Harbaugh RE, Shaffrey C, Couldwell WT, Berger MS (2015) Neurosurgery knowledge update. Thieme, New York
Rafailidis V, Li X, Chryssogonidis I, Rengier F, Rajiah P, Wieker CM et al (2018) Multimodality imaging and endovascular treatment options of subclavian steal syndrome. Can Assoc Radiol J 69(4):493–507. https://doi.org/10.1016/j.carj.2018.08.003
Potter BJ, Pinto DS (2014) Subclavian steal syndrome. Circulation 129(22):2320–2323. https://doi.org/10.1161/CIRCULATIONAHA.113.006653
Zaidat OO, Lazzaro MA, Liebeskind DS, Janjua N, Wechsler L, Nogueira RG et al (2012) Revascularization grading in endovascular acute ischemic stroke therapy. Neurology 79(13 Suppl 1):S110–S116. https://doi.org/10.1212/WNL.0b013e3182695916
Dargazanli C, Fahed R, Blanc R, Gory B, Labreuche J, Duhamel A et al (2018) Modified thrombolysis in cerebral infarction 2C/thrombolysis in cerebral infarction 3 reperfusion should be the aim of mechanical thrombectomy: insights from the ASTER trial (contact aspiration versus stent retriever for successful revascularization). Stroke 49(5):1189–1196. https://doi.org/10.1161/STROKEAHA.118.020700
Tsai JP, Albers GW (2015) Reperfusion versus recanalization: the winner is. Stroke 46(6):1433–1434. https://doi.org/10.1161/STROKEAHA.115.009268
Kosior JC, Buck B, Wannamaker R, Kate M, Liapounova NA, Rempel JL et al (2019) Exploring reperfusion following endovascular thrombectomy. Stroke 50(9):2389–2395. https://doi.org/10.1161/STROKEAHA.119.025537
Kannath SK, Rajan JE, Sylaja PN, Sarma PS, Sukumaran S, Sreedharan SE et al (2018) Dwell time of stentriever influences complete revascularization and first-pass TICI 3 revascularization in acute large vessel occlusive stroke. World Neurosurg 110:169–173. https://doi.org/10.1016/j.wneu.2017.10.155
Simon S, Langan S, Cooke J (2016) Increasing efficacy of thrombectomy by using digital subtraction angiography to confirm stent retriever clot integration. Cureus 8(4):e559. https://doi.org/10.7759/cureus.559
Kabbasch C, Mpotsaris A, Chang DH, Hiss S, Dorn F, Behme D et al (2016) Mechanical thrombectomy with the Trevo ProVue device in ischemic stroke patients: does improved visibility translate into a clinical benefit? J Neurointerv Surg 8(8):778–782. https://doi.org/10.1136/neurintsurg-2015-011861
Francoeur CL, Mayer SA (2016) Management of delayed cerebral ischemia after subarachnoid hemorrhage. Crit Care 20(1):277. https://doi.org/10.1186/s13054-016-1447-6
Molyneux A (2002) International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 360(9342):1267–1274. https://doi.org/10.1016/S0140-6736(02)11314-6
Molyneux AJ, Kerr RS, Birks J, Ramzi N, Yarnold J, Sneade M et al (2009) Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol 8(5):427–433. https://doi.org/10.1016/s1474-4422(09)70080-8
Molyneux AJ, Birks J, Clarke A, Sneade M, Kerr RS (2015) The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT). Lancet 385(9969):691–697. https://doi.org/10.1016/s0140-6736(14)60975-2
Thompson BG, Brown RD Jr, Amin-Hanjani S, Broderick JP, Cockroft KM, Connolly ES Jr et al (2015) Guidelines for the management of patients with unruptured intracranial aneurysms: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 46(8):2368–2400. https://doi.org/10.1161/STR.0000000000000070
Acker G, Schlinkmann N, Piper SK, Onken J, Vajkoczy P, Picht T (2018) Stereoscopic versus monoscopic viewing of aneurysms: experience of a single institution with a novel stereoscopic viewing system. World Neurosurg 119:e491–e501. https://doi.org/10.1016/j.wneu.2018.07.189
