Abstract
An aqueous two-phase flotation (ATPF) based on short-chain alcohol and salt was a preconcentration, separation and analysis method of chloramphenicol (CAP) coupled with high-performance liquid chromatography with ultraviolet–visible detector. The influences of salt concentration, flotation time, flow rate and the volume of n-propanol on the flotation efficiency of CAP were discussed. Response surface methodology was employed to optimize the experimental conditions. Under the optimal conditions, this method has been applied to quantitative determination of CAP in food with detection limit of 0.12 ng g−1 and quantification limit of 0.4 ng g−1 and the recoveries were in the range of 91.53–103.95 %. This ATPF used low cost of organic solvents, had high concentration coefficient and supplied a moderate and biocompatible environment, which is suitable for biomolecules.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As an environmentally friendly and economically viable pretreatment technique, aqueous two-phase systems (ATPSs) have been widely applied for separation and purification of proteins [1], antibiotics [2], metal ions [3] and other biomolecules [4, 5]. Our laboratory is interested in aqueous two-phase extraction (ATPE) [6–8], an efficient separation technique with advantages of high extraction efficiency, no use of toxic solvents and biocompatible environment containing abundant of water. Solvent sublation (SS) is a type of adsorptive bubble separation technique. The target molecules in water are adsorbed on the bubble surfaces of an ascending gas stream and then collected in an organic layer placed on the top of the water column [9]. Recently, a new preconcentration and separation technique that combined SS with ATPE was established to determine antibiotics, which was defined as aqueous two-phase flotation (ATPF) with a higher concentration coefficient and reduced consumption of organic solvents. In our previous report [10, 11], the concentration and separation of antibiotics were used ionic liquid/salt ATPF systems. Ionic liquids (ILs) are “green solvent,” but they are still of high cost and hard to recover the ILs. In recent years, alcohol/salt ATPS has attracted much attention in several fields [12, 13], due to the alcohol is low price and can be recycled by distillation. There is no report on separation and extraction of antibiotics with alcohol/salt ATPF.
CAP first isolated from the bacterium Streptomyces venezuelae is active against vast Gram-positive and Gram-negative bacteria [14] in both humans and animals [15]; however, it is often associated with harmful side effects in human, such as bone marrow depression and fatal aplastic anemia [16]. CAP is still widely used in animal farming due to its easy access and low cost, although it is prohibited from application to food production in the EU [17]; the methods for the determination of CAP include microbial assay [18], enzyme-linked immunosorbent assay (ELISA) [19, 20], fluorometric screening method [21] and chromatographic methods [22, 23]. However, these methods are high cost, short of the necessary sensitivity or time-consuming. Furthermore, because of the complexity and low concentration of CAP residues in food, the sample requires pretreatment technique before CAP analysis. The pretreatment methods mainly are solid-phase extraction (SPE) [24] and liquid–liquid extraction (LLE) [25] at present. However, SPE requires a solvent desorption step, which is time-consuming and complicated; both methods need toxic and volatile organic solvents. Therefore, it is necessary to develop a simple, rapid and sensitive method for sample pretreatment and CAP analysis.
The one-variable-at-a-time [26], an optimization technique, is while only one parameter is changed, others are kept constant. This technique does not represent the whole effects of the parameters on the response because it does not include the interactions among the variables studied. In order to overcome this problem, the optimization of experiment has been carried out using response surface methodology (RSM), which is a multivariate statistic technique. The RSM has advantages of giving abundance of messages from a small amount of experiments and understanding the connection between the independent variables and the response. The objective was to simultaneously optimize the levels of these variables to achieve the best system performance. Among RSM designs, the most popular experimental designs are Box-Behnken design, central composite design, three-level factorial and Plackett-Burman design. Box-Behnken design (BBD) [27, 28] has been widely applied in analytical chemistry [29]. The combinations of factors of BBD are not simultaneously at their highest or lowest levels that experiments performed under extreme conditions, which unsatisfactory results may occur can avoid.
In this paper, the factors of concentration of K2HPO4, flotation time, flow rate and volume of n-propanol influencing the flotation efficiency of CAP in ATPF were investigated. On the basis of single-factor experiment, the BBD method was used to optimize the main factors influencing flotation efficiency. Under the optimal conditions, this ATPF coupled with HPLC was successfully applied to the separation and determination of trace CAP in food.
