Abstract
An 89-year-old Japanese man on peritoneal dialysis (PD) was suspected of having a PD-associated catheter infection. He visited the hospital because of the discharge of pus from the exit site of his catheter. Gram staining of the pus showed Gram-positive bacilli, but these were acid-fast bacilli. The rapidly growing nontuberculous mycobacteria, Mycobacterium abscessus, was isolated. PD catheter removal and debridement were immediately performed. The patient received combination antibiotic therapy. His clinical course was good, but he required hemodialysis due to the discontinuation of PD. However, the patient and his family chose not to continue hemodialysis even when the symptoms of uremia appeared. Best supportive care was arranged by his primary care physician. M. abscessus is a rare causative organism for PD-associated catheter infections and is difficult to treat. In our case, a rapid and precise diagnosis was made using acid-fast staining and Mycobacterium culture. The risk of nontuberculous mycobacterial infections should be considered in patients on PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peritoneal dialysis (PD)-associated catheter infection is one of the most serious complications, because it leads to the discontinuation of PD [1]. Exit-site infection (ESI) is a major predisposing factor for peritonitis [2]. Nontuberculous mycobacteria (NTM) also cause PD-associated catheter infection, but the prevalence is relatively low. The first case of NTM peritonitis was reported in a PD patient in 1982 [3]. NTM is a collective term for describing acid-fast bacilli other than Mycobacterium tuberculosis and Mycobacterium leprae [4]. They are abundant in the natural environment. NTMs are usually not virulent unlike M. tuberculosis. However, PD-associated catheter infections due to rapidly growing NTMs (RGNTMs) are associated with a high rate of catheter loss [5]. Mycobacterium abscessus is an RGNTM and is difficult to treat, because it is one of the most resistant species to anti-microbial drugs. Combination antibiotic therapy is needed, but this infection can be refractory to treatment [6]. M. abscessus peritonitis is characterized by a relatively higher complication rate than other NTM peritonitis and a 3-month mortality rate [7]. The duration of therapy remains unclear for patients with PD-associated catheter infection due to M. abscessus, and catheter removal is one of the radical treatments. The diagnosis of M. abscessus infection is challenging, because early diagnosis is the key to appropriate treatment. Here, we present a case of PD-associated catheter infection caused by M. abscessus, which was successfully treated with catheter removal because of a prompt diagnosis.
Case report
An 89-year-old Japanese man on continuous ambulatory peritoneal dialysis (CAPD) visited the hospital because of the discharge of pus from the exit site of the catheter. One month prior to admission, routine follow-up revealed that PD fluid cell counts were increased. The white blood cell (WBC) count of the PD fluid was 74/µL (monocytes 57/µL; polynuclear leukocytes 17/µL). Blood tests showed that the C-reactive protein (CRP) level was elevated (5.45 mg/dL). However, the patient was asymptomatic at that time. We considered PD-associated peritonitis despite the fact that the WBC count of the PD fluid was 100/µL, and it was not polynuclear leukocyte predominant, indicating a less likely possibility of peritonitis [8]. The patient refused hospitalization and chose outpatient follow-up. Levofloxacin was prescribed. Culture of the skin smear from the exit site and the PD fluid were also performed, but both were negative. During the treatment, the patient did not develop a fever or abdominal pain; his bowel movements were also good. One month later, the patient visited the hospital because of pus from the exit site.
Due to end-stage renal disease (ESRD), PD had been initiated at 6 months prior to the current admission. He did not experience any PD-associated issues before this admission. He had a history of diabetes, hypertension, angina pectoris, and congestive heart failure. An ointment containing bacitracin and fradiomycin sulfate was used for exit-site care.
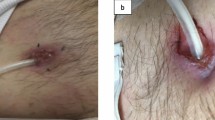
At the initial presentation, the patient was afebrile and asymptomatic. His vital signs were stable. There was no tenderness on abdominal examination. However, pus was being discharged from the exit site. Reddening and swelling of the skin around the exit site and induration of the skin from the exit site up to the area above the second cuff was also noted (Fig. 1). The PD fluid was not cloudy. The WBC count of the PD fluid was decreased to 8/µL (monocytes 5/µL; polynuclear leukocytes 3/µL). Complete blood count revealed that his WBC count was 5700/µL; red blood cell count 374 × 104µ/L; hemoglobin level 10.2 g/dL; and platelet count, 15.8 × 104 µ/L. Blood chemistry tests showed that the levels of Na were 137 mEq/L; K 3.1 mEq/L; Cl 97 mEq/L; P, 3.6 mg/dL; Ca 8.4 mg/dL; albumin 2.5 g/dL; blood urea nitrogen (BUN) 47.4 mg/dL; creatinine 4.75 mg/dL; aspartate aminotransferase 12 U/L; alanine aminotransferase, 7 U/L; and HbA1c 5.7%. CRP levels were decreased but still slightly elevated (1.87 mg/dL). Computed tomography (CT) showed an increased attenuation of fatty tissue around the PD catheter from the area of the second cuff to the exit site.
