Abstract
Biocontrol agents such as parasitic wasps use long-range volatiles and host-associated cues from lower trophic levels to find their hosts. However, this chemical landscape may be altered by the invasion of exotic insect species. The spotted-wing drosophila (SWD), Drosophila suzukii (Diptera: Drosophilidae), is a highly polyphagous fruit pest native to eastern Asia and recently arrived in South America. Our study aimed to characterize the effect of SWD attack on the volatile organic compounds (VOCs) of blueberries, a common host fruit, and to correlate these odor changes with the olfactory-mediated behavioral response of resident populations of Trichopria anastrephae parasitoids, here reported for the first time in Uruguay. Using fruit VOC chemical characterization followed by multivariate analyses of the odor blends of blueberries attacked by SWD, we showed that the development of SWD immature stages inside the fruit generates a different odor profile to that from control fruits (physically damaged and free of damage). These differences can be explained by the diversity, frequency, and amounts of fruit VOCs. The behavioral response of T. anastrephae in Y-tube bioassays showed that female wasps were significantly attracted to volatiles from SWD-attacked blueberries when tested against both clean air and undamaged blueberries. Therefore, T. anastrephae females can use chemical cues from SWD-infested fruits, which may lead to a successful location of their insect host. Since resident parasitoids are able to locate this novel potential host, biological control programs using local populations may be plausible as a strategy for control of SWD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global trade is one of the main causes of the expansion of the range of pest species. The flow of agricultural products between countries facilitates the movement of species to novel environments (Anderson et al. 2004; Lantschner et al. 2019), threatening not only the production of goods but also the local biodiversity (Harvey and Fortuna 2012). When exotic insect species arrive in local natural communities, the native food webs can be altered, as is the entire ecosystem by cascading chemical, physiological and ecological changes across trophic levels (Chabaane et al. 2015). The presence or absence of antagonists (i.e., predators or parasitoids) is a key component in the success or failure of non-native species in a novel range, involving both top–down and bottom–up biotic influences among multiple trophic levels (Schultz et al. 2019). In this context, understanding how non-native pest species establish and interact with the native community is critical to forecast their success and to devise pest management strategies.
Chemical cues and signals are essential mediators in the ecological interactions of insects. Trophic interactions within native communities often rely on these cues and are therefore susceptible to chemical landscape alterations that may result from the invasion of exotic insect species (Chabaane et al. 2015; Mair and Ruther 2019; Rombaut et al. 2017). A common third trophic level involved in plant defense involves parasitic wasps, hymenopteran insects whose immature life stages occur in or on other arthropods, mostly other insects (Cusumano et al. 2020; Schultz et al. 2019). To find adequate habitats for potential mates or hosts, parasitic wasps use long-range volatiles and host-associated cues from lower trophic levels (Desurmont et al. 2020; Mumm and Hilker 2005). While searching for hosts, parasitic wasps need to integrate specific chemical cues with context background odors, which may be classified as irrelevant odors (no role in foraging behavior), masking odors (interfere and neutralize an attractive odor source) and enhancing odors (increase the attractiveness of an odor source) (Schröder and Hilker 2008). In this sense, mixtures of host-associated volatiles and background odors may be important mediators in tritrophic interactions involved in parasitoid host location (Desurmont et al. 2020; Schröder and Hilker 2008). In agricultural systems, these synergistic effects may be necessary to enhance the behavioral response of parasitic wasps to find their hosts efficiently (Liu et al. 2019).
Even though invasion ecology is a growing field, studies seldom focus on the effect of new exotic insects on multitrophic interactions in native communities (Carrasco et al. 2017; Chabaane et al. 2015,). Our study system involved three trophic levels: a local population of the parasitoid wasp Trichopria anastrephae Lima (Hymenoptera: Diapriidae), a potential new insect host that has recently arrived in South America, Drosophila suzukii (Matsumura) (Diptera: Drosophilidae), and one of its soft-skin fruit hosts, Vaccinium corymbosum (blueberries). D. suzukii, known as the spotted wing drosophila (SWD), is a highly invasive insect native to eastern Asia (Walsh et al. 2011). In the last decade, it became a risk for soft-skin fruits affecting a variety of cherry and berry crops (i.e. strawberries, blueberries) (Bolda et al. 2010; Walsh et al. 2011). SWD invasions were initially detected in Europe and North America in 2008 (Cini et al. 2012) and between 2012 and 2015 arrived in South America spreading from Brazil to the Patagonia region in southern Argentina (de la Vega and Corley 2019, de la Vega et al. 2020). The fly presents an important difference with most Drosophila species, which oviposit in decaying or overripe fruit, in that SWD females have a serrated ovipositor to pierce fruit skin, allowing them to lay eggs inside undamaged fresh and ripening fruit (Atallah et al. 2014). Before D. suzukii invaded agroecosystems, wounds on fruits such as grapes were due to climatic factors (i.e., hail, heat shock, heavy rain) or physical damages by birds or wasps (Rombaut et al. 2017). Consequently, the attack of SWD facilitates D. melanogaster infestation. As a consequence, rather than competing with closely related species, the invader makes available otherwise non-accessible resources, hence opening a new ecological niche for native, fructivorous insects (Rombaut et al. 2017).
