Abstract
Abnormal metabolism of phosphatidylcholine (PC) leads to diseases such as cardiovascular, respiratory distress syndrome, chronic inflammatory bowel, and Alzheimer. In this study, we established an efficient colorimetric catalytic cascade system to measure PC value. In the first stage, PC is hydrolyzed to choline and phosphatidic acid by phospholipase D (PLD). Then, choline is used to produce hydrogen peroxide by choline oxidase (ChO). Finally, ABTS (2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)) produces green color in the presence of hydrogen peroxide and deoxyribozyme (DZ). Kinetic study of DZ showed that the value of Km was 0.190 and 0.078 mM for ABTS and hydrogen peroxide, respectively. Assay optimization showed that pH 7.5 and 30 °C were the best conditions for the sensing of PC. In addition, 0.075 μM DZ, 0.16 U/mL ChO, and 12 U/mL PLD were the best concentration for PC detection. The interfering study revealed that the constituents had no apparent absorbance value. The linear regression equation for PC was identified as A = 0.0052C + 0.0233 with a correlation coefficient of 0.9923. The limit of detection of this catalytic cascade system was obtained as 0.58 μM. PC evaluation in serum samples showed that the mean percentage recovery of PC was 97.98%. Taken together, good recovery and precision of PC detection designated that this colorimetric sensing approach is applicable for PC detection in real samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phosphatidylcholine (PC) which composed of two fatty acids and one phosphocholine molecule bound to glycerol is a major class of glycerophospholipids. This metabolite, which contains about 70% of the total phospholipids in circulation, has an essential role in membrane structure. It is known as the primary source of signaling molecules such as phosphatidic acid (PA), diacylglycerol (DAG), lyso-phosphatidylcholine, and arachidonic acid [1]. Choline is a participant of the B-vitamin complex that helps the body to make acetylcholine which is valuable for some diseases, for instance, Alzheimer, and memory loss [2,3,4]. The natural concentration of phosphatidylcholine in human plasma is about 50–200 mg/dl. Decrease synthesis of PC can start the caspases cascade through its intermediaries and leads to apoptosis [1]. Also, abnormal metabolism of phosphatidylcholine leads to diseases such as cardiovascular, respiratory distress syndrome, chronic inflammatory bowel, dementia, and Alzheimer [5,6,7]. For this goal, a lot of investigation has been performed to find the relationship between levels of phosphatidylcholine as a biomarker in various types of cancers such as prostate, colorectal, breast, epithelial ovarian, thyroid papillary, cervical, lung pancreatic, and other abnormalities [8,9,10,11,12,13,14,15,16,17,18,19,20]. Considering the importance of phosphatidylcholine in creating a wide range of diseases, it seems that this metabolite has the potential to be used as a biomarker [6]. Since 1987, some methods are used to measure PC in biological samples like enzymatic/amperometric, nuclear magnetic resonance (NMR), and chromatography [6, 21, 22]. Today, along with older methods, hydrophilic interaction liquid chromatography coupled to electrospray ionization mass spectrometry (HILIC–ESI–MS), ultra-performance liquid chromatography–tandem mass spectrometer UHPLC-MS, matrix-assisted laser desorption ionization–imaging mass spectrometry (MALDI–IMS), etc., have been widely used. Despite many advantages, these methods suffer from some disadvantages, such as the need for an extraction step with organic solvents, the need for complex and expensive machines, the need for expert equipment, time-consuming, and not sensitive [10,11,12,13,14,15,16,17, 23,24,25]. It seems that finding an affordable, easy-to-use, sensitive method like colorimetric could be more efficient [26]. But natural peroxidases which are used in these colorimetric systems have some downsides, for example, catalytic activity in just a certain temperature range, the need for complex preparation and purification, high cost, susceptible to denaturation, and activity loss [27, 28]. Recently, enzyme mimics such as deoxyribozymes, metal oxide, and metal organic framework have been found attractive interest in several areas such as bio-sensing, ecological analysis, and therapeutic treatment [28,29,30].
Deoxyribozymes are nucleic acid aptamers that are specifically linked to cofactors like hemin and induce enzymatic properties. They are also known as deoxyribozymes that are single-stranded DNA molecules with catalytic capabilities obtained by in vitro selection. Deoxyribozymes were found for catalytic reactions, including ligand synthesis, DNA phosphorylation, RNA cleavage, and nucleotide link formation [31, 32]. Compared with protein enzyme, deoxyribozyme has less sensitivity to temperature and maintains its activity even at high temperatures. The most significant progress in the deoxyribozyme branch is the finding of peroxidase-mimicking activity of some DNA–hemin complexes [31,32,33,34,35,36]. In recent years, considerable efforts have been made to assess a variety of deoxyribozymes for innovation-driven applications ranging from bio-sensing to gene regulation [37, 38].
