Abstract
In South America, the resident pupal parasitoid Trichopria anastrephae Costa Lima (Hymenoptera: Diapriidae) is a potential biological control agent of the pest Drosophila suzukii Matsumura (Diptera: Drosophilidae). In the present study, we (1) examined the behavior of T. anastrephae towards different host (D. suzukii) and host-substrate (strawberry) cues in choice and non-choice bioassays in laboratory, and (2) examined the density-dependent parasitism of T. anastrephae in D. suzukii-infested strawberries in a greenhouse. When given a choice, female parasitoids walked longer over chambers with fruits infested with eggs, larvae, or pupae of D. suzukii, when compared to healthy uninfested strawberries, and over overripe fruits when compared to unripe or ripe fruits. In the greenhouse assay, we observed an increase in parasitism and a decrease in the number of D. suzukii emerging per fruit with an increase in the number of parasitoids released. Our results allow a better understanding of the behavior and parasitism of T. anastrephae in D. suzukii-infested strawberries and provide useful data for potential biological control programs using this parasitoid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The spotted wing drosophila, Drosophila suzukii (Matsumura) (Diptera: Drosophilidae), is an invasive pest species, native to Southeast Asia, that in the last years expanded its range and, now, is spread in Europe, North and South America, and Africa (Bolda et al. 2010; Calabria et al. 2012; Cini et al. 2012; Deprá et al. 2014; dos Santos et al. 2017; Boughdad et al. 2021). After its range expansion, D. suzukii rapidly became a major pest in small fruit production, causing an annual economic impact estimated in hundreds of millions of USD, due to yield losses and increased production costs (Goodhue et al. 2011; Knapp et al. 2021). The potential to cause high damage is mainly due to the female’s sclerotized and serrated ovipositor that enables them to lay eggs in ripe and ripening healthy fruits (Lee et al. 2011), short life cycle and high fecundity (Emiljanowicz et al. 2014; Tochen et al. 2014), and its large host range (Lee et al. 2015; Poyet et al. 2015; Kenis et al. 2016). Currently, D. suzukii has been controlled by spraying insecticides successively, which can cause insecticide resistance and impacts in non-target species (Desneux et al. 2007; Gress and Zalom 2018). Thus, research effort has been focused on finding alternative approaches, including biological control (Schetelig et al. 2018; Lee et al. 2019; Wang et al. 2020).
Biological controls, such as predators, parasitoids, nematodes, bacteria, fungi, and viruses, were studied and demonstrated potential to be used in Integrated Pest Management programs against D. suzukii (Garcia et al. 2017; Schetelig et al. 2018; Lee et al. 2019; Garcia 2020). Biological control agents could reduce D. suzukii populations in both crop and non-crop areas promoting a sustained suppression throughout the seasons (Lee et al. 2019). Although resident larval parasitoids associated with D. suzukii were found in almost all continents where the pest is present (Rossi Stacconi et al. 2013; Miller et al. 2015; Wollmann et al. 2016; Knoll et al. 2017; Matsuura et al. 2018), few species can overcome D. suzukii immune responses. Due to its high hemocyte load, D. suzukii can encapsulate eggs from different species of larval parasitoids (Kacsoh and Schlenke 2012). Thus, the most promising parasitoids that can successfully reproduce on D. suzukii are pupal parasitoids.
To date, only a few species of parasitoids have been found associated with D. suzukii in South America (Wollmann et al. 2016; De la Vega et al. 2021; Garcia et al. 2022), including Trichopria anastrephae Costa Lima (Hymenoptera: Diapriidae), a resident pupal parasitoid (Cruz et al. 2011). Since its association with D. suzukii, several aspects of T. anastrephae parasitism were studied, including parasitism ability in different laboratory conditions (Krüger et al. 2019; Vieira et al. 2019, 2020), competition with another parasitoid (Oliveira et al. 2021), and toxicological effects of insecticides and essential oils (Schlesener et al. 2019). Results from all these studies demonstrated the potential of T. anastrephae as a biological control agent of D. suzukii and encourage further studies.
Although most studies were performed using naked pupae only, a recent study showed that T. anastrephae can discriminate among D. suzukii-infested and non-infested blueberry, indicating that this parasitoid can use chemical cues from the interaction among host (D. suzukii) and host substrate (fruit) to locate the pest (De La Vega et al. 2021). Similarly, Trichopria drosophilae Perkins (Hymenoptera: Diapriidae), a pupal parasitoid found parasitizing D. suzukii in North and Central America and Europe, is also able to discriminate among non-crop fruits infested and non-infested by D. suzukii (Wolf et al. 2020). Furthermore, semi-field and field experiments showed that released T. drosophilae can locate and parasitize D. suzukii in blueberry, cherry, and raspberry (Rossi Stacconi et al. 2018, 2019; Gonzaléz-Cabrera et al. 2019).
In Brazil, D. suzukii imposes a great risk for strawberry production, and heavy economic losses were already reported (Santos 2014; Andreazza et al. 2016), and T. anastrephae could be an alternative to control this pest in strawberry orchards. However, it is necessary to evaluate the performance of this parasitoid in D. suzukii-infested strawberries. Thus, aiming to evaluate the ability of T. anastrephae to parasitize D. suzukii in strawberries, we conducted two experiments. In the first experiment, we examined the behavior of this parasitoid towards several host (D. suzukii) and host-substrate (strawberry) cues, to identify if T. anastrephae can discriminate between infested and non-infested strawberries. In the second experiment, we examined the density-dependent parasitism of T. anastrephae in D. suzukii-infested strawberries in a confined environment (greenhouse).