Soft-skin fruits such as blueberries change their profile of volatile organic compounds (VOCs) as they ripen or senesce (Farneti et al. 2017). Therefore, fruit VOCs may potentially be used by D. melanogaster and D. suzukii to find different fruit stages (Karageorgi et al. 2017; Keesay et al. 2015). A host preference shift from rotten to fresh fruit has been proposed for SWD (Keesay et al. 2015), an ecological shift that may have had an impact on higher trophic levels as well. In this scenario, understanding multitrophic chemical ecology aspects related to the invasion of D. suzukii to new environments represents an opportunity to understand its effect on established populations of natural enemies. In turn, this understanding may result in improvements for integrated pest management programs.
There is growing interest in the development of environmentally friendly pest management methods to reduce the application of harmful pesticides (Kruitwagen et al. 2018). Thus, both larval and pupal parasitic wasps have been tested as biological control agents for SWD. The former group includes species of the genus Asobara (Hymenoptera: Braconidae), Ganaspis (Hymenoptera: Figitidae), and Leptopilina (Hymenoptera: Figitidae). Pupal parasitoids include Pachycrepoideus vindemiae Rondani (Hymenoptera: Pteromalidae), Spalangia erythromera Förster (Hymenoptera: Pteromalidae), Trichopria drosophilae (Perkins), and T. anastrephae Lima (Hymenoptera: Diapriidae). These were all able to parasitize D. suzukii under laboratory conditions (Ibouh et al. 2019; Vieira et al. 2019). While potential biological control agents may be identified in SWD’s native range (Lee et al. 2019), complex international regulations and biodiversity risks associated with the introduction of exotic natural enemies underline the need for improving the efficacy of resident species of natural enemies (Kruitwagen et al. 2018). In this sense, the presence of T. anastrephae populations in Latin America has been reported since 2001 (summarized in Vieira 2019), although biological control studies against D. suzukii are still only starting in the region (i.e., Wollmann et al. 2016; Vieira et al. 2019), with no studies focusing on the chemical ecology of these interactions. In this particular system, understanding if parasitoids have an innate ability to find and exploit fruit infested by SWD by using volatile cues bears potential implications for the biological control of this fruit pest. More specifically, if the parasitoid shows plasticity in exploiting volatile cues from various infested fruits, it is then capable of switching its preference patterns toward non-native host fruits or laboratory artificial substrates, which becomes also important for the development of rearing strategies for pest management programs (Biondi et al. 2017).
Using a chemical ecology approach, we here explored two ecological questions in our tritrophic study system. First, we investigated the effects of SWD infestation on the VOCs of ripening blueberries. Second, we addressed the behavioral response of female parasitoid wasps of a local population of T. anastrephae to fruits infested with this novel insect host.
Methods and Materials
Fruits
Organic grown blueberry fruits (Vaccinium corymbosum var. O'Neil and Blue Jay) were used to analyze the effect of SWD attack on VOC profiles. The fruits were harvested weekly from multiple plants between December 2019 and January 2020 in a local organic farm (La Micaela, Canelones, Uruguay, http://lamicaelaorganico.com/). They were harvested before the fully ripe stage, still on the red stage as described by Gilbert et al. (2013) and Farneti et al. (2017) (see Supplementary Data Fig. S1). The fruits were harvested in the morning (0900–1200 h) and VOC sampling was performed in the laboratory the same afternoon.
Insect Rearing
Drosophila suzukii adults came from a laboratory colony established from flies collected locally in April 2019. The rearing was maintained on common cornmeal diet (504 ml distilled water, 66 g sucrose, 6 g bread yeast, 2.3 g agar, 52 g corn-flour, 1.3 ml propionic acid, 0.8 g nipagin), in vials (12 cm high, 2.5 cm diam.) placed in an incubator under controlled conditions (21.5 ± 1 °C, 65 ± 5% relative humidity, 12:12 h photoperiod).
The parasitoid, T. anastrephae, also came from a laboratory rearing established at the same time (April 2019) from field-collected insects. It should be noted that, while T. anastrephae is well known at the regional level, these field collections represent the first report of the presence of this parasitoid in Uruguay. To work with wasps naïve with respect to SWD, the parasitoid rearing was maintained continuously on D. melanogaster, under the same conditions as described above for SWD.
Collection of Fruit Volatiles
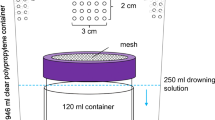
Blueberries free from external damages or irregularities (25 g) were placed in glass collecting chambers (20 cm length, 8 cm diam.) for dynamic headspace volatile collection. VOCs were collected by passing air pushed from an air compressor at a flow rate of 0.5 l min−1. The air was previously dehumidified with silica gel and filtered through activated carbon. VOCs were adsorbed in glass tubes filled with 50 mg of HaySep Q (Alltech, USA) for 24 h. VOC collections were made at a temperature of 25 ± 2 °C, 70 ± 5% relative humidity, and a photoperiod of 12:12 h (L:D). Adsorbed volatiles were eluted with 1 ml hexane, concentrated to 150 μl under a gentle flow of nitrogen, and stored in 250 μl vial inserts at − 20 °C until GC–MS analysis. An internal standard solution (100 μl) was added prior to concentrating the sample (tridecane in hexane, 1 μg/ml).