Experimental
Chemicals
The standard sample of CAP was bought from the Chinese National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). The alcohols and salt of analytical grade and methanol of HPLC grade were supplied by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). They were all used without further purification. The stock solution of CAP was prepared by dissolving in methanol at a concentration of 500 μg mL−1 and should be replaced every 2 months. Standard working solutions of CAP were prepared by appropriately diluting the stock solution with deionized water. All solutions were stored at 4 °C.
Apparatus
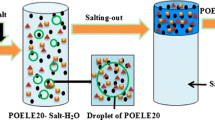
A BS124S electron balance (Beijing Sartorius instrument Co., Ltd., Beijing, China) was used for weighting. The analysis of variance was calculated using the Design-Expert.V.8.0.5.b. An Agilent 1200 HPLC (Agilent, USA) equipped with a quaternary pump and an ultraviolet–visible (UV) detector was used for analysis of extraction products. An analytical reversed-phase column was used for chromatographic separations at the column temperature of 25 °C. The instrument control and data processing were actualized using Agilent ChemStation software. Figure 1 shows the ATPF apparatus. The apparatus consists of a glass cylinder equipped with a sintered glass disk (G4 porosity) at the bottom to generate small bubbles. The disk is connected to a N2 gas cylinder with a pressure regulator by a fine pressure needle valve for controlling the gas flow. A glass column was used as the sublation column. It was 52 cm in height and 2.0 cm in inside diameter with three access ports. The bottom access with a microporous plate was applied to introduce air bubbles into the aqueous phase, the middle one for bulk aqueous solution sample and the upper one for the outlet of the alcohol phase.
Method
To a 50 mL colorimetric tube, 45 g K2HPO4 and 0.1 mg CAP were added, and then, deionized water was added. The solution was shaken for 10 min until dissolve fully and then transferred to the flotation cell (Fig. 1, calibration A). About 3 mL of alcohol was added on the top of the flotation cell, and then, the gas flow rate was adjusted at 40 mL min−1 for 50 min. After flotation, the CAP was transferred into the alcohol-rich phase (the top phase). All separation processes were performed at room temperature.
CAP in the top phase was determined by HPLC after flotation without any treatment. The ratio of mobile phase of methanol and water was 43:57 at the flow rate of 1.0 mL min−1. The injected volume was 20 μL, and the column effluent was monitored at a wavelength of 276 nm.
Preparation of real samples
Beef
The beef purchased from local retail market was stored at −10 °C and thawed several hours at ambient temperature before using. About 1.5 g of minced beef was placed into a 100 mL polypropylene tube, added in CAP working solutions. Then, trichloroacetic acid (10 mL, 15 % in water) was added, and the mixture was thoroughly mixed using a homogenizer–disperser till it was in homogeneity. The solution was centrifuged at 4,000 rpm for 30 min; finally, the supernatant was filtered through microfilter with a pore size of 0.45 µm to remove the denatured proteins. The extracts were stored at 4 °C for future use.
Milk
The milk was purchased from a local supermarket. Approximately 10 g of homogenized milk was weighted in a 100 mL polypropylene tube, added in CAP working solutions. Then, trichloroacetic acid (10 mL, 15 % in water) was added, and the solution was shaken and centrifuged at 4,000 rpm for 30 min and finally filtered through microfilter with a pore size of 0.45 µm to remove the denatured proteins. The extracts were stored at 4 °C for future use.
Milk powder
Milk powder was purchased from a local supermarket. Approximately 5 g of milk powder was weighted in a 100 mL polypropylene tube, added in CAP working solutions. Then, trichloroacetic acid (10 mL, 15 % in water) was added, and the mixture was shaken and centrifuged at 4,000 rpm for 30 min and finally filtered through microfilter with a pore size of 0.45 µm to remove the denatured proteins. The extracts were stored at 4 °C for future use.
Analysis of samples
The flotation efficiency (F, %) of CAP was calculated by
where C t represented equilibrium concentration of CAP in the top phase, V t was the volume of the top phase, and m s was the mass of CAP initially added.