Material and methods
Insects rearing
Trichopria anastrephae and D. suzukii rearing were conducted under controlled conditions (23 ± 2°C, 70 ± 10% RH, and 12 h of light) at the Laboratory of Entomology, Embrapa Clima Temperado, Brazil. As described elsewhere (Vieira et al. 2019), colonies were initiated from flies and parasitoids collected in nearby organic farms, with new material periodically introduced to maintain colony vigor. Flies were reared on a cornmeal-based diet (Schlesener et al. 2018), and adult flies were held in plastic cages (262 × 177 × 147 mm) (length × height × width), with two side openings (80 × 100 mm), and an opening in the lid (155 × 50 mm), covered with voile cloth. Parasitoids were reared on D. suzukii pupae, and adults were held in plastic cages, as the ones described here for D. suzukii, and fed pure honey (ad libitum).
Behavioral assays
To test the response of T. anastrephae females towards different host and host substrate cues, we conducted behavioral assays in a circular four-chamber olfactometer (adapted from Steidle and Schöller (1997), and previously used for T. drosophilae by Wolf et al. (2020)). The olfactometer used had a 15-cm diameter and 6-cm height and was divided into four equal-sized chambers. One strawberry (~ 10 g) was placed in one (no choice) or two opposite (choice) chambers of the olfactometer. A plastic mesh was used to cover the olfactometer and a plate (20-cm diameter and 1-cm height) was placed above the mesh. The plate had one hole in the center, where an open 1.5-mL tube containing one parasitoid female (3 to 5 days old, naïve, and mated) was inserted. The parasitoid was placed in the tubes 30–60 min before the assay. After leaving the tube, the parasitoid was able to walk freely in the plate, above the chambers, without contacting the fruits. During the tests, the olfactometer was kept in a white plastic basin, and placed under a light source, for evenly distributed light.
For no-choice and choice bioassays strawberry fruits containing different potential cues were analyzed (Table 1). All fruits were obtained from plants (cv. San Andreas) cultivated without any pesticide spray and showed no signs of insect infestation or fungal contamination before they were treated. For treatments containing D. suzukii cues, fruits were collected and individually placed in plastic containers (200 mL) with two couples of D. suzukii. After 24 h, D. suzukii adults were removed. This treatment induced an infestation of 6–10 eggs per fruit. Infested strawberries were kept for 1 (fruits with eggs), 5 (fruits with larvae), or 8 days (fruits with pupae) before they were used in the bioassays. Non-infested fruits for choice bioassays were collected on the same day, but they were not exposed to D. suzukii adults. Artificially damaged fruits were collected and perforated ten times with a “00” entomological pin, which was pierced through the skin only about 1 mm. Fruits with inoculated pupae were kept in the lab for 8 days, then perforated and inoculated with ten 24-h-old D. suzukii pupae. Strawberries in different ripening stages were also tested. Unripe (1/3 of the fruit was pink) and ripe (completely red fruit) strawberries were collected the day before being used for bioassays, while for overripe strawberries, fruits were marked in the plant when they were ripe (completely red), but only collected 7–9 days later, when showing signs of overripening (losing their firmness).
Each parasitoid female was observed for 300 s, and the observation began when the female left the tube. If a female did not leave the tube for 50 s or moved for less than 150 s during the observation period, it was considered non-responsive and removed from the analysis. The period that each female walked over each chamber was logged using the open-source software Boris (Behavioral Observation Research Interactive Software) (Friard and Gamba 2016). Each female was used only once. After each observation, the olfactometer was rotated 90° and the plate over the olfactometer was exchanged. Each fruit and olfactometer was used three times, and then, the fruits were removed and the olfactometers were cleaned (with distilled water and neutral soap, rinsed with 70% ethanol, and dried overnight). For each treatment, in both choice and no choice bioassays, between 20 and 37 responsive females were observed. All bioassays were conducted at 23 ± 2°C, 70 ± 10% RH, and between 09:00 and 14:00 h.
Greenhouse assay
We tested the parasitism of different densities of T. anastrephae released in walk-in cages (360 × 220 × 200 cm) (length × height × width), placed inside a greenhouse. Inside each walk-in cage, we placed 18 potted strawberry plants (cv. San Andreas), each plant containing at least 3 ripe fruits. An artificial infestation of D. suzukii (60 couples, 3–5 days old) was performed in each cage. Since T. anastrephae is a pupal parasitoid, they were released inside each cage 5 days later than D. suzukii infestation, to allow host development. Parasitoids were released in each cage according to treatments (T1- no release (control), T2- 60 parasitoids, T3- 120 parasitoids, T4- 240 parasitoids, and T5- 360 parasitoids), in the same sex ratio as the maintenance rearing (0.7).
To assess fruit infestation and parasitism, strawberry fruits were collected 5, 10, and 15 days after parasitoid release. In each fruit sampling, 20 fruits were randomly collected and individually placed in plastic containers (250 mL), containing a fine layer (~ 2 cm) of vermiculite). The number of flies and parasitoids from each fruit was recorded 30 days after fruit sampling. The experiment was replicated 4 times between October 2019 and February 2021. No pesticide was sprayed during experiments.