Soize S, Gawlitza M, Raoult H, Pierot L (2016) Imaging follow-up of intracranial aneurysms treated by endovascular means: why, when, and how? Stroke 47(5):1407–1412. https://doi.org/10.1161/STROKEAHA.115.011414
Stapleton CJ, Torok CM, Rabinov JD, Walcott BP, Mascitelli JR, Leslie-Mazwi TM et al (2016) Validation of the Modified Raymond-Roy classification for intracranial aneurysms treated with coil embolization. J NeuroInterv Surg 8(9):927. https://doi.org/10.1136/neurintsurg-2015-012035
Thompson BG, Brown RD, Amin-Hanjani S, Broderick JP, Cockroft KM, Connolly ES et al (2015) Guidelines for the management of patients with unruptured intracranial aneurysms. Stroke 46(8):2368–2400. https://doi.org/10.1161/STR.0000000000000070
Angle JF, Nida BA, Matsumoto AH (2014) Endovascular treatment of large vessel arteritis. Tech Vasc Interv Radiol 17(4):252–257. https://doi.org/10.1053/j.tvir.2014.11.006
Hong JM, Lee SE, Lee SJ, Lee JS, Demchuk AM (2017) Distinctive patterns on CT angiography characterize acute internal carotid artery occlusion subtypes. Medicine (Baltimore) 96(5):e5722. https://doi.org/10.1097/MD.0000000000005722
Tian H, Parsons MW, Levi CR, Lin L, Aviv RI, Spratt NJ et al (2019) Influence of occlusion site and baseline ischemic core on outcome in patients with ischemic stroke. Neurology 92(23):e2626–e2643. https://doi.org/10.1212/WNL.0000000000007553
de Jong S, Lodder J, Luijckx GJ (2004) Is cerebral angiography redundant in undetermined cause of stroke in patients below 50 years when the stroke is lacunar? J Neurol Sci 222(1–2):83–85. https://doi.org/10.1016/j.jns.2004.04.007
Jansen IGH, Berkhemer OA, Yoo AJ, Vos JA, Lycklama ANGJ, Sprengers MES et al (2016) Comparison of CTA- and DSA-based collateral flow assessment in patients with anterior circulation stroke. AJNR Am J Neuroradiol 37(11):2037–2042. https://doi.org/10.3174/ajnr.A4878
Singer OC, Berkefeld J, Nolte CH, Bohner G, Haring HP, Trenkler J et al (2015) Mechanical recanalization in basilar artery occlusion: the ENDOSTROKE study. Ann Neurol 77(3):415–424. https://doi.org/10.1002/ana.24336
Liu F, Chen C, Hong L, Shen H, Cao W, Dong Q et al (2020) Lenticulostriate arteries appearance before thrombectomy predicts good outcome in acute middle cerebral artery occlusion. BMC Neurol 20(1):139. https://doi.org/10.1186/s12883-020-01716-1
van Velzen TJ, Kuhrij LS, Westendorp WF, van de Beek D, Nederkoorn PJ (2021) Prevalence, predictors and outcome of carotid stenosis: a sub study in the preventive antibiotics in stroke study (PASS). BMC Neurol 21(1):20. https://doi.org/10.1186/s12883-020-02032-4
Silvennoinen HM, Ikonen S, Soinne L, Railo M, Valanne L (2007) CT angiographic analysis of carotid artery stenosis: comparison of manual assessment, semiautomatic vessel analysis, and digital subtraction angiography. AJNR Am J Neuroradiol 28(1):97–103
Kwon OK, Oh CW, Park H, Bang JS, Bae HJ, Han MK et al (2011) Is fasting necessary for elective cerebral angiography? AJNR Am J Neuroradiol 32(5):908–910. https://doi.org/10.3174/ajnr.A2408
Pooley RA, McKinney JM, Miller DA (2001) The AAPM/RSNA physics tutorial for residents. Radiographics 21(2):521–534. https://doi.org/10.1148/radiographics.21.2.g01mr20521
Ge B, Wei Y (2020) Comparison of transfemoral cerebral angiography and transradial cerebral angiography following a shift in practice during four years at a single center in China. Med Sci Monit 26:e921631. https://doi.org/10.12659/MSM.921631