We initially suspected bacterial ESI and tunnel infection according to the clinical manifestations and CT findings [9]. We performed gram staining and culture of the pus and PD fluid. Gram staining of the pus revealed Gram-positive bacilli. Culture of the PD fluid was negative. Although cefazolin had been administered since admission, the amount of pus was markedly increased and included a small amount of blood on day 3. It seemed that cefazolin was not an effective antibiotic against the infection. On day 4, we performed acid-fast staining, which was positive. According to the bacterial growth rate, we considered RGNTM. We initiated combination antibiotic therapy using amikacin, imipenem, and clarithromycin on day 4. M. abscessus was isolated from the pus culture on day 8 by using DNA–DNA hybridization (DDH Mycobacterium “KYOKUTO,” KYOKUTO PHARMACEUTICAL INDUSTRIAL CO., LTD., Tokyo, Japan).
We consulted urologists in our hospital and decided to perform catheter removal surgery, because M. abscessus infections are usually difficult to treat and fatal. The operation was performed on day 14. During the operation, pus and inflammatory changes were unremarkable along the subcutaneous tunnel. Debridement and lavage using normal saline were also performed. Part of the infection-suspected skin was surgically removed. Incisions at the exit site and second cuff remained open wounds to be cleaned after the operation. Mycobacterium cultures of the tip of the catheter, first cuff, and second cuff were examined, and the results were negative, negative, and positive for M. abscessus, respectively. According to culture results, ESI and tunnel infection were more likely than peritonitis. After catheter removal surgery, the patient’s condition stabilized. On day 16, antibiotic sensitivity testing revealed that the M. abscessus isolated from the patient was resistant to almost all types of antibiotics, including isoniazid, rifampicin, streptomycin, enviomycin, ethionamide, ethambutol, cycloserine, and levofloxacin.
The patient needed to continue renal replacement therapy; however, PD was not recommended in this case considering the risk of recurrent infections, because M. abscessus is ubiquitous in natural environments such as soil and water [10]. The PD catheter was not replaced due to a high rate of PD catheter loss (80%) in PD patients with M. abscessuss infections [6]. We talked with the patient and his family and the patients decided on initiating hemodialysis. Arteriovenous graft surgery was performed on day 19. A few days after hemodialysis was initiated, the patient refused to continue dialysis due to severe boring pain. He also exhibited a depressive mood. Finally, the patient and his family decided not to continue dialysis even when the symptoms of uremia appeared. Imipenem was switched to faropenem, and amikacin was discontinued on day 29 due to respect for the patient’s opinion. Then, we continued to administer faropenem and clarithromycin until day 47. He was discharged on day 48, and we arranged a primary care physician to provide the best supportive care for the patient.
Discussion
Mycobacterium abscessus is an RGNTM categorized in Runyon Group IV, which includes M. fortuitum and M. chelonae [11]. These organisms require only 3–7 days to grow. M. abscessus was first isolated from a knee abscess by Moore et al. [12]. It was named after its ability to produce deep subcutaneous abscesses. M. abscessus and M. chelonae were initially considered to belong to the same group; however, M. abscessus was reclassified as an individual species in 1992 [13]. It commonly causes skin infections, soft tissue infections, and respiratory infections, but it can also cause various infections in almost all human organs [14].
Song et al. [15] reviewed 57 cases of PD-associated NTM peritonitis. Among them, the most prevalent organism was M. fortuitum (38.6%), followed by M. chelonae (14.0%). The incidence of M. abscessus was relatively low (8%). The PD catheter needed to be removed in 52 (92.9%) of 56 cases. Renaud et al. [6] reported that ESIs occurring due to RGNTMs constituted 3% of all ESIs. Infections due to M. abscessus are refractory, because this organism is intrinsically resistant to currently available anti-tuberculous drugs and antibiotics. Drugs recommended for the treatment of M. abscessus infections are a combination therapy of clarithromycin with one aminoglycoside and one other injectable drug such as cefoxitin or imipenem [16]. Macrolides are the only active oral antibiotic against M. abscessus [17].