Results and discussion
Selection of alcohols
Changing the type of alcohols is known to influence the forming of ATPS, and then further influences the partition behaviors of the target. Hence, it is desirable to study the effect of alcohols on the partitions of CAP in alcohol-based ATPF. The partitions of CAP were discussed in ATPSs of K2HPO4 with n-propanol, isopropanol or ethanol. In Fig. 2, the flotation efficiencies of CAP all increased with the increase in salt concentration, and then decreased. The phase-separation ability of alcohols is n-propanol > isopropanol > ethanol, and the maximum flotation efficiency was in n-propanol/K2HPO4 ATPF. Apparently, it was the best choice to use n-propanol as flotation solvent in the subsequently experiments.
Effect of the concentration of K2HPO4
With K2HPO4 concentration increase, the flotation efficiency of CAP first increased, and then kept above 90 % in 0.75–0.95 g mL−1. This is because with the increase in salt concentration, the solubility of CAP decreased in the bottom phase, resulting in the transfer of CAP to the top phase. With K2HPO4 concentration continuously increase, the flotation efficiency reduced slightly. The salt solution with high viscosity could weaken the mass transfer of CAP to air–water interface of rising bubble, which results in the decrease in flotation efficiency.
Effect of flotation time
From Fig. 3, we can see that the flotation efficiency of CAP reached 80 % when flotation time was 10 min. With flotation time increase, the flotation efficiency increased. This is because the increase in flotation time results in fully contact between the targets and bubbles; more targets were transferred to the top phase. Not the longer of flotation time was, the higher flotation efficiency can reach. When flotation time was higher than 50 min, flotation efficiency slightly decreased.
Effect of gas flow rate
The flotation efficiency with low gas flow rate is lower than that with a high one. Mass transfer of solute to air–water interface of rising bubble in the aqueous phase is the dominant transport process strongly affecting the flotation efficiency in ATPF. Under invariable mean radius of bubbles, air–water interface area of the same air volume at high flow rate is larger than that at the low one. As a result, more solute would be adsorbed or attached to the interface and entrained to the top phase with high flow rate, resulting in higher flotation efficiency. But under higher flow rate, the water–alcohol interface was drastically disrupted and some drops of the top layer could return to the water phase. Although the increased gas flow rate can improve the flotation efficiency, if the flow rate is quite high, the gas currents would disrupt the water–alcohol interface. In our experiment, with the increase in gas flow rate, the flotation efficiency of CAP first increased, and then decreased.
Effect of initial volume of n-propanol
The effect of initial volume of n-propanol on n-propanol/K2HPO4 ATPF was discussed in this part. When the initial volume of n-propanol was from 1.0 to 3.0 mL, the flotation efficiency of CAP was increased from 89.16 to 95.20 %. With continuous increase in the volume of n-propanol, the flotation efficiency increased slowly. In consideration of the flotation efficiency and experimental cost, 3.0 mL n-propanol was appropriate for CAP flotation.
Experimental design
A three-level factorial BBD was chosen for optimizing the process parameters affecting CAP flotation. Based on the results of a series of one-variable-at-a-time experiments, several variables that could potentially affect the extraction efficiency were chosen. The three factors that were selected to optimize the parameters by BBD were the concentration of K2HPO4 (A, 0.75–0.95 g mL−1), flotation time (B, 30–60 min) and flow rate (C, 10–50 mL min−1). The factor levels were coded as −1 (low), 0 (central point) and 1 (high).
According to the experimental design, the results from the experimental research were analyzed and tabulated in Table 1. The second-order polynomial equations in terms of coded factors were established as follows:
The coefficients of the equation were procured by regression analysis of the experimental data, where YF was the response of flotation efficiency.
An analysis of variance (ANOVA) about the response was shown in Table 2. The model was significant. There was only a 0.01 % chance that the model could occur due to noise. Values of “Prob > F” <0.05 indicate the model terms are significant, while values >0.10 indicate the model terms are not significant. In this case, A, AC, A 2, B 2 and C 2 were significant model terms, and A, A 2, B 2 and C 2 were highly significant model terms (p < 0.001). The nonsignificant lack of fit (p > 0.05) showed that the model was significant for the response. The determination coefficient (R 2) of 0.9975 and the adjusted R 2 (R 2adj ) of 0.9879 demonstrated a good degree of correlation between the experimental and the predicted data of the response [30].