Statistical analysis
For data collected in behavioral experiments, the time that female wasps spent walking over chambers containing fruit vs no fruit, in no choice bioassays, or over the two different treatments, in choice bioassays, was analyzed with Wilcoxon signed rank tests (p ≤ 0.05). And for data collected in the greenhouse trials, we used a generalized linear model (GLM), with quasipoisson error distribution, to assess the effects of parasitoid release density and time after parasitoid release on a number of D. suzukii or T. anastrephae per fruit (p ≤ 0.05). In case of significance, Tukey’s post hoc test was run to compare treatments (p ≤ 0.05). All statistical analyses were performed in the software R version 4.0.3 (R Core Team 2020).
Results
Behavioral assays
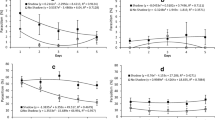
Parasitoid females walked significantly longer over olfactometer chambers with infested strawberry fruits containing larvae, pupae, or inoculated pupae when compared to empty chambers (Fig. 1). Similarly, T. anastrephae females also walked longer over chambers containing overripe strawberries than over chambers without a fruit (Fig. 1). However, damaged fruits, fruits containing D. suzukii eggs, and unripe and ripe fruits did not affect the length that female parasitoids walked over each chamber of the olfactometer (Fig. 1).
Length of time (seconds) that Trichopria anastrephae females spent walking over olfactometer chambers containing a fruit with different potential cues vs. no fruit (empty). Values are mean ± SE. Sample size is indicated in the brackets. ns indicates no significant difference, while *, **, and *** indicate a significant difference at 5, 1, and 0.1% levels, respectively (Wilcoxon signed rank tests)
When offered a combination of two fruits containing two different potential cues, parasitoid females walked longer over chambers containing fruits naturally infested with eggs, larvae, or pupae of D. suzukii, when compared to healthy uninfested fruits (Fig. 2). Females also walked longer over fruits with naturally infested pupae than inoculated pupae, and over overripe fruits when compared to unripe or ripe fruits (Fig. 2).
Length of time (seconds) that Trichopria anastrephae females spent walking over olfactometer chambers containing fruits with different potential cues. Values are mean ± SE. Sample size is indicated in the brackets. ns indicates no significant difference, while * and ** indicate a significant difference at 5 and 1% levels, respectively (Wilcoxon signed rank tests)
Greenhouse assay
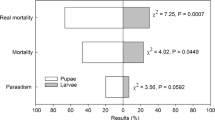
Based on the number of insects emerging from strawberry fruits, we did not observe an interactive effect between the number of parasitoids released and time after parasitoid release for either number of D. suzukii (χ2 = 1735.20, p = 0.3828) or T. anastrephae (χ2 = 338.97, p = 0.1592). However, the number of D. suzukii significantly decreased with the increase in the parasitoid release density (χ2 = 1842.50, p < 0.0001, Fig. 3). Compared to the control (no parasitoid release), the number of D. suzukii adults emerging per fruit decreased by 21.12, 48.20, 59.48, and 66.38% when 60, 120, 240, or 360 parasitoids were released, respectively. On the other hand, the number of T. anastrephae adults emerging per fruit increased with the increase in the release density (χ2 = 374.71, p < 0.0001, Fig. 3). As expected, no parasitoid emerged from the control, while 0.38 parasitoids/fruit emerged when 360 parasitoids were released.
Number of Drosophila suzukii and Trichopria anastrephae adults emerging per fruit collected from strawberry plants treated with different release densities of T. anastrephae. Values are mean ± SE. Letters are comparing bars of the same color; different letters indicate a significant difference according to the Tukey test (p ≤ 0.05)
The number of D. suzukii and T. anastrephae were affected by the time after the parasitoid was released (χ2 = 1776.20, p < 0.0001 and χ2 = 350.44, p < 0.0001, respectively, Fig. 4). When fruits were sampled 5 days after parasitoid release, we observed the highest numbers of D. suzukii (1.91 ± 0.20) and T. anastrephae (0.30 ± 0.06) adults emerging from fruits. However, the number of emerging insects started to decrease for both species 10 days after parasitoid release (1.36 ± 0.19 D. suzukii/fruit and 0.15 ± 0.03 T. anastrephae/fruit), and number of D. suzukii emerging adults reached its lowest 15 days after parasitoid release (0.95 ± 0.17), while the number of T. anastrephae were stable after 10 days.
Discussion
In the present study, we evaluated the behavior of T. anastrephae, a potential biocontrol agent of D. suzukii in Latin America, towards D. suzukii-infested and non-infested strawberries, as well as the density-dependent parasitism of D. suzukii pupae in strawberries cultivated in a greenhouse. Our results indicate that female parasitoids can recognize fruits infested with larvae and pupae (naturally and artificially infested) or in overripe stage. Moreover, when given a choice, female parasitoids showed preference towards fruits infested with eggs, larvae, and pupae over healthy undamaged fruits, and towards overripe fruits over unripe or ripe fruits. Also, T. anastrephae was able to parasitize D. suzukii-infested strawberries in the greenhouse and reduce the pest emergence, in a density-dependent manner.