Effect of Attack by SWD Females on Blueberries
After the initial 24-h VOC collection the blueberry samples were assigned to the different treatments. To obtain SWD-attacked blueberries, the fruits were enclosed for 24 h with ten SWD couples ranging 2–7 days old. After 24 h the flies were anesthetized with CO2 and removed, and oviposition was confirmed under a stereo microscope by the presence of egg breathing tubes (9 ± 6 egg in each blueberry). The fruits were stored in clean glass containers covered with a fine mesh, under the same conditions as described for the insect rearing. Ten days later, a period that correlates with egg to pupae development of SWD (Tochen et al. 2014), fruit VOCs were collected under the same conditions as described above (SWD-attacked samples). Two controls were performed to differentiate the effect of SWD attack on fruit VOCs from the effect of physical damage and the natural ripening of the fruit. Physical damage (Physically damage samples) was mimicked by gently punching all fruits in the 25 g cluster with a 5 μm diam. microcapillary tube (three holes per fruit). The fruits were punctured ten days before VOC collection to match the maturation time of SWD-attacked fruit. Natural ripening was allowed under the same conditions with no treatment as an additional control treatment (undamaged control samples).
Chemical Analyses
Blueberry VOCs were analyzed by gas chromatography coupled with mass spectrometry (GC–MS) using a Shimadzu QP 2010 PLUS (Shimadzu Corp., Tokyo, Japan) equipped with a Rtx®-5MS column (30 m, 0.25 mm i.d, 0.25 μm film thickness; Alltech, USA). Samples (1 μl) were injected in the splitless mode with He as carrier gas at a flow rate of 1 ml/min (49.7 kPa). The oven temperature was programmed from 40 °C for 4 min, then increased to 150 °C at 5 °C/min and to 250 °C at 10 °C/min and held for 10 min. Injector and MS transfer line temperatures were both set at 250 °C.
Volatile compounds were identified and quantified using the GCMS Solution software (Shimadzu GCMS Solution V 4.45SP1). The chromatograms were analyzed first by comparison with a system blank (without blueberries) for background volatiles, then by comparison among the fruit VOC samples under the three treatments (SWD-attacked, physical damage, undamaged control). VOCs were identified from their mass spectra and retention indices, using the NIST08 and Adams’ MS databases (Adams 2007). Amounts of Individual compounds present were calculated relative to the internal standard by peak area comparison and are hence expressed as μg/25 g/24 h.
Olfactometer Bioassays
The behavioral responses of T. anastrephae females to volatiles from blueberries were evaluated using a glass Y-tube olfactometer consisting of two arms (6 cm long by 0.6 cm internal diameter) connected to chambers (9 cm long by 5 cm internal diameter) for the volatile stimuli. The chambers were located so that no visual contact was possible from the Y-tube. Humidified, charcoal-filtered air was pushed through the stimuli and olfactometer with a pump at a rate of 0.5 l/min. The olfactometer was laid horizontally on a glass surface homogeneously trans-illuminated with cold white LED lights (3600 lumens). To further eliminate visual cues, the olfactometer was fully enclosed in a box made from white corrugated plastic with a hole on top to allow video recording. All bioassays were conducted at 22 ± 2 °C, 70 ± 5% RH, and performed between 09:00 and 18:00 h. After each trial, the olfactometer arms were rotated to avoid position bias. At the end of the day the olfactometer was washed with distilled water and neutral soap, rinsed with ethanol and acetone, and oven-dried at 100 °C for 24 h.
Trichopria anastrephae females were used 2–5 days after hatching from D. melanogaster pupae. Upon hatching, females and males were placed in glass containers with access to honey-water (50:50) absorbed in cotton balls, until their use in the experiments. To conduct the bioassay, each female wasp was individually placed at the base of the common section of the Y-tube, and a 5-min period was video recorded. From this video, we measured the time spent in each arm, the first arm selected, and the position at the end of the recording period. For the three variables, the assignment of the wasp position was defined relative to a perpendicular line at the base of each arm.
Three olfactometer experiments were performed: one bioassay tested undamaged blueberries versus clean air, the second tested SWD-attacked blueberries versus clean air, and the third compared SWD-attacked blueberries versus undamaged blueberries. Physically-damaged blueberries used as a control in VOC chemical analyses were not used in the behavioral bioassays because the VOC profiles from undamaged and physically-damaged blueberries were not different (see “Results”). For the experiments with SWD-damaged fruit, 25 g of blueberries were exposed to oviposition by SWD under the same conditions as described for the VOC collections, and similarly kept for 10 days prior to the bioassays. Undamaged blueberries were stored under identical conditions. Blueberries for olfactometer bioassays were of commercial origin (Vaccinium corymbosum, Frusan, Frutera San Fernando, Chile); they were used fresh upon purchase and gently washed with distilled water.
Statistical Analyses
All statistical analyses were performed using R (Version 3.6–2) (R Core Development Team 2019).