Three-dimensional (3D) surface plots and contour plots were constructed as shown in Fig. 4. The 3D surface plots showed visually the effects and interaction between two independent variables on the responding variable as third independent variable was fixed at the central experimental level of zero.
From the results of BBD, the optimal conditions were obtained when the concentration of K2HPO4, flotation time and flow rate were 0.84 g mL−1, 45.75 min and 30.07 mL min−1, respectively. Under this condition, the flotation efficiency of CAP could reach 97.42 %.
Characterization of the method
The analytical curve was performed by adding standard CAP in the range of 0.4–40 ng mL−1 to ATPF. After phase separation, the top phase was determined by HPLC–UV method. The analytical curve for CAP was Area = 4.19418875 × c −0.2112819 with R 2 = 0.9999, where c represented the concentration of CAP (ng mL−1), and R 2 was the correlation coefficient. The limit of detection (LOD) was a signal value of three times the noise, and the limit of quantification (LOQ) was a signal value of ten times the noise. The LOD obtained was 0.12 ng g−1, and the LOQ was 0.4 ng g−1. The LOD for CAP is lower than the minimum required performance limit (0.3 ng g−1) established by the European Commission. Successive seven-time extraction and analysis of a 200 ng mL−1 standard solution of CAP were performed to check the repeatability of this method. The relative standard deviation (RSD) was 0.97 %.
In this study, comparative study of our developed method with other reported sample preparation procedures was performed and the results are presented in Table 3. Compared with the reported methods, lower LOD and LOQ could be obtained in the proposed method than other methods. The experimental and comparative results well indicate that the ATPF method can be used to effectively analyze strongly CAP in food samples.
Recovery studies in real samples
Under the optimum conditions, three kinds of food were analyzed to demonstrate the applicability of the proposed extraction technique. At detectable levels, no contamination of CAP residues was found in food before CAP was added. The recoveries of CAP were studied by adding known concentration of CAP standard solution into food samples. After phase separation, CAP in food was extracted to the top phase and determined by HPLC–UV (Fig. 5 and Table 4). As shown in Table 3, the recoveries of CAP were in the range of 91.53–103.95 % when the samples were spiked with 2–8 ng mL−1 CAP. The results showed that the reproducibility and recovery of the method were satisfactory for CAP determination, and the method can be gratifyingly applied to quantitative analysis of CAP in food.
Conclusions
This study demonstrated that n-propanol/K2HPO4 ATPF coupled with HPLC–UV was a good method for CAP flotation from food. The advantages of ATPF are a higher concentration coefficient and reduced consumption of organic solvents. The factors influencing the partitions of CAP were studied, and three factors containing the concentration of K2HPO4, flotation time and flow rate were chosen for further evaluating the experimental conditions and optimizing the process parameters by BBD. As a viable pretreatment technique, the separation method coupled with HPLC has been successfully applied to determining trace CAP in food.
References
S. Dreyer, P. Salim, U. Kragl, Biochem. Eng. J. 46, 176 (2009)
X. Xie, Y. Wang, J. Han, Y. Yan, Anal. Chim. Acta 687, 61 (2011)
L. Bulgariu, D. Bulgariu, Sep. Purif. Technol. 118, 209 (2013)
A.M. Azevedo, A.G. Gomes, P.A.J. Rosa, I.F. Ferreira, A.M.M.O. Pisco, M.R. Aires-Barros, Sep. Purif. Technol. 65, 14 (2009)