The ability of a parasitoid to search for a host directly affects its efficacy as a biological control agent, and this behavior is often mediated by compounds emitted by the host substrate (plants), the host, and/or interaction among them (Vet and Dicke 1992). Our results show that infested fruits with larvae or pupae of D. suzukii and overripe fruits elicit a response in female parasitoids since they preferred to walk over chambers containing these treatments as compared to empty controls. In fact, as a pupal parasitoid, T. anastrephae has a better chance of finding a host in fermenting fruits, explaining their preference for fruits in a more advanced decaying stage over empty chambers, even when the fruits were not infested. The use of cues from both uninfested and infested fruits by female parasitoids was shown in the tephritid parasitoid Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae) (Silva et al. 2007; Segura et al. 2012). This larval parasitoid was attracted to fruit cues alone, but the presence of its host larvae in the fruit enhanced the attraction (Silva et al. 2007). Another experiment with this species showed that even using a larval medium elicits a positive response in female parasitoids, suggesting that byproducts from larval activity are an important source of chemical cues (Segura et al. 2012).
When given a choice, T. anastrephae showed a preference for infested strawberries (containing eggs, larvae, or pupae) over healthy fruits. Similarly, this species also shows a preference for infested blueberries when compared to non-infested fruits (De la Vega et al. 2021). The authors showed that the development of D. suzukii immature stages inside the fruit results in changes in the odor profile of the blueberry, which may result in a higher attraction of T. anastrephae to infested fruits. Another species of the pupal parasitoid, T. drosophilae, is also able to distinguish between D. suzukii-infested and non-non-infested fruits, in at least five different wild non-crop fruits (Wolf et al. 2020). The larval parasitoid Asobara japonica Belokobylskij (Hymenoptera: Braconidae) shows a preference for D. suzukii-infested cherries, strawberries, and blackberries when compared to non-infested fruits (Biondi et al. 2017). Other larval parasitoids, Leptopilina japonica Novkovic & Kimura and Ganaspis brasiliensis (Ihering) (Hymenoptera: Figitidae), also respond to fruit volatile cues associated with the presence of D. suzukii (Biondi et al. 2021).
We also tested fruits containing inoculated pupae. Fruits for this treatment were picked, brought to the lab, and only inoculated and tested 8 days after, to mimic the time taken by D. suzukii to become pupae in naturally infested fruits. In no-choice experiments, female parasitoids showed a preference to walk over chambers containing fruits with inoculated pupae compared to empty chambers. But they also spent more time on fruits with naturally infested pupae and overripe fruits than on empty chambers. Thus, it was not clear if females were behaving towards fruits with inoculated pupae due to the presence of pupae or the age of the strawberry. When given a choice between inoculated pupae or healthy fruit (both tested 8 days after picking), female parasitoids did not show any preference, suggesting that the sole presence of host pupae does not seem to be sufficient to elicit a response when compared to old fruits. However, female parasitoids spent more time walking over fruits with naturally infested pupae when compared to fruits with inoculated pupae. For a biological control agent, the foraging process includes host habitat (or substrate) location, host location, host acceptance, and host suitability (Vinson 1976, 1985). Our results suggest that T. anastrephae uses cues from overripe strawberries, which may be indirectly associated with the fly infestation since infested fruit become rotten more rapidly; thus, overripe/rotten fruits are more likely to contain hosts than unripe/ripe fruits. In a longer range, volatile compounds released by overripe fruits may direct parasitoids to habitats with a higher probability of host occurrence (Segura et al. 2012).
Artificially damaged fruits and fruits with eggs did not elicit a response in T. anastrephae. These cues are associated with the initial phases of infestation, and, as T. anastrephae is a pupal parasitoid, they may not be capable of identifying the egg stage (Hoffmeister and Gienapp 1999; Segura et al. 2012). On the other hand, infested fruits, containing larvae or pupae of D. suzukii, elicit a positive response in the parasitoid. The pupal stage, used by T. anastrephae as a host, is motionless and does not feed, resulting in a minimum odor emission (Fischer et al. 2001), and our results indicate that the combination of larvae feeding and pupating in the fruit affect the wasp. Probably, the cues responsible for promoting parasitoid response result from the combination of the host substrate (fruit), the host, and the host-associated microorganisms and/or byproducts (Vet and Dicke 1992; Hamby and Becher 2016).
In the greenhouse trial, to guarantee infestation of D. suzukii, we released adults of this pest, before releasing parasitoids. The average infestation in our control cage (no parasitoid release) was of 2.32 emerging D. suzukii per fruit. This infestation can be considered a low infestation, as found in the early season, since in the same region in Brazil, the average number of emerging D. suzukii during the growing season ranges between 5 and 10 adults per strawberry (Santos 2014; Wollmann et al. 2020). The management of D. suzukii in the early season is indicated to reduce or delay pest outbreaks (Rossi-Stacconi et al. 2016). Previous studies demonstrated that early augmentative releases of T. drosophilae have the potential to suppress the population of D. suzukii and reduce pest damage on crops (Rossi-Stacconi et al. 2019). On the other hand, when T. drosophilae was tested in a greenhouse with a high infestation of D. suzukii, the parasitoid was not effective (Trottin et al. 2014). Here, we showed that T. anastrephae can reduce D. suzukii emergence depending on the number of parasitoids released, but, ideally, this parasitoid should be also tested in future studies in higher levels of D. suzukii infestation.