In ESRD patients, uremia is associated with immune dysfunction of both the innate and adaptive immune systems alternately, leading to a high infection rate in patients with ESRD [18]. In PD patients, impaired maturation of helper T-cells has been seen, which might be the cause of susceptibility to infection [19]. In our case, the patient was old (aged 89 years) and had diabetes without medication; in addition, he was on CAPD. These could be the causes for the susceptibility to NTM infections. Bacitracin and fradiomycin ointment use might be a risk factor for NTM infections in this case, considering that a retrospective observational study comparing the incidence of NTM infections between patients receiving prophylactic gentamicin cream (2.71%) and those receiving alternative exit-site care (0.102%) reported that gentamicin cream might be a risk factor for atypical mycobacterial infection because of selective pressure on other micro-organisms [20]. Conversely, other double-blind randomized controlled trials have demonstrated no statistical difference between topical mupirocin and gentamicin cream [21]. Further clinical researches will be needed to provide a better exit-site care for preventing NTM infections.
In this study, we concluded that our case was limited to tunnel infection, according to the results of PD fluid analysis. The patient did not have either a fever or abdominal pain, which was compatible with the diagnosis of tunnel infection [22, 23]. If there has been a delay in diagnosis, the infection would have progressed to peritonitis, and treatment would have been difficult. We identified nine cases of ESIs and/or tunnel infections due to M. abscessus [6, 20, 24, 25] in the literature (Table 1). Our patient was the oldest, with the shortest duration of PD among patients with ESI due to M. abscessus. Making an accurate diagnosis of PD-associated catheter infection due to NTM is challenging. Maeda et al. reported a case of PD-associated peritonitis due to M. abscessus [26]. In that case, M. abscessus was isolated after 2 years of recurrent ESIs. In patients with refractory or recurrent PD-associated ESI and peritonitis, NTMs should be considered as causative organisms.
Acid-fast staining is not routinely performed in patients with PD-associated catheter infections in our faculty, but it was a key diagnostic examination in this case. We should consider NTM infections in PD patients. For an early diagnosis of NTM infections, acid-fast staining is highly recommended, because it can quickly evaluate the existence of NTM. After making a diagnosis, immediate catheter removal was effective in our case. Although the patient’s refusal for hemodialysis was an unexpected event, other renal replacement therapies and the consequences of PD catheter removal should also be considered.
References
Akoh JA. Peritoneal dialysis associated infections: an update on diagnosis and management. World J Nephrol. 2012;1:106–22.
Van Diepen AT, Tomlinson GA, Jassal SV. The association between exit-site infection and subsequent peritonitis among peritoneal dialysis patients. Clin Am Soc Nephrol. 2012;8:1266–71.
Band JD, Ward JI, Fraser DW, Peterson NJ, Silcox VA, Good RC, et al. Peritonitis due to mycobacterium chelonei-like organism associated with intermittent chronic peritoneal dialysis. J Infect Dis. 1982;145:9–17.
Jarzembowski JA, Young MB. Nontuberculous mycobacterial infections. Arch Pathol Lab Med. 2008;8:1333–41.
Jeon K, Kwon OJ, Lee NY, Kim BJ, Kook YH, Lee SH, et al. Antibiotic treatment of Mycobacterium abscessus lung disease: a retrospective analysis of 65 patients. Am J Respir Crit Care Med. 2009;9:896–902.
Renaud CJ, Subramanian S, Tambyah PA, Lee EJ. The clinical course of rapidly growing nontuberculous mycobacterial peritoneal dialysis infections in Asians: a case series and literature review. Nephrology (Carlton). 2011;2:174–9.
Yang TK, Lee JJ, Lu PL, Kuo HT, Kuo MC, Chen C. Peritoneal dialysis-associated peritonitis caused by Mycobacterium abscessus. Perit Dial Int. 2015;3:369–71.
Kofteridis DP, Valachis A, Perakis K, Maraki S, Daphnis E, Samonis G. Peritoneal dialysis-associated peritonitis: clinical features and predictors of outcome. Int J Infect Dis. 2010;6:e489–93.