C. Kepka, J. Rhodin, R. Lemmens, F. Tjerneld, P.E. Gustavsson, J. Chromatogr. A 1024, 95 (2004)
J. Han, Y. Wang, C. Yu, Y. Yan, X. Xie, Anal. Bioanal. Chem. 399, 1295 (2011)
C. Li, J. Han, Y. Wang, Y. Yan, X. Xu, J. Pan, Anal. Chim. Acta 653, 178 (2009)
X. Xie, Y. Wang, J. Han, Y. Yan, Anal. Chim. Acta 687, 61 (2011)
M. Li, H. Dong, Sep. Purif. Technol. 73, 208 (2010)
J. Han, Y. Wang, C. Yu, C. Li, Y. Yan, Y. Liu, L. Wang, Anal. Chim. Acta 685, 138 (2011)
Y. Wang, X. Xu, J. Han, Y. Yan, Desalination 266, 114 (2011)
C.W. Ooi, T.B. Ti, S.L. Hii, S.M.M. Kamal, J.C.W. Lan, A. Ariff, T.C. Ling, Process. Biochem. 44, 1083 (2009)
Y.K. Lin, C.W. Oci, J.S. Tan, P.L. Show, A. Ariff, T.C. Ling, Sep. Purif. Technol. 120, 362 (2013)
L.K. Sørensen, T.H. Elbek, H. Hansen, J. Assoc. Off. Anal. Chem. 86, 703 (2003)
K. Vivekanandan, M.G. Swamy, S. Prasad, R. Mukherjee, Rapid Commun. Mass Spectrom. 19, 3025 (2005)
I. Shalit, M.I. Marks, Drug 28, 281 (1984)
European Commission, Regulation 2002/ 657/EC, 12 August, Implementing council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Commun. L211, 8 (2002)
R.J. Shakila, R. Saravanakumar, S.A.P. Vyla, G. Jeyasekaran, Innov. Food Sci. Emerg. 8, 515 (2007)
L. Wang, Y. Zhang, X. Gao, Z.J. Duan, S. Wang, J. Agric. Food Chem. 58, 3265 (2010)
G. Biancotto, L. Contiero, C. Benetti, M. Calligaris, E. Tibaldi, L. Cerni, M. Francese, Anal. Chim. Acta 637, 173 (2009)
Y. Liu, B. Danielsson, Microchim. Acta 153, 133 (2006)
L. Santos, J. Barbosa, M.C. Castilho, F. Ramos, C.A.F. Ribeiro, M.I.N. da Silveira, Anal. Chim. Acta 529, 249 (2005)
P. Mottier, V. Parisod, E. Gremaud, P.A. Guy, R.H. Stadler, J. Chromatogr. A 994, 75 (2003)
M. Bononi, F. Tateo, J. Food Compos. Anal. 21, 84 (2008)
H.X. Chen, H. Chen, J. Ying, J.L. Huang, L. Liao, Anal. Chim. Acta 632, 80 (2009)
R.S. Nicolich, E. Werneck-Barroso, M.A.S. Marques, Anal. Chim. Acta 565, 97 (2006)
M.A. Bezerra, R.E. Santelli, E.P. Oliveira, L.S. Villar, L.A. Escaleira, Talanta 76, 965 (2008)
F. Francis, A. Sabu, K.M. Nampoothiri, S. Ramachandran, S. Ghosh, G. Szakac, A. Pandey, Biochem. Eng. J. 15, 107 (2003)
S.L.C. Ferreira, R.E. Bruns, H.S. Ferreira, G.D. Matos, J.M. David, G.C. Brandão, E.G.P. da Silva, L.A. Portugal, P.S. dos Reis, A.S. Souza, W.N.L. dos Santos, Anal. Chim. Acta 597, 179 (2007)
S.N. Su, H.L. Nie, L.M. Zhu, T.X. Chen, Bioresour. Technol. 100, 2336 (2009)
T. Wanf, J. Tong, M. Sun, L. Chen, J. Sep. Sci. 34, 1886 (2011)
Y. Li, J. Han, Y. Wang, J. Ma, Y. Yan, L. Cao, J. Braz. Chem. Soc. 24, 669 (2013)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 21206059 and 21207051), the Natural Science Foundation of Jiangsu Province (Nos. BK2011529 and BK20131258), PhD Programs Foundation of Ministry of Education of China (Nos. 20123227120015 and 20133227120006), China Postdoctoral Science Foundation funded project (No. 2013M531284), Jiangsu Postdoctoral Science Foundation funded project (No. 1202039C), Zhenjiang Social Development project (SH201306), the Programs of Senior Talent Foundation of Jiangsu University (Nos. 11JDG029 and 12JDG079), Siping Science and Technology Development Program Foundation (No. 2012042), the Natural Science Foundation of Jilin Province (No. 20130101179JC_15) and Science and Technology Research Foundation of Jilin Province Department of Education (No. 2014_158).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, J., Wang, Y., Luo, L. et al. Optimization of separation and determination of chloramphenicol in food using aqueous two-phase flotation coupled with HPLC. J IRAN CHEM SOC 11, 1775–1782 (2014). https://doi.org/10.1007/s13738-014-0463-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-014-0463-1