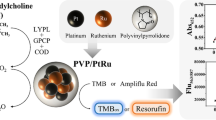
In this study, we want to establish an efficient colorimetric catalytic cascade system to measurement phosphatidylcholine value. In the first stage, phosphatidylcholine is hydrolyzed to choline and phosphatidic acid by phospholipase D. Then, choline is used to produce hydrogen peroxide in a catalytic reaction by choline oxidase. Finally, ABTS produces a green color in the presence of deoxyribozyme and hydrogen peroxide.
Materials and methods
Material
Hemin, ABTS, phospholipase D (from Streptomyces sp.), choline oxidase (from Alcaligenes sp.), MES buffer, and H2O2 were obtained from Sigma. Reagents such as Triton X-100, Tris–HCl, KCl, MgCl2, and DMSO were acquired from Merck. Hemin solution stock (12.5 mM) was equipped in TBST buffer and kept in the dark at − 20 °C.
Preparation of deoxyribozyme
At first, the G4-DNA (TGGGTAGGGCGGGTTGGGAAA) molecules synthesized by custom oligo synthesis (Bioneer company). These oligos were liquefied in a 30 µL of deionized water to the final concentration of 0.5 mM [38]. The G4-DNA solutions (25 µM, 40 µL) were heated to 95 °C for 5 min and then cooled to 25 °C gradually to guarantee the G4 arrangement. Then, 40 µL of buffer solution (50 mM Tris–HCl, 100 mM MES, 40 mM KCl, (induced deoxyribozyme structure) 0.05% (w/v) Triton X-100, pH 7.0) was added and incubated in room temperature for 1 h. Lastly, the obtained mixture was mixed with 80 µL hemin solution (12.5 µM) in dimethyl sulfoxide (DMSO) and maintained at room temperature for 1 h to form a deoxyribozyme structure [29].
Measurement of peroxidase activity of DZ
To measure the peroxidase activity of DZ, H2O2-ABTS system was used at room temperature. Before using this catalysis cascade system, the stock solution of ABTS (1.2 mM) prepared in TBST buffer (2 ml) composed of Tris–HCl buffer (20 mM, pH 7.0), 1.6 mM KCl, and 0.8 mM MgCl2 [39]. The assay mixture contains 8 µL of 1.2 mM H2O2, 8 µL of 0.075 μM DZ, 168 µL of 1.2 mM ABTS, and 16 µL TBST buffer solution (50 mM Tris–HCl, 40 mM KCl, 0.05% (w/v) Triton X-100, pH 7.0). The reaction was maintained at room temperature for 5 min. The appearance of green color was considered by UV–vis spectroscopy at 414 nm.
Optimization of DZ activity
To improve the best performance of this catalytic cascade system, a different concentration of ABTS and H2O2 was considered. To optimize the ABTS value, different concentrations of ABTS (0.0–0.816 mM) have been investigated in the presence of 0.28 mM H2O2 and 0.075 μM DZ in the mentioned buffer (pH 7.5). In this study, to optimize the H2O2 value different concentrations of H2O2 (0.0–1000 µM) have been investigated in the presence of 0.48 mM ABTS and 0.075 μM DZ in the mentioned buffer.
Catalytic cascade system for phosphatidylcholine measurement
To confirm the capacity of this catalytic cascade system to detection of phosphatidylcholine, phospholipase D (4 µL, 12 unit/mL, added to start the reaction), choline oxidase (6 µL, 0.16 unit/mL) were added into quartz cell including 0.48 mM ABTS (168 µL), 0.075 μM DZ (8 µL), and 100 μM phosphatidylcholine in Tris/HCl buffer (16 µL, 20 mM, pH 7.5) which contains 1.6 mM KCl, 0.01% (v/v) Triton X-100, and 0.8 mM MgCl2 The absorption changes were measured by a Cary 50 UV–Vis spectrophotometer (at 414 nm) from Agilent Technologies after 30 min of incubation at 30 °C.