To visualize VOC profiles in the different treatments a nonmetric multidimensional scaling (NMDS) was performed on the amount of VOCs matrix by using a Bray–Curtis distance matrix (Vegan package V2.4-6 in R). The data include many zero values, so it was fourth-root transformed (Hervé et al. 2018). To test for significant differences in the chemical composition of VOCs produced among the treatments (SWD-attacked, physical damage, and control) a permutational multivariate analysis of variance (perMANOVA) was also carried out on the distance matrix based on 9999 permutations. The analysis was performed using the adonis function in R (Vegan package V2.4-6).
The behavioral response of parasitoid wasps was analyzed by a paired t-test for the time spent in each arm, and an exact binomial test both for the first arm chosen and the position at the end of the bioassay. The exact binomial test performs an exact test of a simple null hypothesis about the probability that the number of wasps for first choice of either olfactometer arm had a 50:50 distribution. Also, to test the position at the end of the bioassay we performed the same test with a probability of 0.33 for the number of wasps present in either olfactometer arm or the common section. Females that did not respond were excluded from the analyses.
Results
Effects of Attack by SWD on Blueberry VOCs
Analyses of collection of volatiles from blueberries before initiation of the treatments and visualization by NMDS confirmed that the batches assigned to treatments were homogenous (Supplementary Data Fig. S2). We then measured VOCs from 15 samples of SWD-attacked blueberries, 10 samples of physically damaged fruit, and 15 samples of undamaged control fruits. Twenty-four compounds were identified in the VOC extracts from blueberries (Table 1, Fig. 1, Supplementary Data Table S1). Of these, nine compounds were exclusively present in the VOCs from blueberries attacked by D. suzukii (SWD-attacked) (Table 1). Taking into account the frequency in which it was found and the relative amount, isoamyl acetate was one of the main compounds that separates VOCs of SWD-attacked blueberries from VOCs of the fruit treatments (Table 1). Overall, volatiles produced in the highest amounts were short-chain aliphatic esters (Table 2).
Typical GC–MS chromatogram (TIC) of VOC collections from blueberries attacked by SWD (red), physically damaged control (blue), undamaged control (grey) and ambient background volatiles (black). Numbers indicate compounds as described in Tables 1 and S1. Non-numbered peaks correspond to background volatiles. Missing numbers are blueberry VOCs not found in the samples chosen for the Figure (IS internal standard 100 ng tridecane)
Multivariate analyses also showed that the VOC profiles of SWD-attacked blueberries differed from the VOC profiles of both control treatments (perMANOVA: F2,37 = 1.91 P = 0.04, permutation = 9999). The NMDS ordination of the VOC composition partially separated VOCs of SWD-attacked fruits from physically damaged and control VOCs (Fig. 2). Further, the multivariate pairwise comparison showed significant differences between the VOCs from SWD-attacked blueberries and those from physically attacked controls (perMANOVA: F1,23 = 2.63, P = 0.03, permutation = 9999), as well from SWD-attacked blueberries and undamaged control fruit VOCs (perMANOVA: F1,28 = 2.26, P = 0.04, permutation = 9999). Finally, the NMDS ordination did not graphically-show differences between VOCs from physically damaged and undamaged blueberries, nor did the multivariate analyses (perMANOVA: F1,23 = 0.34, P = 0.83, permutation = 9999).
Non-metric multidimensional scaling (NMDS) ordination based on Bray–Curtis dissimilarities of the volatile organic compounds (VOCs) from blueberries attacked by Drosophila suzukii (SWD-attacked, N = 15); VOCs from physically-damaged blueberries (N = 10) and from undamaged fruit (Control, N = 15). Stress value = 0.1615
Olfactory Responses of Trichopria anastrephae to Blueberry Volatiles
In the Y-tube bioassay, females of T. anastrephae reared on D. melanogaster responded preferentially to volatiles from blueberries attacked by D. suzukii, in comparison to undamaged blueberries or clean air. In both bioassays involving SWD-attacked fruits (vs. air-control and vs. undamaged blueberries), about 60% of females made a choice during the 5-min test period (72 out of 109 and 53 out 92, respectively). In contrast, in the bioassay comparing undamaged control blueberries vs. clean air only around 30% of the females made a choice (28 out of 103). In general, the behavioral responses of T. anastrephae females showed a tendency to prefer the blueberry-treated arm. Parasitoid wasps were significantly attracted to SWD-attacked blueberries when compared to clean air in all three variables measured (first choice binomial test P = 0.01; final position binomial test P = 0.0003 and time in each arm t52 = 2.52, P = 0.01) (Figs. 3, 4). Also, T. anastrephae females were significantly attracted to the volatiles from SWD-infested blueberries relative to undamaged blueberries when considering their final position (binomial test P = 0.002), but not in the first arm choice (binomial test P = 0.1) or the time spent in each olfactometer arm (t71 = 1.28, P = 0.20). In the case of undamaged blueberries compared to clean air, there were no significant differences in any of the three variables measured (first choice binomial test P = 0.28; final position binomial test P = 0.33 and time in each arm t27 = 1.31, P = 0.19).