Ghani MR, Busa V, Dardeir A, Marudhai S, Patel M, Abdelmoneim YM et al (2020) Mechanical thrombectomy in patients with acute ischemic stroke: a comparison of transradial versus transfemoral cerebral angiography. Cureus. https://doi.org/10.7759/cureus.10919
Chen SH, Snelling BM, Sur S, Shah SS, McCarthy DJ, Luther E et al (2019) Transradial versus transfemoral access for anterior circulation mechanical thrombectomy: comparison of technical and clinical outcomes. J Neurointervent Surg 11(9):874–878. https://doi.org/10.1136/neurintsurg-2018-014485
Bhat FA, Changal KH, Raina H, Tramboo NA, Rather HA (2017) Transradial versus transfemoral approach for coronary angiography and angioplasty—a prospective, randomized comparison. BMC Cardiovasc Disord 17(1):23. https://doi.org/10.1186/s12872-016-0457-2
Shah R, Patel D, Abbate A, Cowley M, Jovin I (2016) Comparison of transradial coronary procedures via right radial versus left radial artery approach: a meta-analysis. Cathet Cardiovasc Intervent 88(7):1027–1033. https://doi.org/10.1002/ccd.26519
Han H-C (2012) Twisted blood vessels: symptoms, etiology and biomechanical mechanisms. J Vasc Res 49(3):185–197. https://doi.org/10.1159/000335123
Applegate RJ, Grabarczyk MA, Little WC, Craven T, Walkup M, Kahl FR et al (2002) Vascular closure devices in patients treated with anticoagulation and IIb/IIIa receptor inhibitors during percutaneous revascularization. J Am Coll Cardiol 40(1):78–83. https://doi.org/10.1016/s0735-1097(02)01924-1
Geyik S, Yavuz K, Akgoz A, Koc O, Peynircioglu B, Cil B et al (2007) The safety and efficacy of the Angio-Seal closure device in diagnostic and interventional neuroangiography setting: a single-center experience with 1443 closures. Neuroradiology 49(9):739–746. https://doi.org/10.1007/s00234-007-0249-6
Beyer-Enke SA, Söldner J, Zeitler E (1996) Immediate sealing of arterial puncture site following femoropopliteal angioplasty: a prospective randomized trial. Cardiovasc Intervent Radiol 19(6):406–410. https://doi.org/10.1007/bf02577628
Lin A, Rawal S, Agid R, Mandell DM (2018) Cerebrovascular imaging: which test is best? Neurosurgery 83(1):5–18. https://doi.org/10.1093/neuros/nyx325
Mujagić S (2013) The inner diameter of arteries of the circle of Willis regarding gender and age on magnetic resonance angiography. Acta Med Salin 42:6–12
Guimaraes M, Schönholz C, Uflacker R, Huda W (2009) Comprehensive vascular and endovascular surgery. Mosby, Philadelphia
Kortman HG, Smit EJ, Oei MT, Manniesing R, Prokop M, Meijer FJ (2015) 4D-CTA in neurovascular disease: a review. AJNR Am J Neuroradiol 36(6):1026–1033. https://doi.org/10.3174/ajnr.A4162
Mazighi M, Labreuche J, Gongora-Rivera F, Duyckaerts C, Hauw JJ, Amarenco P (2008) Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke 39(4):1142–1147. https://doi.org/10.1161/STROKEAHA.107.496513
Lufft V, Hoogestraat-Lufft L, Fels LM, Egbeyong-Baiyee D, Tusch G, Galanski M et al (2002) Contrast media nephropathy: intravenous CT angiography versus intraarterial digital subtraction angiography in renal artery stenosis: a prospective randomized trial. Am J Kidney Dis 40(2):236–242. https://doi.org/10.1053/ajkd.2002.34501
Gupta RK, Bang TJ (2010) Prevention of contrast-induced nephropathy (CIN) in interventional radiology practice. Semin Intervent Radiol 27(4):348–359. https://doi.org/10.1055/s-0030-1267860