Laboratory optimization for phosphatidylcholine measurement
Effect of pH on the catalytic cascade system
To consider the effect of pH on the catalytic cascade system, a universal buffer with the pH range of 4–10 with different compositions (20 mM sodium acetate, 20 mM buffer potassium phosphate buffer, and 20 mM Tris/base buffer) was prepared. Then, the optimal pH for this catalytic cascade system was considered in the presence of 0.16 U/mL of choline oxidase, 12 U/mL of phospholipase D, 0.075 μM DZ, 100 μM phosphatidylcholine, and 0.480 mM ABTS. The absorption changes were measured by a spectrophotometer (at 414 nm) after 30 min of incubation at 30 °C.
Effect of temperature on the catalytic cascade system
The function of this catalysis cascade system was considered at different temperatures (15, 20, 30, 40, 50, and 60 °C). The value of all components was the same as the section of “Measurement of peroxidase activity of DZ.” The absorption changes were measured at 414 nm by a spectrophotometer after 30 min of incubation at the mentioned temperatures.
Optimization of the catalyst concentration
The function of this cascade catalytic system was considered at different concentrations of DZ (0.0, 0.005, 0.125, 0.025, 0.0375, 0.05, 0.075, 0.1, 0.2 μM). The value of all components was the same as the section of “Measurement of peroxidase activity of DZ.” The absorption changes were measured at 414 nm by a spectrophotometer after 30 min of incubation at 30 °C.
The function of this catalytic cascade system was considered at different concentrations of choline oxidase (0, 0.01, 0.02, 0.04, 0.08, 0.12, 0.16, 0.24 unit/mL). The value of all components was the same as the section of “Measurement of peroxidase activity of DZ.” The absorption changes were measured at 414 nm by a spectrophotometer after 30 min of incubation at 30 °C.
Different concentrations of phospholipase D (0, 1, 2, 4, 8, 12, 18, and 24 unit/mL) were equipped in Tris–HCl buffer (20 mM, pH 7.5), and the ideal value of phospholipase D for this catalytic cascade system was investigated. In all the mentioned assay, the value of all components was the same as the section of “Measurement of peroxidase activity of DZ.” The absorption changes were measured at 414 nm by a spectrophotometer after 30 min of incubation at 30 °C.
Colorimetric detection of PC in the catalytic cascade system
The function of this catalytic cascade system was investigated at different concentrations of phosphatidylcholine (0.0–160 μM) which prepared in Tris–HCl buffer (20 mM, pH 7.5) containing 0.01% (v/v) Triton X-100, 0.8 mM MgCl2, and 1.6 mM KCl. The other components in assay reaction were phospholipase D (12 unit/mL, added to start the reaction), choline oxidase (0.16 unit/mL), 0.480 mM ABTS substrate, and 0.075 μM DZ. The absorption changes in 414 nm were measured and recorded by a spectrophotometer after 30 min of incubation at 30 °C.
Effect of interface substrates on phosphatidylcholine assay
To consider the value of interfering substrates existing in plasma, some probable interfering components (alanine, asparagine, uric acid, ascorbic acid, galactose, glutamic acid, glucose, glutamine, glycine, maltose, phenylalanine, citric acid, cysteine, tryptophan, tyrosine, cholesterol, urea, and their mixture (without PC)) were examined in activity reaction. The concentration of all these interfering components was the same as PC. The value of all components was the same as the section of “Measurement of peroxidase activity of DZ.” The absorption changes in 414 nm were measured and recorded by a spectrophotometer after 30 min of incubation at the mentioned temperatures.
Biological samples collection and pretreatment
We finally tested this catalyst cascade system for the detection of the PC amount in serum samples. At first, solid materials of plasma samples were removed by centrifugation (5000 g, 10 min). Diverse quantities of PC (30, 60, 90, 120, 150 mg/dl) were combined with serum samples, and finally, PC amount in these plasma samples was calculated. The recovery of this catalytic cascade system was also calculated as following [29]:
Results and discussion
Peroxidase-mimicking assay of deoxyribozyme
To consider the peroxidase-mimicking assay of DZ, ABTS oxidation was measured in the existence of H2O2. As exhibited in Fig. 1, the UV–visible oxidation plot of the ABTS in the attendance of DZ, H2O2, and Tris/HCl buffer was compared with control samples. The control samples contain all constituents except DZ and ABTS. Remarkably, an absorbance peak was obtained for ABTS-H2O2 in the existence of DZ with the highest absorbance at 414 nm. But, control tests revealed no absorbance value at a similar wavelength, which nominated that no ABTS oxidation happened. Also, a picture of these three tests is shown in Fig. 1B. These results indicated that DZ has bright peroxidase-mimicking activity, like commonly used horseradish peroxidase (HRP). Moreover, Fig. 1A showed the peroxidase-like activity of DZ after 5 min of incubation. The developed green color was stable up to 30 min of incubation.