Despite the initial pest population, the number of released parasitoids is another crucial factor for the success of biological control. In our experiments, the different parasitoid release rates resulted in different impacts on D. suzukii and T. anastrephae emergence. Although no difference was seen between the control and the lowest number of parasitoids released tested, the emergence of D. suzukii decreased when 120 parasitoids or more were released. A previous study showed that releasing up to 3000 T. drosophilae adults/ha is not sufficient to impact D. suzukii population density; however, releasing 4500 parasitoids/ha resulted in a reduction of 50% of the pest population in a berry field in Mexico (Gonzales-Cabrera et al. 2019). In our study, releasing 240 and 360 parasitoids per cage resulted in a decrease of approximately 60% in D. suzukii emergence. On the other hand, the number of parasitoids emerging per fruit was low (an average of < 1 parasitoid/fruit) even in the highest release ratio. However, it is important to note that this number may be underestimated since we only considered parasitoids emerging from pupae located in fruits collected from plants. Although there were no fruits on the ground to be collected, D. suzukii can pupate in the soil (Woltz and Lee 2017), and such pupae could also be parasitized.
Here, the timing of parasitoid release was planned according to the development of D. suzukii, to ensure that pupae would be available for a higher parasitoid success. In nature, it is more difficult to predict the most susceptible period of the host since there are several biotic and abiotic factors involved. However, simulated predictions of pest populations were performed for regions in the Northern Hemisphere and allowed researchers to predict periods when populations are composed of different stages, including pupae (Wiman et al. 2014). This information is useful to obtain the highest efficiency from parasitoid releases and to plan early season management of D. suzukii (Pfab et al. 2018; Rossi-Stacconi et al. 2019). Such simulations are not yet available for the Southern Hemisphere, but they would help plan biological control programs.
The results presented in this study provide some initial data that can be useful for biological control strategies using T. anastrephae. Our results allow a better understanding of the cues used by T. anastrephae in host searching, and their ability to parasitize infested strawberries. This indigenous biocontrol agent, despite being generalist, is an alternative to more specialist parasitoid species identified in D. suzukii’s native range since the introduction of exotic natural enemies may be challenging due to international regulations and risks imposed to resident biodiversity. Moreover, as a local parasitoid, T. anastrephae is expected to be well adapted to local environmental conditions and may not only parasitize D. suzukii pupae found in the targeted crop but also in unmanaged adjacent crops and wild fruits, that may serve as a reservoir of D. suzukii.
References
Andreazza F, Haddi K, Oliveira EE, Ferreira JAM (2016) Drosophila suzukii (Diptera: Drosophilidae) arrives at Minas Gerais state, a main strawberry production region in Brazil. Fla Entomol 99:796–798. https://doi.org/10.1653/024.099.0439
Biondi A, Wang X, Miller JC, Miller B, Shearer PW, Zappalà L, Siscaro G, Walton VW, Hoelmer KA, Daane KM (2017) Innate olfactory responses of Asobara japonica toward fruits infested by the invasive spotted wing drosophila. J Insect Behav 30:495–506. https://doi.org/10.1007/s10905-017-9636-y
Biondi A, Wang X, Daane KM (2021) Host preference of three Asian larval parasitoids to closely related Drosophila species: implications for biological control of Drosophila suzukii. J Pest Sci 94:273–283. https://doi.org/10.1007/s10340-020-01272-0
Bolda MP, Goodhue RE, Zalom FG (2010) Spotted wing drosophila: potential economic impact of a newly established pest. Agric Resour Econ Update 13:5–8
Boughdad A, Haddi K, El Bouazzati A, Nassiri A, Tahiri A, El Anbri C, Eddaya T, Zaid A, Biondi A (2021) First record of the invasive spotted wing Drosophila infesting berry crops in Africa. J Pest Sci 94:261–271. https://doi.org/10.1007/s10340-020-01280-0
Calabria G, Máca J, Bächli G, Serra L, Pascual M (2012) First records of the potential pest species Drosophila suzukii (Diptera: Drosophilidae) in Europe. J Appl Entomol 136:139–147. https://doi.org/10.1111/j.1439-0418.2010.01583.x
Cini A, Ioriatti C, Anfora G (2012) A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bull Insectology 65:149–160
Cruz PP, Neutzling AS, Garcia FRM (2011) Primeiro registro de Trichopria anastrephae, parasitoide de moscas-das-frutas, no Rio Grande do Sul. Ciência Rural 41:1297–1299. https://doi.org/10.1590/S0103-84782011000800001
