Abstract
Improvements in the exit-site care for peritoneal dialysis (PD) patients have uncovered a trend for increasing incidence of rapidly growing nontuberculous mycobacterium exit-site infections (ESI). Among these, Mycobacterium abscessus is unique in terms of its high morbidity and treatment failure rates. The international society of PD guidelines encourage PD catheter removal in patients with M. abscessus peritonitis but, do not have evidence-based recommendations for the management of ESIs related to this organism. We report an unusual case in which an asymptomatic end-stage renal disease patient with multiple favorable clinical characteristics, i.e., no apparent immunodeficiency, sensitivity pattern showing possibility of treatment with multiple antibiotics, no evidence of peritonitis, and early clinical response, was treated with a 9-month combination antimicrobial regimen administered orally and intraperitoneally. Despite excellent clinical response with a resolution of the ESI, our patient relapsed quickly, within 30 days of stopping antimicrobial therapy and required PD catheter removal. Our case, taken together with available published case reports, highlights the futility of the conservative approach towards the M. abscessus ESI and makes the cases for early PD catheter removal in these patients.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Infectious complications are among the leading causes of peritoneal dialysis (PD) failure in persons with end-stage renal disease (ESRD) [1]. A majority of these PD failures are associated with PD peritonitis. Catheter loss due to pure exit-site infection (ESI) in a stable, immunocompetent patient with ESRD is uncommon. Either Gram-positive or Gram-negative organisms cause most ESIs, and routinely prescribed topical creams and systemic antimicrobial agents are highly effective in the prevention and treatment of ESI [2, 3]. Current guidelines recommend evaluation for atypical organisms in ESIs either refractory to, or slowly responding to the conventional therapy.
Mycobacterium abscessus (M. abscessus) complex is a rapidly growing group of multidrug-resistant, nontuberculous mycobacteria (NTM) that are commonly found in soil and water supplies and are responsible for increasing occurrences of infections in the skin and soft tissues, respiratory tract, bloodstream, and central nervous system [4]. ESIs caused by NTM and M. abscessus, in particular, are relatively uncommon in otherwise immunocompetent PD patients, though a few recent reports have highlighted a growing problem [5]. Published reports have demonstrated that M. abscessus ESIs are challenging and difficult to treat, though successful outcomes have been reported with extended courses of antimicrobial therapy [6, 7].
We report an unusual and insightful case of M. abscessus ESI in an individual who did not acquire peritonitis, was otherwise immunocompetent, and showed an early and sustained response to an extended course of combined intraperitoneal and oral antimicrobial therapy. Despite the apparent clinical resolution, the patient relapsed quickly after discontinuation of treatment and required PD catheter removal.
Case report
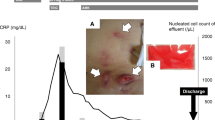
A 55-year-old male engineer with a history of ESRD secondary to IgA nephropathy, on PD for 12 months, presented for his routine PD clinic visit with a granulating ulcer in the circumferential area at the PD exit site with mild purulent discharge. The patient had an independent lifestyle with extended duration excursions at sea in a personal sailboat. He used gentamicin cream for exit-site care and was treated for an episode of culture-negative PD peritonitis with intraperitoneal antibiotics (vancomycin and cefepime) approximately a month before his current presentation. Physical examination revealed stable hemodynamics and no signs of peritonitis. There was a granulating, circumferential ulcer at the catheter exit site without any swelling, induration, erythema, or tenderness along the tunnel (Fig. 1). Pertinent laboratory findings included PD fluid white blood cells (WBC) count of 8/mm3, blood worked showing WBC count of 7.1 (reference range 4.0–10.0/mm3), ESR of 19 mm/h (reference range 0–10 mm/h), and C-reactive protein of 0.1 (reference range 0.1–0.4 mg/dl). A possible ESI was diagnosed, and antimicrobial therapy was initiated with oral ciprofloxacin after specimens from the exit site and PD fluid was sent for bacterial, fungal, and mycobacterial culture. During the subsequent weeks, PD fluid cultures remained negative for any kind of growth, and exit-site cultures yielded growth of M. abscessus.