A ABTS oxidation by deoxyribozyme in TBST buffer (pH7.5): a) in the presence of 0.48 mM ABTS, and 0.28 mM H2O2, b) in the absence of deoxyribozyme and ABTS, c) in the presence of deoxyribozyme, 0.48 mM ABTS, and 0.28 mM H2O2. B Comparison of color of these mentioned tests, C Time course of peroxidase-mimicking activity of DNAzyme: a) in the presence of 0.48 mM ABTS, and 0.28 mM H2O2, b) in the absence of deoxyribozyme and ABTS, c) in the presence of deoxyribozyme, 0.48 mM ABTS, and 0.28 mM H2O2
The kinetic activity of deoxyribozyme
Kinetic factors of peroxidase-mimicking activity of deoxyribozyme were obtained. Due to the lack of optimization in the previous reports to increase the enzyme efficiency, in this work, we examined the effects of various concentrations of ABTS and H2O2 on the activity of deoxyribozyme. As revealed in Figs. 2 and 3, the curves of initial velocity against diverse values of ABTS and hydrogen peroxide observed Michaelis–Menten behaviors. Results indicated that the quantity of Km was 0.190 and 0.078 mM for ABTS and hydrogen peroxide, respectively. It is stated that the Km is an indication of enzyme affinity toward substrates (ABTS/H2O2). Results exhibited that Km value of DZ with ABTS is lesser than HRP (as a natural peroxidase enzyme) and MIL-53(Fe) (as peroxidase-mimicking artificial enzyme) [40]. It is mentioned that Pan et al. 2018 stated that Vmax of Ficin-Heme complex in the presence of TMB and H2O2 was about 0.8 and 0.58 MS−1, respectively [41].
Investigation of the effect of different concentrations of ABTS on the peroxidase activity of deoxyribozyme in TBST buffer. The concentration and volume of the other reagents were the same as described in the section of “Measurement of peroxidase activity of DZ.” a Michaelis–Menten plot, b Line weaver Burk plot, c Green color of different concentrations of ABTS oxidation
Investigation of the effect of different concentrations of H2O2 on the peroxidase activity of deoxyribozyme in TBST buffer. The concentration and volume of the other reagents were the same as described in the section of “Measurement of peroxidase activity of DZ.” a Michaelis–Menten plot, b Line weaver Burk plot, c Green color of different concentrations of ABTS oxidation
Quantitative determination of PC
As shown in Scheme 1, there were three catalytic cascade stages for the colorimetric detection of PC levels: (1) bacterial phospholipase D (PLD, specific for PC) hydrolyzed PC substrate to choline and phosphatidic acid; (2) choline oxidase (ChO) hydrolyzed choline to produce hydrogen peroxide; and (3) hydrogen peroxide was used with ABTS, and DZ to produce a green color with an ideal peak absorption at 414 nm.
Moreover, the absorbance value of the oxi-ABTS product in the reaction solution is linearly associated with the value of hydrogen peroxide. Accordingly, the proposed colorimetric catalytic system using PLD, ChO, and DZ could be used to consider PC quantity. Figure 4 shows the brilliance UV–visible curve of the phosphatidylcholine detection system (containing PC, PLD, ChO, DZ, and ABTS). No apparent peak is seen in four control tests, which confirmed that the specificity of this catalytic cascade system for the phosphatidylcholine. In this catalytic cascade method, hydrogen peroxide which formed from enzymatic activity of PLD, ChO on PC was used as a substrate of DZ to form radical oxidized ABTS with green color after 30 min of incubation at 30 °C, in which Hojjati and Jiang [27] reported maximum absorbance of oxidized ABTS with green color obtained after 45 min of incubation at 37 °C.
Investigation of the capacity of cascade catalytic system for detection of PC. The function of this cascade catalytic system was investigated in the presence of phosphatidylcholine (100 μM) which prepared in Tris–HCl buffer (20 mM, pH 7.5) containing 0.01% (v/v) Triton X-100, 0.8 mM MgCl2, and 1.6 mM KCl. The other components in assay reaction were phospholipase D (12 unit/mL, added to start the reaction), choline oxidase (0.16 unit/mL), 0.480 mM ABTS substrate, and 0.075 μM DZ. The absorption changes in 414 nm were measured by a spectrophotometer after 30 min of incubation at 30 °C. Results are presented as mean ± SD (n = 3)
Optimization the function of the catalytic cascade system
Consideration of the pH effect
pH is one of the most important parameters on the function of enzymes. Consequently, the effect of pH on the function of catalytic cascade system has been examined in the area of 4.0 to 10.0. Results in Fig. 5A showed that the absorbance value at 414 nm improved in the pH 4.0 to 7.5, whereas it diminished at higher pH. Therefore, pH 7.5 was chosen as the best pH for the PC determination in this catalytic cascade system. As this pH is near to the biological environment, it marks it an appreciated system to quantity phosphatidylcholine value in the real trials. Li and coworkers were also reported that pH 8.0 was the best pH for the detection of cholesterol [42]. In our previous study, we report that pH 8.0 is the best pH for choline measurement in the choline oxidase/DZ catalytic system [30].