De la Vega GJ, Triñanes F, González A (2021) Effect of Drosophila suzukii on blueberry VOCs: chemical cues for a pupal parasitoid, Trichopria anastrephae. J Chem Ecol 47:1014–1024. https://doi.org/10.1007/s10886-021-01294-7
Deprá M, Poppe JL, Schmitz HJ, De Toni DC, Valente VLS (2014) The first records of the invasive pest Drosophila suzukii in the South American continent. J Pest Sci 87:379–383. https://doi.org/10.1007/S10340-014-0591-5
Desneux N, Decourtye A, Delpuech JM (2007) The sublethal effects of pesticides on beneficial arthropods. Ann Rev Entomol 52:81–106. https://doi.org/10.1146/annurev.ento.52.110405.091440
Dos Santos LA, Mendes MF, Krüger AP, Blauth ML, Gottschalk MS, Garcia FRM (2017) Global potential distribution of Drosophila suzukii (Diptera: Drosophilidae). PLoSOne 12:e0174318. https://doi.org/10.1371/journal.pone.0174318
Emiljanowicz LM, Ryan GD, Langille A, Newman J (2014) Development, reproductive output, and population growth of the fruit fly pest Drosophila suzukii (Diptera: Drosophilidae) on artificial diet. J Econ Entomol 107:1392–1398. https://doi.org/10.1603/EC13504
Fischer S, Samietz J, Wäckers FL, Dorn S (2001) Interaction of vibrational and visual cues in the parasitoid host location. J Comp Physiol A 187:785–791. https://doi.org/10.1007/s00359-001-0249-7
Friard O, Gamba M (2016) BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods in Ecol Evol 7:1325–1330. https://doi.org/10.1111/2041-210X.12584
Garcia FRM, Wollmann J, Krüger AP, Schlesener DCH, Teixeira CM (2017) Biological control of Drosophila suzukii (Matsumura, 1931) (Diptera: Drosophilidae): state of the art and prospects. In: Davenport L (ed) Biological control: methods, applications, and challenges. Nova Science Publishers Inc, New York, pp 1–28
Garcia FRM, Lasa R, Funes C, Buzzetti K (2022) Drosophila suzukii management in Latin America: current status and perspectives. J Econ Entomol 115:1008–1023. https://doi.org/10.1093/jee/toac052
Garcia FRM (2020) Basis for area-wide management of Drosophila suzukii in Latin America. In: Garcia FRM (ed) Drosophila suzukii management. Springer, Cham, pp 93–110. https://doi.org/10.1007/978-3-030-62692-1_5
Gonzalez-Cabrera J, Moreno-Carrillo G, Sanchez-Gonzalez JA, Mendoza-Ceballos MY, Arredondo-Bernal HC (2019) Single and combined release of Trichopria drosophilae (Hymenoptera: Diapriidae) to control Drosophila suzukii (Diptera: Drosophilidae). Neotrop Entomol 48:949–956. https://doi.org/10.1007/s13744-019-00707-3
Goodhue RE, Bolda M, Farnsworth D, Williams JC, Zalom FG (2011) Spotted wing Drosophila infestation of California strawberries and raspberries: economic analysis of potential revenue losses and control costs. Pest Manag Sci 67:1396–1402. https://doi.org/10.1002/ps.2259
Gress BE, Zalom FG (2018) Identification and risk assessment of spinosad resistance in a California population of Drosophila suzukii. Pest Manag Sci 75:1270–1276. https://doi.org/10.1002/ps.5240
Hamby KA, Becher PG (2016) Current knowledge of interactions between Drosophila suzukii and microbes, and their potential utility for pest management. J Pest Sci 8:621–630. https://doi.org/10.1007/s10340-016-0768-1
Hoffmeister TS, Gienapp P (1999) Exploitation of the host’s chemical communication in a parasitoid searching for concealing host larvae. Ethology 105:223–232
Kacsoh BZ, Schlenke TA (2012) High hemocyte load is associated with increased resistance against parasitoids in Drosophila suzukii, a relative of D. melanogaster. PLoSOne 7:1–16. https://doi.org/10.1371/journal.pone.0034721
Kenis M, Tonina L, Eschen R, van der Sluis B, Sancassani M, Mori N, Haye T, Helsen H (2016) Non-crop plants used as hosts by Drosophila suzukii in Europe. J Pest Sci 89:735–748. https://doi.org/10.1007/s10340-016-0755-6
Knapp L, Mazzi D, Finger R (2021) The economic impact of Drosophila suzukii perceived costs and revenue losses of Swiss cherry, plum, and grape growers. Pest Managt Sci 77:3597–3597. https://doi.org/10.1002/ps.6421
Knöll V, Ellenbroek T, Romeis J, Collatz J (2017) Seasonal and regional presence of hymenopteran parasitoids of Drosophila in Switzerland and their ability to parasitize the invasive Drosophila suzukii. Sci Rep 7:40697. https://doi.org/10.1038/srep40697
Kruger AP, Scheunemann T, Vieira JGA, Morais MC, Bernardi D, Nava DE, Garcia FRM (2019) Effects of extrinsic, intraspecific competition and host deprivation on the biology of Trichopria anastrephae (Hymenoptera: Diapriidae) reared on Drosophila suzukii (Diptera: Drosophilidae). Neotrop Entomol 6:1–9. https://doi.org/10.1007/s13744-019-00705-5