Percent time spent by Trichopria anastrephae females in each arm of an olfactometer tube in choice bioassays testing volatile cues produced by blueberries attacked by Drosophila suzukii (SWD-attacked), control blueberries (undamaged) or clean air. Paired t-test are reported and error bars show Standard Error
Percent Trichopria anastrephae females in each arm of an olfactometer tube in choice bioassays with volatile cues produced by blueberries attacked by Drosophila suzukii (SWD-attacked), undamaged control or clean air. The upper and lower panels show the first arm choice and the final position at the end of a 5-min test period, respectively. P values for the binomial test are reported
Discussion
The aim of our study was to characterize the effect of SWD attack on the VOCs of ripening blueberries, and to correlate these odor changes with the olfactory-mediated behavioral responses of local populations of T. anastrephae female parasitoids. Our study is the first to use a chemo-ecological approach to test the capacity of T. anastrephae to find D. suzukii-infested fruit.
Multivariate analyses of the odor blends produced by SWD-attacked blueberries showed that the development of SWD immature stages inside the fruit generates a different odor profile in comparison with physically damaged and undamaged control fruits. Ten days after SWD infestation, a period that correlates with SWD larval development (Tochen et al. 2014), the odor differences among SWD-attacked and control blueberries (physically damaged and undamaged) were significant and observable in the diversity, frequency, and amounts of volatile compounds. These differences in the blueberry VOC profiles may be the result of various factors associated with SWD infestation, such as fruit tissue collapse due to larval feeding, larval metabolic wastes and their associated microorganisms, and opportunistic microorganisms associated with oviposition wounds (Hamby and Becher 2016; Rombaut et al. 2017).
Short-chain aliphatic esters, particularly ethyl 3-methylbutanoate, constituted the most abundant group of chemicals in all three treatments. Along with the esters, the blueberries emitted sesqui- and monoterpenes as relatively minor components. Among the sesquiterpenes, delta-elemene was the most abundant and frequent, while limonene was the most abundant monoterpene in the control treatments. In SWD-attacked blueberries, however, the amount of limonene was similar to that of anhydrolinalool oxide, a probable fungal biotransformation metabolite. VOCs from SWD-attacked fruits also contained 2-phenylethanol and ethyl benzoate, volatiles commonly found in flowers and fruits and also typical from fermentation processes in the case of the former. These aromatic volatiles were not found in either of the control treatments. Finally, SWD-attacked blueberries emitted seven additional compounds that were not found in the VOC collections from both control treatments. Among these, the most abundant were 3-methylbutyl 3-methylbutanoate (isoamyl isovalerate) and isoamyl acetate. The latter was also the most consistently found among compounds exclusive to SWD-attacked fruits.
Compounds such as hexanol, (Z)-linalool oxide and linalool are probably produced throughout ripening and preserved during the last maturation phases, reaching high amounts in the overripe stage (Farneti et al. 2017; Horvat et al. 1996). Other compounds seem to be emitted by unripe fruits and drastically reduced during ripening (e.g. caryophyllene), while compounds such as δ-elemene are stable during all ripening phases (Farneti et al. 2017). In contrast, esters such as ethyl 3-methylbutanoate (ethyl isovalerate), ethyl acetate, and methyl 2-methylbutanoate are exclusively produced in the last phase of ripening, increasing as the blueberries overripe (Farneti et al. 2017). In this scenario, our results suggest that SWD attack results in volatile emissions that resemble overripe fruit.
Fruit volatiles are important in the chemical ecology of drosophilids and may play a role in niche differentiation among sympatric species. Even though cosmopolitan Drosophila species are host generalists, different species may separate along resource-based niche dimensions such as fruit maturation time (Nunney 1996). SWD may be attracted to leaf volatile cues to mate-finding and also fresh unripe fruits odors to locate areas for oviposition (Cloonan et al. 2018). As fruits ripen, other drosophilids such as Drosophila simulans, D. melanogaster, and Drosophila immigrans may further colonize this rotten fruit, following a preference order for increasing maturation stages (Atallah et al. 2014; Nunney 1996; Rombaut et al. 2017). Fruit volatile esters may provide cues for ripening stages and may encode enough information to enable drosophilid flies to detect and discriminate their niches (Scheidler et al. 2015). For instance, isoamyl acetate, a “fruity” odor often present in ripening, ripe, and early fermenting fruits, is known to attract many drosophilids (Stökl et al. 2010). This compound, along with isobutyl acetate and ethyl hexanoate, were present in headspace VOC samples of fruit-associated yeasts and caused antennal responses in D. melanogaster and D. suzukii (Scheidler et al. 2015). Moreover, isoamyl acetate was one of the EAD-active compounds from wild blueberries, attractive to D. suzukii both individually and as part of a blend (Urbaneja-Bernat et al. 2021). However, tested in formulated blends for trapping SWD, isoamyl acetate showed no attraction and even a decrease in SWD adult captures (Cha et al. 2012). In this case, it is possible that concentration modulates SWD response to volatiles such as isoamyl acetate, since high concentrations may signal an overripe fruit that is not a preferred oviposition site for SWD females (Revadi et al. 2015).