Investigation of the effect of different pH (4.0–10.0) and temperature (15–60 °C) on the PC detection by cascade catalytic system. The concentration and volume of all reagents were the same as described in the section of “Measurement of peroxidase activity of DZ.” Results are presented as mean ± SD (n = 3)
Consideration of the temperature effect
Temperature is the other significant issues for enzyme activity. Therefore, the effect of temperature on the assay reaction was considered in the area of 15–70 °C. Results in Fig. 5B showed that the absorbance value improved in the area of 15–30 °C, whereas it is decreased at upper temperatures of 30 °C. So, 30 °C was designated as the optimal temperature for the following study. It might be related to the stability of mesophilic ChO and PLD, which showed less thermal stability in previous reports [30]. In our previous study, we described that 25 °C is the optimal temperature for the choline measurement in the choline oxidase/DZ catalytic system. Li and coworkers [41], Song and coworkers [43] were also described that 30 and 35 °C were the ideal temperature for the biosensors functionality, respectively. These results specified that our catalytic cascade system is a potential phosphatidylcholine biosensor, particularly in point of care investigation.
Consideration of the deoxyribozyme value
DZ has peroxidase-mimicking activity, which catalyzes the oxidation of hydrogen peroxide in the attendance of ABTS. Therefore, its concentration is significant in this catalytic cascade system. So, the amount of DZ from 0.005 to 0.16 μM was equipped in Tris–HCl buffer (40 mM, pH 8.0), and then their effect on the phosphatidylcholine detection has been investigated. As revealed in Fig. 6A, the absorbance value has improved with a rise of DZ from 0.005 to 0.075 μM, but there is not a linear correlation between DZ concentration and absorbance at the upper values. Therefore, 0.075 μM DZ was used for the following tests. This DZ value is lower than the reports of Wang et al. [44] and Zhou et al. [45]. They reported that the concentration of deoxyribozyme in assay reaction was 0.35 and 0.3 μM, respectively. Furthermore, we previously reported that the 0.25 μM DZ is the optimal concentration for choline detection in the ChO/DZ catalytic system [29]. It is directed that this catalytic cascade-based DZ is low-cost for bio-sensing of phosphatidylcholine.
Investigation of the effect of different catalyst value (a; DZ, b; choline oxidase, c; phospholipase D) on the PC detection by cascade catalytic system. The concentration and volume of all reagents were the same as described in the section of “Measurement of peroxidase activity of DZ.” Results are presented as mean ± standard deviation (n = 3)
Consideration of the choline oxidase value
Choline oxidase oxidizes choline and produces H2O2 in this catalytic cascade system. Consequently, its concentration optimization is very vital to find the best assay condition. Therefore, the value of choline oxidase from 0.00 to 0.24 U/mL has been considered. Results in Fig. 6B showed that the activity increased from 0.01 to 0.16 U/mL ChO, while at a greater value, the absorbance value retained approximately constant. Thus, 0.16 U/mL ChO was chosen for the following study in this buffering system. It is minor than the reports of Takayama and coworkers [46] and Campanella et al. [47], which showed that 1.0 and 10 U/mL amount of ChO to the quantity of serum choline, respectively. Hidaka and coworkers used 0.07 U choline oxidase in assay solution for detection of PC by a procedure which performed using either microplate or automatic analyzer technology [25]. Furthermore, we previously reported that 0.75 U/mL ChO was the optimal concentration for choline detection in the ChO/DZ catalytic system [29]. Due to the high price of choline oxidase, the minor use of this enzyme in this optimized catalytic cascade system signifies good system optimization, particularly in bulky scale consumption.