Lee JC, Bruck DJ, Dreves AJ, Ioriatti C, Vogt H, Baufeld P (2011) In Focus: Spotted wing drosophila, Drosophila suzukii, across perspectives. Pest Manag Sci 67:349–1351. https://doi.org/10.1002/ps.2271
Lee JC, Dreves AJ, Cave AM, Kawai S, Isaacs R, Miller JC, van Timmeren S, Bruck DJ (2015) Infestation of wild and ornamental noncrop fruits by Drosophila suzukii (Diptera: Drosophilidae). Ann Entomol Soc Am 108:117–129. https://doi.org/10.1093/aesa/sau014
Lee JC, Wang X, Daane M., Hoelmer K, Isaacs R, Sial AA, Walton VM (2019) Biological control of spotted-wing Drosophila (Diptera: Drosophilidae) – current and pending tactics. J Integr Pest Manag 10:1–9. https://doi.org/10.1093/jipm/pmz012
Matsuura A, Mitsui H, Kimur M (2018) A preliminary study on distributions and oviposition sites of Drosophila suzukii (Diptera: Drosophilidae) and its parasitoids on wild cherry tree in Tokyo, central Japan. Appl Entomol Zool 53:47–53. https://doi.org/10.1007/s13355-017-0527-7
Miller B, Anfora G, Buffington M, Daane KM, Dalton DT, Hoelmer KM, Rossi Stacconi MV, Grassi A, Loni A, Mille JC, Ouantar M, Walton VM, Wiman NG, Wang X, Ioriatti C (2015) Resident parasitoids associated with Drosophila suzukii and their seasonal occurrence in two small fruit production regions. Bull Insectology 68:255–263
Oliveira DC, Stupp P, Martins LN, Wollmann J, Geisler FCS, Cardoso TDN, Bernardi D, Garcia FRM (2021) Interspecific competition in Trichopria anastrephae (Hymenoptera: Diapriidae) and Pachycrepoideus vindemmiae (Hymenoptera: Pteromalidae) parasitism on pupae of Drosophila suzukii (Diptera: Drosophilidae). Phytoparasitica 49:207–215. https://doi.org/10.1007/s12600-020-00843-2
Pfab F, Stacconi MVR, Anfora G, Grassi A, Walton V, Pugliese A (2018) Optimized timing of parasitoid release: a mathematical model for biological control of Drosophila suzukii. Theor Ecol 11:489–501. https://doi.org/10.1007/s12080-018-0382-3
Poyet M, Le Roux V, Gibert P, Meirland A, Prévost G, Eslin P, Chabrerie O (2015) The wide potential trophic niche of the Asiatic fruit fly Drosophila suzukii: The key to its invasion success in temperate Europe? Plos One 10:e0142785. https://doi.org/10.1371/journal.pone.0142785
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Rossi Stacconi MV, Grassi A, Dalton DT, Miller B, Ouantar M, Loni A, Ioriatti C, Walton VM, Anfora G (2013) First field records of Pachycrepoideus vindemiae as a parasitoid of Drosophila suzukii in European and Oregon small fruit production areas. Entomologia 1:11–16
Rossi Stacconi MV, Grassi A, Ioriatti C, Anfora G (2019) Augmentative releases of Trichopria drosophilae for the suppression of early season Drosophila suzukii populations. BioControl 64:9–19. https://doi.org/10.1007/s10526-018-09914-0
Rossi-Stacconi MV, Kaur R, Mazzoni V, Ometto L, Grassi A, Gottardello A, Rota-Stabelli O, Anfora G (2016) Multiple lines of evidence for reproductive winter diapause in the invasive pest Drosophila suzukii: useful clues for control strategies. J Pest Sci 89:689–700. https://doi.org/10.1007/s10340-016-0753-8
Rossi-Stacconi MVR, Amiresmaeili N, Biondi A, Carli C, Caruso S, Dindo ML, Francati S, Gottardello A, Grassi A, Lupi D, Machetti E, Mazzetto F, Mori N, Pantezzi T, Travella L, Garzia GT, Tonina L, Vaccari G, Anfora G, Ioriatti C (2018) Host location and dispersal ability of the cosmopolitan parasitoid Trichopria drosophilae released to control the invasive spotted wing Drosophila. Biol Control 117:188–196. https://doi.org/10.1016/j.biocontrol.2017.11.013
Santos RSS (2014) Ocorrência de Drosophila suzukii (Matsumura, 1931) (Diptera: Drosophilidae) atacando frutos de morango no Brasil. Comunicado Técnico, Embrapa, Bento Gonçalves. http://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/992353. Acessed 2 June 2021
Schetelig MF, Lee KZ, Otto S, Talmann L, Stokl J, Degenkolb T, Vilcinskas A, Halitschke R (2018) Environmentally sustainable pest control options for Drosophila suzukii. J Appl Entomol 142:3–17. https://doi.org/10.1111/jen.12469
Schlesener DCH, Wollmann J, Krüger AP, Nunes AM, Bernardi D, Garcia FRM (2018) Biology and fertility life table of Drosophila suzukii on artificial diets. Experiment Appl Entomol 166:932–936. https://doi.org/10.1111/eea.12736
Schlesener DCH, Wollmann J, Pazini JB, Padilha A, Grützmacher AD, Garcia FRM (2019) Insecticide toxicity to Drosophila suzukii (Diptera: Drosophilidae) parasitoids: Trichopria anastrephae (Hymenoptera: Diapriidae) and Pachycrepoideus vindemmiae (Hymenoptera: Pteromalidae). J Econ Entomol 112:1197–1206. https://doi.org/10.1093/jee/toz033
Segura DF, Viscarret MM, Ovruski SM, Cladera JL (2012) Response of the fruit fly parasitoid Diachasmimorpha longicaudata to host and host-habitat volatile cues. Entomol Exp Appl 143:164–172. https://doi.org/10.1111/j.1570-7458.2012.01246.x