The dynamics of fruit volatile blends associated with the temporal separation of Drosophila species may provide host-finding cues to the next trophic level (Vet and Dicke 1992). Using behavioral bioassays, we demonstrated that resident populations of T. anastrephae, a pupal parasitoid reared in the laboratory on D. melanogaster, responded differentially to volatiles emitted by blueberries infested by SWD. We measured three variables to characterize female wasp responses to the volatiles of blueberries: the first choice, the time spent in each arm, and the final position after five minutes of bioassay. Females of T. anastrephae showed a preference in all three variables when SWD-attacked fruits were tested against clean air. When the bioassay compared SWD-attacked and undamaged blueberries, a preference was only found in the position of the wasp at the end of the tested period, a variable that may correlate with active seeking behavior of the preferred source. These results are in line with those obtained from a related parasitoid, T. drosophilae. Using an olfactometer arena with four chambers and testing seven wild non-crop fruits as stimuli, Wolf et al. (2020) showed that female T. drosophilae spent more time walking over chambers with SWD-infested fruits compared to clean air. When comparing SWD-infested fruits with non-infested fruits, significant preferences were not so consistent, indicating that fruit odors alone may not be sufficient for host location in these fruits (Wolf et al. 2020). The preference showed by T. anastrephae females towards volatiles of SWD-infested blueberries appear to be innate, since they have had no access to SWD or SWD-infested fruit prior to the tests. SWD infestation of blueberries resulted in an increase of typical volatile compounds that other Drosophila species use for locating food, mating, and oviposition sites. It is then likely that T. anastrephae females use these same general odorant cues to locate their established drosophilid hosts. The foraging females may switch hosts if other alternatives are more abundant (Jaworski et al. 2013) or if they find earlier infested fruits that are more likely to contain pupae. In support of this, the generalist parasitoid T. drosophilae showed no differential preference for D. melanogaster or D. suzukii pupae (Wang et al. 2016). Similarly, a recent study by Biondi et al. (2021) showed that the larval parasitoids, Asobara japonica Belokobylskij (Hymenoptera: Braconidae), Leptopilina japonica Novković & Kimura, and Ganaspis brasiliensis (Ihering) (Hymenoptera: Figitidae), respond to fruit volatile cues associated with the presence of either D. suzukii or D. melanogaster (Biondi et al. 2021).
From an applied perspective, our results represent a relevant contribution to the development of a biological control program for SWD, since it deepens our understanding of how SWD impact blueberry VOCs, and how a parasitoid responds to the presence of its potential pest host (Biondi et al. 2017). Our study highlights the potential use of established populations of T. anastrephae since they are naturally able to cue on VOCs from SWD-attacked fruit and use SWD as a viable host (Vieria et al. 2020). Although most SWD pupae are found in the soil (Wolz et al. 2017), by using fruit VOCs as a long-range cue, parasitoids could target the right ecological niche to then refine their search. Understanding the behavior of this parasitoid in challenging environments is important to obtain higher efficiency in biological control programs against SWD (Krüger et al. 2019), stressing the need for more studies on the biology of T. anastrephae in the region. In line with this, the absence of management in adjacent crops or wild fruits could be reservoir of SWD where resident populations of parasitoids could have a greater importance. Our finding of local parasitoid populations suggests that the wasp is well adapted to local environmental conditions, which further underlines its potential as a biological control agent also in unmanaged adjacent crops or nearby wild fruits, which could serve as reservoirs for SWD (Krüger et al. 2019). In a broader sense, studies on these local parasitoid populations at the regional level may offer opportunities to manage SWD in Latin America without the new introduction of exotic species. Hence, a non-native organism such as SWD in Latin America may become controlled by a regulating mechanism in the introduced range, which limits its density and expansion (Schulz et al. 2019).
Availability of Data and Material
Data is available from the corresponding authors on request.
Code availability.
Code is available from the corresponding authors on request.
Code Availability
Code is available from the corresponding authors on request.
References
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry, 4th edn. Allured Publ, Carol Stream
Anderson PK, Cunningham AA, Patel NG et al (2004) Emerging infectious diseases of plants: pathogen pollution, climate change and agrotechnology drivers. Trends Ecol Evol 19:535–544. https://doi.org/10.1016/j.tree.2004.07.021