Consideration of the phospholipase D value
As shown in Scheme 1, phospholipase D has a central role in phosphatidylcholine detection in this catalytic cascade system. So, finding the best concentration of PLD is important for the function of this catalytic cascade system. Therefore, the amount of PLD from 0.0 to 24 U/mL has been considered. As revealed in Fig. 6C, in the area of 0.0 to 12 U/mL PLD the activity improved while at the greater value, the absorbance value retained on the maximum value, approximately. Thus, 12 U/mL PLD was used for the following study. Hidaka and coworkers [25] used 18 U of PC-specific PLD in assay solution by a procedure performed using either microplate or automatic analyzer technology. Like choline oxidase, due to the high price of PLD, the minor using of this enzyme in this catalytic cascade system signifies the good system optimization, principally in bulky scale consumption.
Quantitative determination of PC
Cascade enzymatic detection of PC levels was approved using the novel three-step method (Fig. 7). Results in Fig. 6 revealed that the absorbance amount of the oxidized ABTS at the diverse value of PC by using this catalytic cascade system. Results displayed that the absorbance amount improved gradually with growing the PC value from 0.02 to 100 μM (Fig. 7A). As revealed in Fig. 7C, the green color of the catalytic system linearly increased with increasing in PC concentration. The linear regression equation for phosphatidylcholine was identified as A = 0.0052C + 0.0233 with a correlation coefficient of 0.9923 (Fig. 7b), where C is the PC value, A defines the absorbance amount. The limit of detection (LOD) of this catalytic cascade system was obtained as 0.58 μM. Results in Table 1 showed that the linear area for PC was from 1.0 to 25 μM, in which Hojjati and Jiang [26] reported that the linear area for PC detection was 2.5–20 µg.
a Investigation of the effect of different concentrations of PC by cascade catalytic system. The reactions were incubated at 30 °C for 30 min. The appearance of green color was considered by UV–vis spectroscopy at 414 nm. b The linear tuning plot for PC substrate. Results are presented as mean ± standard deviation (n = 3)
Consideration the effect of the interfering substrate
To investigate the selectivity of PC measurement by catalytic cascade system (PLD, ChO, and DZ), the absorbance amounts of this catalytic cascade system were considered. Results in Fig. 8 revealed that these constituents had no apparent absorbance value. The absorbance of green color of the oxABTS reduced the presence of alanine, asparagine, uric acid, ascorbic acid, galactose, glutamic acid, glucose, glutamine, glycine, maltose, phenylalanine, citric acid, cysteine, tryptophan, tyrosine, cholesterol, urea, and their mixture (without PC). Equally, the mix of all noisy materials with PC showed a sharp absorbance value.
Assay of cascade catalytic system in the presence of some interfacing substrates. The concentration and volume of all reagents were the same as described in the section of “Measurement of peroxidase activity of DZ,” except, of interfacing substrates used instead of PC. Error bars indicate the standard deviation from three equivalent tests. Results are presented as mean ± SD (n = 3)
Because the plasma sphingomyelin concentration is significantly lower than the PC concentrations, the hemolytic plasma may significantly interfere with the absorption reading of the sphingomyelin assay. Hojjati and Jiang [26] tested the hemolytic plasma in the sphingomyelin assay. In this report, we just established the mentioned assay for PC sensing. However, to investigate this effect, we incubated plasma samples (as the blank) without PLD at the same condition to PC assay and then measured their absorption at 414 nm. Finally, the blank results were subtracted from the sample results to obtain the actual results. Therefore, this catalytic cascade system based on PLD, ChO, and DZ exhibited high selectivity for PC quantity in biological samples.
Consideration of PC in serum samples
For evaluation of the operability of the phosphatidylcholine assay in serum samples, we investigate the precision of this catalytic cascade method. In this procedure, we added the known quantities of PC to plasma sample, and then recoveries have been calculated. The results in Table 2 showed the mean percentage recovery of PC was 97.98%. Hojjati and Jiang [26] reported that the mean percentage recovery of PC was 96.6%. Good recovery and precision of PC detection designated that this customary colorimetric sensing approach is applicable for PC detection in real samples.
Conclusion
Considering the importance of phosphatidylcholine in creating a wide range of diseases, it seems that this metabolite has the potential to be used as a biomarker. Despite many advantages of traditional approaches for PC measurement, these methods suffer from some disadvantages such as the need for an extraction step with organic solvents, need for complex and expensive machines, need for expert equipment, time-consuming, and not sensitive. But the catalysis cascade system is a simple, rapid, specific, sensitive, and high-throughput method that has been established for plasma PC measurement. The maximum function of this system has been obtained near physiological conditions, which is appreciated for the PC measurement in the biological samples. Good recovery and precision of PC detection designated that this customary colorimetric sensing approach is applicable for the PC detection in point of care diagnostic.