Silva JWP, Bento JMS, Zucchi RA (2007) Olfactory response of three parasitoid species (Hymenoptera: Braconidae) to volatiles of guavas infested or not with fruit fly larvae (Diptera: Tephritidae). Biol Control 41:304–311. https://doi.org/10.1016/j.biocontrol.2007.03.005
Steidle JL, Schöller M (1997) Olfactory host location and learning in the granary weevil parasitoid Lariophagus distinguendus (Hymenoptera: Pteromalidae). J Insect Behav 10:331–342. https://doi.org/10.1007/BF02765601
Tochen S, Dalton DT, Wiman N, Hamm C, Shearer PW, Walton VM (2014) Temperature-related development and population parameters for Drosophila suzukii (Diptera: Drosophilidae) on cherry and blueberry. Environm Entomol 43:501–510. https://doi.org/10.1603/EN13200
Trottin Y, Paulhiac E, Zicot A, Baffert V, Leyre J, Weydert C, Poyet M, Ris N, Gibert P (2014) Experimental studies on Drosophila suzukii in protected strawberry crops: biology of the pest and effectiveness of a parasitoid of pupa in field conditions. In: Proceeding of the IOBC VIII workshop on integrated soft fruit production, Trento, pp 26–28
Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a tri-trophic context. Ann Rev Entomol 37:141–172. https://doi.org/10.1146/annurev.en.37.010192.001041
Vieira JGA, Krüger AP, Scheuneumann T, Morais MC, Speriogin HS, Garcia FRM, Nava DE, Bernardi D (2019) Some aspects of the biology of Trichopria anastrephae (Hymenoptera: Diapriidae), a resident parasitoid attacking Drosophila suzukii (Diptera: Drosophilidae) in Brazil. J Econ Entomol 113:81–87. https://doi.org/10.1093/jee/toz270
Vieira JGA, Krüger AP, Scheunemann T, Garcez AM, Morais MC, Garcia FRM, Nava DE, Bernardi D (2020) Effect of temperature on the development time and lifetime fecundity of Trichopria anastrephae parasitizing Drosophila suzukii. J Appl Entomol 114:857–865. https://doi.org/10.1111/jen.12799
Vinson SB (1976) Host selection by insect parasitoids. Ann Rev Entomol 21:109–133. https://doi.org/10.1146/annurev.en.21.010176.000545
Vinson SB (1985) The behavior of parasitoids. In: Kerkut GA, Gilbert LI (eds) Comprehensive Insect Physiology, Biochemistry, and Pharmacology, vol 9. Pergamon Press, New York, NY, USA, pp 417–46
Wang X, Daane KM, Hoelmer KA, Lee JC (2020) Biological control of spotted-wing Drosophila: an update on promising agents. In: Garcia FRM (eds) Drosophila suzukii Management. Springer, Cham, pp 143–167. https://doi.org/10.1007/978-3-030-62692-1_8
Wiman N, Walton VM, Dalton DT, Anfora G, Burrack HJ, Chiu JC, Daane KM, Grassi A, Miller B, Tochen S, Wang XG, Ioriatti C (2014) Integrating temperature-dependent life table data into a matrix projection model for Drosophila suzukii population estimation. Plos One 9:e106909. https://doi.org/10.1371/journal.pone.0106909
Wolf S, Boycheva-Woltering S, Romeis J, Collatz J (2020) Trichopria drosophilae parasitizes Drosophila suzukii in seven common non-crop fruits. J Pest Sci 93:627–638. https://doi.org/10.1007/s10340-019-01180-y
Wollmann J, Schlesene DCH, Ferreira MS, Garcia FRM (2016) Parasitoids of Drosophilidae with potential for parasitism on Drosophila suzukii in Brazil. DIS 99:38–42
Wollmann J, Schlesener DCH, Mendes SR, Krüger P, Martins N, Bernar D, Garcia MS, Garcia FRM (2020) Infestation index of Drosophila suzukii (Diptera: Drosophilidae) in small fruit in southern Brazil. Arq Inst Biol 87:1–9. https://doi.org/10.1590/1808-1657000432018
Woltz MJ, Lee JC (2017) Pupation behavior and larval and pupal biocontrol of Drosophila suzukii in the field. Biol Control 110:62–69. https://doi.org/10.1016/j.biocontrol.2017.04.007
Acknowledgements
The authors appreciate the financial support provided by the National Council of Scientific and Technological Development (CNPq) and the Higher Education Improvement Coordination (CAPES, finance code 001). To the National Research Council for the DEN and FRMG Research Productivity Grant. International Atomic Energy Agency for project support.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the conception and design of the work. All authors contributed to the acquisition of data. All authors contributed to the analysis and interpretation of data. All authors whose names appear on the submission drafted the work or revised it critically for important intellectual content, approved the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Edited by Eugenio E de Oliveira
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Krüger, A.P., Garcez, A.M., Scheunemann, T. et al. Trichopria anastrephae as a Biological Control Agent of Drosophila suzukii in Strawberries. Neotrop Entomol 53, 216–224 (2024). https://doi.org/10.1007/s13744-023-01113-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-023-01113-6