Atallah J, Teixeira L, Salazar R, Zaragoza G, Kopp A (2014) The making of a pest: the evolution of a fruit-penetrating ovipositor in Drosophila suzukii and related species. Proc Biol Sci 281(1781):20132840. https://doi.org/10.1098/rspb.2013.2840
Biondi A, Wang X, Miller JC et al (2017) Innate olfactory responses of Asobara japonica toward fruits infested by the invasive spotted wing drosophila. J Insect Behav 30:495–506. https://doi.org/10.1007/s10905-017-9636-y
Biondi A, Wang X, Daane KM (2021) Host preference of three Asian larval parasitoids to closely related Drosophila species: implications for biological control of Drosophila suzukii. J Pest Sci 94:273–283. https://doi.org/10.1007/s10340-020-01272-0
Bolda MP, Goodhue RE, Zalom FG (2010) Spotted wing drosophila: potential economic impact of newly established pest. Giannini Foundation of Agricultural Economics, University of California. Agric Resour Econ Update 13:5–8
Carrasco D, Desurmont GA, Laplanche D, Proffit M, Gols R et al (2017) With or without you: Effects of the concurrent range expansion of an herbivore and its natural enemy on native species interactions. Glob Change Biol 24:631–643
Cha D, Adams T, Rogg H, Landolt PJ (2012) Identification and field evaluation of fermentation volatiles from wine and vinegar that mediate attraction of spotted wing drosophila, Drosophila suzukii. J Chem Ecol 38:1419–1431
Chabaane Y, Laplanche D, Turlings TC, Desurmont GA (2015) Impact of exotic insect herbivores on native tritrophic interactions: a case study of the African cotton leafworm, Spodoptera littoralis and insects associated with the field mustard Brassica rapa. J Ecol 103:109–117
Cini A, Ioriatti C, Anfora G (2012) A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. B Insectol 65:149–160
Cloonan KR, Abraham J, Angeli S et al (2018) Advances in the chemical ecology of the spotted wing drosophila (Drosophila suzukii) and its applications. J Chem Ecol 44:922–939. https://doi.org/10.1007/s10886-018-1000-y
Cusumano A, Harvey JA, Bourne ME, Poelman EH, de Boer JG (2020) Exploiting chemical ecology to manage hyperparasitoids in biological control of arthropod pests. Pest Man Sci 76:432–443
de la Vega GJ, Corley JC (2019) Drosophila suzukii (Diptera: Drosophilidae) distribution modelling improves our understanding of pest range limits. Int J Pest Manage 65:217–227. https://doi.org/10.1080/09670874.2018.1547460
de la Vega GJ, Corley JC, Soliani C (2020) Genetic assessment of the invasion history of Drosophila suzukii in Argentina. J Pest Sci 93:63–75. https://doi.org/10.1007/s10340-019-01149-x
Desurmont GA, von Arx M, Turlings TCJ, Schiestl FP (2020) Floral odors can interfere with the foraging behavior of parasitoids searching for hosts. Front Ecol Evol 8:148. https://doi.org/10.3389/fevo.2020.00148
Farneti B, Khomenko I, Grisenti M et al (2017) Exploring blueberry aroma complexity by chromatographic and direct-injection spectrometric techniques. Front Plant Sci 8:617
Gilbert JL, Schwieterman ML, Colquhoun TA, Clark DG, Olmstead JW (2013) Potential for increasing southern highbush blueberry flavor acceptance by breeding for major volatile components. HortScience 48:835–843
Hamby KA, Becher PG (2016) Current knowledge of interactions between Drosophila suzukii (Diptera: Drosophilidae) and microbes, and their potential utility for pest management. J Pest Sci 89:621–630
Harvey JA, Fortuna TM (2012) Chemical and structural effects of invasive plants on herbivore–parasitoid/predator interactions in native communities. Entomol Exp Applic 144:14–26
Hervé MR, Nicolè F, Lê Cao K (2018) Multivariate Analysis of multiple datasets: a practical guide for chemical ecology. J Chem Ecol 44:215–234. https://doi.org/10.1007/s10886-018-0932-6
Horvat RJ, Schlotzhauer WS, Chortyk OT et al (1996) Comparison of volatile compounds from rabbiteye blueberry (Vaccinium ashei) and deerberry (V. stamineum) during maturation. J Essent Oil Res 8:645–648
Ibouh K, Oreste M, Bubici G et al (2019) Biological control of Drosophila suzukii: efficacy of parasitoids, entomopathogenic fungi, nematodes and deterrents of oviposition in laboratory assays. Crop Prot 125:104897
Jaworski CC, Bompard A, Genies L, Amiens-Desneux E, Desneux N (2013) Preference and prey switching in a generalist predator attacking local and invasive alien pests. PLoS ONE 8(12):e82231
Karageorgi M, Brńcker LB, Lebreton S et al (2017) Evolution of multiple sensory systems drives novel egg-laying behavior in the fruit pest Drosophila suzukii. Curr Biol 27:847–853
Keesey IW, Knaden M, Hansson BS (2015) Olfactory specialization in Drosophila suzukii supports an ecological shift in host preference from rotten to fresh fruit. J Chem Ecol 41:121–128
Krüger AP, Scheunemann T, Vieira JGA et al (2019) Effects of extrinsic, intraspecific competition and host deprivation on the biology of Trichopria anastrephae (Hymenoptera: Diapriidae) reared on Drosophila suzukii (Diptera: Drosophilidae). Neotrop Entomol 48:957–965. https://doi.org/10.1007/s13744-019-00705-5
Kruitwagen A, Beukeboom LW, Wertheim B (2018) Optimization of native biocontrol agents, with parasitoids of invasive pest Drosophila suzukii as an example. Evol Appl 11:1473–1497
Lantschner MV, de la Vega GJ, Corley JC (2019) Modelling the establishment, spread and distribution shifts of pests. Int J Pest Manag 65:187–189. https://doi.org/10.1080/09670874.2019.1575490