References
Z. Cui, M. Houweling, Biochim. Biophys. Acta (BBA) 1585(2), 87 (2002)
R. Ehehalt, A. Braun, M. Karner, J. Füllekrug, W. Stremmel, Biochim. Biophys. Acta (BBA). 1801(9), 983 (2010)
K. Singhal, A. Naithani, V. Prakash, O. Bangar, Int. J. Nutr. Pharmacol. Neurol Dis. 2, 84 (2012)
P. Rahimi, Y. Joseph, Trends Anal. Chem. 110, 367 (2019)
Z. Wang, E. Klipfell, B.J. Bennett, R. Koeth, B.S. Levison, B. DuGar, A.E. Feldstein, E.B. Britt, X. Fu, Y.M. Chung, Y. Wu, P. Schauer, J.D. Smith, H. Allayee, W.H.W. Tang, J.A. DiDonato, A.J. Lusis, S.L. Hazen, Nature 472(7341), 57 (2011)
M.Y. Tsai, E.K. Shultz, P.P. Williams, R. Bendel, J. Butler, H. Farb, G. Wager, E.G. Knox, T. Julian, T.R. Thompson, Clin. Chem. 33(9), 1648 (1987)
E.J. Schaefer, V. Bongard, A.S. Beiser, S. Lamon-Fava, S.J. Robins, R. Au, K.L. Tucker, D.J. Kyle, P.W. Wilson, P.A. Wolf, Arch. Neurol. 63(11), 1545 (2006)
E. Iorio, A. Ricci, M. Bagnoli, M.E. Pisanu, G. Castellano, M. Di Vito, E. Venturini, K. Glunde, Z.M. Bhujwalla, D. Mezzanzanica, S. Canevari, F. Podo, Cancer Res. 70(5), 2126 (2010)
S. Ishikawa, I. Tateya, T. Hayasaka, N. Masaki, Y. Takizawa, S. Ohno, T. Kojima, Y. Kitani, M. Kitamura, S. Hirano, M. Setou, J. Ito, PLoS ONE 7(11), 48873 (2012)
J.W. Lee, K.M. Cho, J.H. Jung, Q. Tran, W. Jung, J. Park, K.P. Kim, Alteration of phospholipids during the mitophagic process in lung cancer cells. Int. J. Microbiol. Biotechnol. 26(10), 1790 (2016)
N. Kurabe, T. Hayasaka, M. Ogawa, N. Masaki, Y. Ide, M. Waki, T. Nakamura, K. Kurachi, T. Kahyo, K. Shinmura, Y. Midorikawa, Y. Sugiyama, M. Setou, H. Sugimura, Cancer Sci. 104(10), 1295 (2013)
Y. Ide, M. Waki, T. Hayasaka, T. Nishio, Y. Morita, H. Tanaka, T. Sasaki, K. Koizumi, R. Matsunuma, Y. Hosokawa, H. Ogura, N. Shiiya, M. Setou, PLoS ONE 8(4), 61204 (2013)
N. Patel, R. Vogel, K. Chandra-Kuntal, W. Glasgow, U. Kelavkar, PLoS ONE 9(3), 88841 (2014)
M.Z. Yin, S. Tan, X. Li, Y. Hou, G. Cao, K. Li, J. Kou, G. Lou, Tumour Biol. 37(4), 5485 (2016)
F. Spadaro, C. Ramoni, D. Mezzanzanica, S. Miotti, P. Alberti, S. Cecchetti, E. Iorio, V. Dolo, S. Canevari, F. Podo, Cancer Res. 68(16), 6541 (2008)
F. Perrotti, C. Rosa, I. Cicalini, P. Sacchetta, P.D. Boccio, D. Genovesi, D. Pieragostino, Int. J. Mol. Sci. 17(12), 1992 (2016)
J. Zhang, J. Xu, H. Lu, J. Ding, D. Yu, P. Li, J. Xiong, X. Liu, H. Chen, Y. Wei, Oncotarget 7(39), 63158 (2016)
M. Zemanova, M. Vecka, L. Petruželka, B. Staňková, A. Žák, M. Zeman, Med. Sci. Monit. 22, 4092 (2016)
Y. Hosokawa, N. Masaki, S. Takei, M. Horikawa, S. Matsushita, E. Sugiyama, H. Ogura, N. Shiiya, M. Setou, PLoS ONE 12(8), 183724 (2017)
T. Kühn, A. Floegel, D. Sookthai, T. Johnson, U. Rolle-Kampczyk, W. Otto, M. Bergen, H. Boeing, R. Kaaks, BMC Med. 14(1), 13 (2016)
J.M. Pearce, J.T. Krone, A.A. Pappas, R.A. Komoroski, Magn. Reson. Med. 30(4), 476–484 (1993)