Ball JB Jr, Lukin RR, Tomsick TA, Chambers AA (1985) Complications of intravenous digital subtraction angiography. Arch Neurol 42(10):969–972. https://doi.org/10.1001/archneur.1985.04060090051013
Shen J, Karki M, Jiang T, Zhao B (2018) Complications associated with diagnostic cerebral angiography: a retrospective analysis of 644 consecutive cerebral angiographic cases. Neurol India 66(4):1154–1158. https://doi.org/10.4103/0028-3886.237018
Hamon M, Gomes S, Oppenheim C, Morello R, Sabatier R, Lognone T et al (2006) Cerebral microembolism during cardiac catheterization and risk of acute brain injury: a prospective diffusion-weighted magnetic resonance imaging study. Stroke 37(8):2035–2038. https://doi.org/10.1161/01.STR.0000231641.55843.49
Liu ZW, Han C, Zhao F, Qiao PG, Wang H, Bao XY et al (2019) Collateral circulation in moyamoya disease: a new grading system. Stroke 50(10):2708–2715. https://doi.org/10.1161/STROKEAHA.119.024487
Rutledge WC, Ko NU, Lawton MT, Kim H (2014) Hemorrhage rates and risk factors in the natural history course of brain arteriovenous malformations. Transl Stroke Res 5(5):538–542. https://doi.org/10.1007/s12975-014-0351-0
Liu L, Ding J, Leng X, Pu Y, Huang LA, Xu A et al (2018) Guidelines for evaluation and management of cerebral collateral circulation in ischaemic stroke 2017. Stroke Vasc Neurol 3(3):117–130. https://doi.org/10.1136/svn-2017-000135
Brown RD Jr, Broderick JP (2014) Unruptured intracranial aneurysms: epidemiology, natural history, management options, and familial screening. Lancet Neurol 13(4):393–404. https://doi.org/10.1016/S1474-4422(14)70015-8
Hoh BL, Sistrom CL, Firment CS, Fautheree GL, Velat GJ, Whiting JH et al (2007) Bottleneck factor and height-width ratio: association with ruptured aneurysms in patients with multiple cerebral aneurysms. Neurosurgery 61(4):716–722. https://doi.org/10.1227/01.NEU.0000298899.77097.BF
Hirotsune N, Meguro T, Kawada S, Nakashima H, Ohmoto T (1997) Long-term follow-up study of patients with unilateral moyamoya disease. Clin Neurol Neurosurg 99(Suppl 2):S178–S181. https://doi.org/10.1016/s0303-8467(97)00043-7
Acknowledgements
We would like to acknowledge the support of the administrative staff and our NSW wide partnering clinicians and investigators.
Funding
Funding for the NSW Brain Clot Bank (Chief Investigator: Dr Bhaskar) from the NSW Ministry of Health (2019–2022) is acknowledged. The funding body has no role in the study design, data collection, analysis, interpretation of findings and manuscript preparation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the affiliated/funding organisation/s.
Author information
Authors and Affiliations
Contributions
SMMB conceived the study, contributed to the planning, draft and revision of the manuscript, supervision of the student. SMMB encouraged SS to investigate and supervised the findings of this work. SS and SMMB wrote the first draft of this paper. All the authors contributed to the revision of the manuscript. All the authors approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All analyses were based on previously published studies; thus, no ethical approval and patient consent are required.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shaban, S., Huasen, B., Haridas, A. et al. Digital subtraction angiography in cerebrovascular disease: current practice and perspectives on diagnosis, acute treatment and prognosis. Acta Neurol Belg 122, 763–780 (2022). https://doi.org/10.1007/s13760-021-01805-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-021-01805-z