Lee JC, Wang X, Daane KM, Hoelmer KA, Isaacs R, Sial AA, Walton VW (2019) Biological control of spotted-wing drosophila (Diptera: Drosophilidae)—current and pending tactics. J Integr Pest Manag 10:13
Liu CM, Matsuyama S, Kainoh Y (2019) Synergistic effects of volatiles from host-infested plants on host-searching behavior in the parasitoid wasp Lytopylus rufipes (Hymenoptera: Braconidae). J Chem Ecol 45:684–692
Mair MM, Ruther J (2019) Chemical ecology of the parasitoid wasp genus Nasonia (Hymenoptera, Pteromalidae). Front Ecol Evol 7:184
Mumm R, Hilker M (2005) The significance of background odour for an egg parasitoid to detect plants with host eggs. Chem Senses 30:337–343
Nunney L (1996) The colonization of oranges by the cosmopolitan Drosophila. Oecologia 108(3):552–561
R Core Development Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Revadi S, Vitagliano S, Rossi Stacconi MV, Ramasamy S, Mansourian S et al (2015) Olfactory responses of Drosophila suzukii females to host plant volatiles. Physiol Entomol 40:54–64
Rombaut A, Guilhot R, Xuéreb A, Benoit L, Chapuis MP, Gibert P, Fellous S (2017) Invasive Drosophila suzukii facilitates Drosophila melanogaster infestation and sour rot outbreaks in the vineyards. Roy Soc Open Sci 4:170117. https://doi.org/10.1098/rsos.170117
Scheidler NH, Liu C, Hamby KA, Zalom FG, Syed Z (2015) Volatile codes: correlation of olfactory signals and reception in Drosophila-yeast chemical communication. Sci Rep 5:14059
Schröder R, Hilker M (2008) The relevance of background odor in resource location by insects: a behavioral approach. AIBS Bull 58:308–316
Schulz AN, Lucardi RD, Marsico TD (2019) Successful invasions and failed biocontrol: the role of antagonistic species interactions. Bioscience 69:711–724. https://doi.org/10.1093/biosci/biz075
Stökl J, Strutz A, Dafni A et al (2010) A deceptive pollination system targeting drosophilids through olfactory mimicry of yeast. Curr Biol 20:1846–1852
Tochen S, Dalton DT, Nik W et al (2014) Temperature-Related Development and Population Parameters for Drosophila suzukii (Diptera: Drosophilidae) on Cherry and Blueberry. Environ Entomol 43:501–510
Urbaneja-Bernat P, Cloonan K, Zhang A et al (2021) Fruit volatiles mediate differential attraction of Drosophila suzukii to wild and cultivated blueberries. J Pest Sci. https://doi.org/10.1007/s10340-021-01332-z
Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritrophic context. Annu Rev Entomol 37:141–172
Vieira JGA, Krüger AP, Scheuneumann T et al (2019) Some aspects of the biology of Trichopria anastrephae (Hymenoptera: Diapriidae), a resident parasitoid attacking Drosophila suzukii (Diptera: Drosophilidae) in Brazil. J Econ Entomol 113:81–87
Vieira JGA, Krüger AP, Scheunemann T et al (2020) Effect of temperature on the development time and life-time fecundity of Trichopria anastrephae parasitizing Drosophila suzukii. J Appl Entomol 144:857–865. https://doi.org/10.1111/jen.12799
Walsh DB, Bolda MP, Goodhue RE, Dreves AJ, Lee J, Bruck DJ (2011) Drosophila suzukii (Diptera: Drosophilidae): invasive pest of ripening soft fruit expanding its geographic range and damage potential. J Integr Pest Manag 2:1–8
Wang X, Kacar G, Biondi A, Daane KM (2016) Life-history and host preference of Trichopria drosophilae, a pupal parasitoid of spotted wing drosophila. Biocontrol 61:387–397
Wolf S, Boycheva-Woltering S, Romeis J, Collatz J (2020) Trichopria drosophilae parasitizes Drosophila suzukii in seven common non-crop fruits. J Pest Sci 93:627–638
Wollmann J, Schlesener DCH, Ferreira MS, Garcia FRM (2016) Parasitoids of Drosophilidae with potential for parasitism on Drosophila suzukii in Brazil. Drosophila Inf Serv 99:38–42
Woltz J, Megan J, Lee C (2017) Pupation behavior and larval and pupal biocontrol of Drosophila suzukii in the field. Biol Control 110:62–69
Acknowledgements
This paper is dedicated to the memory of Professor Wittko Francke, for his generous contribution to the growth of chemical ecology in Latin America. This project was funded by Grants from Agencia Nacional de Investigación e Innovación: FMV-1-2019-1-156089 and PD_NAC_2018_1_150632 (fellowship to GD). We thank Dr. Beatriz Goñi for kind help and advice in field collections, Daniela Mato for assistance in VOC collections, and Finca La Micaela for access to their organic farm.
Funding
This project was funded by grants from Agencia Nacional de Investigación e Innovación (ANII): FMV-1-2019-1-156089 and PD_NAC_2018_1_150632 (fellowship to GD).
Author information
Authors and Affiliations
Contributions
AG and GD conceived and designed research; GD, AG, and FT collected the data; AG identified the chemical compounds; FT led the parasitoid experiments; GD and AG led the analysis and writing of the manuscript. All authors contributed to the drafts and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de la Vega, G.J., Triñanes, F. & González, A. Effect of Drosophila suzukii on Blueberry VOCs: Chemical Cues for a Pupal Parasitoid, Trichopria anastrephae. J Chem Ecol 47, 1014–1024 (2021). https://doi.org/10.1007/s10886-021-01294-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-021-01294-7