L. Campanella, M. Tomassetti, G. De Angelis, M.P. Sammartino, M. Cordatore, Clin. Chim. Acta 169(2–3), 175 (1987)
H. Akita, S.A. Ritchie, I. Takemasa, H. Eguchi, E. Pastural, W. Jin, Y. Yamazaki, D.B. Goodenowe, H. Nagano, M. Monden, M. Mori, Y. Doki, Pancreas 45(10), 1418 (2016)
T. Skotland, K. Ekroos, D. Kauhanen, H. Simolin, T. Seierstad, V. Berge, K. Sandvig, A. Llorente, Eur. J. Cancer 70, 122 (2017)
H. Hidaka, K. Yamauchi, H. Ohta, T. Akamatsu, T. Honda, T. Katsuyama, Clin. Biochem. 41, 1211 (2008)
M.R. Hojjati, X.C. Jiang, J. Lipid Res. 47(3), 673 (2006)
J. Kosman, B. Juskowiak, Anal. Chim. Acta 707(1–2), 7 (2011)
D.K. Yang, C.J. Kuo, L.C. Chen, Anal. Chim. Acta 856, 96 (2015)
N. Nikzad, Z. Karami, Int. J. Biol. Macromol. 115, 1241 (2018)
A. Badoei-dalfard, N. Sohrabi, Z. Karami, G. Sargazi, Biosens. Bioelectron. 141, 111420 (2019)
M. Mahdiannasser, Z. Karami, Biosens. Bioelectron. 107, 123 (2018)
D.M. Kong, Methods 4(3), 199 (2013)
D.M. Kong, J. Xu, H.X. Shen, Anal. Chem. 82(14), 6148 (2010)
Y. Xiang, P. Wu, L.H. Tan, Y. Lu, Biosensors Based on Aptamers and Enzymes. 140, 93 (2013)
D.M. Kong, L.L. Cai, J.H. Guo, J. Wu, H.X. Shen, Biopolymers 91(5), 331 (2009)
D.M. Kong, J. Wu, N. Wang, W. Yang, H.X. Shen, Talanta 80(2), 459 (2009)
E. Zokaei, A. Badoei-Dalfrad, M. Ansari, Z. Karami, T. Eslaminejad, S.N. Nematollahi-Mahani, Appl. Biochem. Biotechnol. 187(3), 708 (2019)
F. Farrokhi, Z. Karami, S. Esmaeili-Mahani, A. Heydari, J. Drug Deliv. Sci. Technol. 47, 477 (2018)
X. Zhu, Y. Cao, Z. Liang, G. Li, Protein Cell 1(9), 842 (2010)
W. Dong, X.D. Liu, W. Shi, Y. Huang, RSC Adv. 5, 17451 (2015)
Y. Pan, Y. Yang, Y. Pang, Y. Shi, Y. Long, H. Zheng, Talanta 185, 433 (2018)
Y. Li, H. Huang, F. Shi, Y. Li, X. Su, Microchim. Acta 180, 1135 (2013)
Z. Song, J.D. Huang, B.Y. Wu, H.B. Shi, J.I. Anzai, Q. Chen, Sens. Actuators B Chem. 115(2), 626 (2006)
Y. Wang, J. Wang, F. Yang, X. Yang, Microchim. Acta 171(1–2), 195 (2010)
X.H. Zhou, D.M. Kong, H.X. Shen, Anal. Chem. 82(3), 789 (2010)
M. Takayama, S. Itoh, T. Nagasaki, I. Tanimizu, Clin. Chim. Acta 79(1), 93 (1977)
L. Campanella, A. Bonanni, A. Magri, A. Sorbo, M. Tomassetti, J. Pharm. Biomed. Anal. 35(2), 399 (2004)
D. Yu, D. Zou, D. Li, X. Wang, X. Zhang, C. Yu, L. Wang, W. Elfalleh, L. Jiang, Food Anal. Methods 12, 229 (2019)
Acknowledgements
The authors express their gratitude to the Research Council of the Shahid Bahonar University of Kerman (Iran) and Iran National Science Foundation (INSF) for their financial support during the course of this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karami, Z., Khaksar, M. Construction of a sensitive cascade catalytic method for measurements of plasma phosphatidylcholine. J IRAN CHEM SOC 17, 2001–2011 (2020). https://doi.org/10.1007/s13738-020-01903-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-020-01903-4