Nozaki K, Kamijo Y, Nakatsuka M, Azuma T, Nakagawa T, Miyazaki H, et al. Computed tomography for the management of exit-site and tunnel infections in peritoneal dialysis patients. Clin Nephrol. 2016;12:328–32.
Brown-Elliott BA, Wallance RJ Jr. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev. 2002;4:716–46.
Ingram CW, Tanner DC, Durack DT, Kernodle GW Jr, Corey GR. Disseminated infection with rapidly growing mucobacteria. Clin Infect Dis. 1993;4:463–71.
Moore M, Frerichs JB. An unusual acid-fast infection of the knee with subcutaneous, abscess-like lesions of the gluteal region; report of a case with a study of the organism, Mycobacterium abscessus, n. sp. J Invest Dermatol. 1953;2:133–69.
Lee MR, Sheng WH, Hung CC, Yu CJ, Lee LN, Hsueh PR. Mycobacterium abscessus complex infections in human. Emerg Infc Dis. 2015;9:1638–46.
Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. Mycobacterium abscessus: a new antibiotic nightmare. J Antimicrob Chemother. 2012;4:810–8.
Song Y, Wu J, Yan H, Chen J. Peritoneal dialysis-associated nontuberculous mycobacterium peritonitis: a systematic review of reported cases. Nephrol Doal Transplant. 2012;4:1639–44.
Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaeo A, Daley C, Holland SM, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial disease. Am J Respir Crit Care Med. 2007;4:367–416.
Choi GE, Shin SJ, Won CJ, Oh T, Hahn MY, Lee K, et al. Macrolide treatment for Mycobacterium abscessus and Mycobacterium massiliense infection and inducible resistance. Am J Respir Crit Care Med. 2012;9:917–25.
Kato S, Chmielewski M, Honda H, Pecoitis-Fiho R, Matsuo S, Yuzawa Y, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;5:1526–33.
Ando M, Shibuya A, Yasuda M, Azuma N, Tsuchiya K, Akiba T, et al. Impairment of innate cellular response to in vitro stimuli in patients on continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant. 2005;11:2497–503.
Lo MW, Mak SK, Wong YY, Lo KC, Chan SF, Tong GM, et al. Atypical mycobacterial exit-site infection and peritonitis in peritoneal dialysis patients on prophylactic exit-site gentamicin cream. Perit Doal Int. 2013;3:267–72.
Bemardini KJ, Bender F, Florio T, Sloand J, Palmmontalbano L, Fried L, et al. Randomizes, double-blined trial of antibiotic exit site cream for prevention of exit site infection in peritoneal dialysis patients. J Am Soc Nephrol. 2005;2:539–45.
Szeto CC, Li PK, Johnson DW, Bernardini J, Dong J, Figueiredo AE, et al. ISPD catheter-related infection recommendation: 2017 Update. Perit Dial Int. 2017;2:141–54.
Li PK, Szeto CC, Piraino B, de Arteaga J, Fan S, Figueiredo AE, et al. ISPD Peritonitis recommendations: 2016 update on prevention and treatment. Perit Dial Int. 2016;5:481–508.
Kleinpeter MA, Krene NK. Treatment of mycobacterial exit-site infections in patients on continuous ambulatory peritoneal dialysis. Adv Perit Dial. 2001;17:172–5.
Ellis EN, Schutze GE, Wheeler JG. Nontuberculous mycobacterial exit-site infection and abscess in a peritoneal dialysis patient. A case report and review of the literature. Pediatr Nephrol. 2005;7:1016–8.
Maeda Y, Uno T, Yoshida A, Takahashi A, Inabe N, Shiigai T. Nontuberculous mycobacterial peritonitis in a patient undergoing continuous ambulatory peritoneal dialysis. J Rural Med. 2009;2:75–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest associated with this manuscript.
Human and animal rights
The article does not contain any studies involving human or animal participants.
Informed consent
Informed consent was obtained from the patient’s family due to the patient’s ineligibility in the present study.
About this article
Cite this article
Hibi, A., Kasugai, T., Kamiya, K. et al. Peritoneal dialysis-associated catheter infection caused by Mycobacterium abscessus in an elderly patient who was successfully treated with catheter removal. CEN Case Rep 6, 175–179 (2017). https://doi.org/10.1007/s13730-017-0270-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13730-017-0270-5