Based on in vitro antimicrobial susceptibility test results and following consultation with the Infectious Disease Service, it was determined that the patient did not require debridement. He was commenced on oral azithromycin (250 mg daily), and intraperitoneal administration of amikacin (bolus dose: 240 mg daily) and imipenem (continuous dose: 50 mg/l on cycler with additional 250 mg/liter with the last dwell). PD catheter removal was discussed but deferred given clinical stability for 2 months, and to accommodate the patient’s social needs and outdoor lifestyle. Amikacin had to be discontinued after 3 weeks for ototoxicity, despite a close plasma monitoring and maintenance of the levels within the acceptable therapeutic range.
The exit site showed significant early improvements in swelling, erythema, and induration at the monthly follow-up visits during the first 2 months, and blood work did not show concerns for worsening infection; PD fluid cell count of 3 WBC/mm3, ESR of 12 mm/h and CRP of 0.1 mg/dl. Favorable progression and consultations with clinicians at the National Institutes of Health (NIH) prompted collaborative decision-making between the patient and the providers to continue oral azithromycin and intraperitoneal imipenem for a protracted period under close clinical monitoring. Over the ensuing months, the exit site continued to show progressive improvements with resolution of the erythema, induration, swelling, and gradual decrease in the size of the granulating ulcer. Antimicrobial therapy was discontinued after 9 months with circumferential healing of the exit-site ulcer having been established for two consecutive months.
The patient suffered a quick relapse of the ESI within a month of antimicrobial discontinuation with expanding erythema and exit-site discharge (ESD). Repeat exit-site cultures were positive for the growth of M. abscessus. At the same time, the simultaneously examined PD fluid yielded Paenibacillus species (a Gram-variable Bacillus found in soil and often associated with plant roots). Other pertinent labs include ESR 51 mm/h and CRP of 0.4 mg/dl. The PD catheter was promptly removed along with surgical debridement of the exit site and catheter tunnel, and the patient was switched to hemodialysis. A complete and lasting resolution of the infection was achieved after an additional 4 months of antimicrobial therapy with intravenous tigecycline (12.5 mg daily), meropenem (1.25 g daily) and oral azithromycin (250 mg daily). The timeline and course of the ESI infection have been depicted in Fig. 2.
Discussion
Routine use of effective topical antimicrobial agents have substantially reduced ESI caused by Gram-positive and Gram-negative microorganisms [8]. The current International Society for Peritoneal Dialysis (ISPD) guidelines suggest catheter removal in ESIs that are refractory to conventional therapy or caused by atypical microorganisms, such as fungi. ESIs caused by NTM, such as M. abscessus, are uncommon, and when they do occur, they often follow a protracted course and are refractory to treatment. We lack a uniform, evidence-based guidelines for the management of such NTM ESIs, and current ISPD guidelines recommend individualizing therapeutic options for each patient [2]. The patient in our report acquired a PD catheter ESI caused by a relatively resistant strain of M. abscessus. Because he remained completely free of constitutional symptoms (fever, chills, rigors, night sweats, and weight loss), did not acquire peritonitis or catheter tunnel involvement, our initial therapeutic response was conservative: robust anti-mycobacterial therapy with the catheter left in situ. Relapse occurred following a period where there was ostensibly complete healing, which led to prompt removal of the catheter.
NTM are classified into Runyon groups I to IV based on the rate of growth, production of yellow pigment in the dark, and on exposure to light. PD catheter ESI due to NTM predominantly involves the Runyon class IV rapidly growing mycobacteria and includes organisms such as M. fortuitum, M. abscessus, and M. chelonae. M. abscessus often appear as Gram-positive rods that resemble diphtheroid and are often mistaken as contaminants [6, 9, 10]. It is a ubiquitous environmental organism found in water and typically causes skin, soft tissue, catheter-related, and lung infections [4]. Among these NTM, M. abscessus is especially conspicuous since it is intrinsically resistant to many of the anti-mycobacterial agents currently available in clinical practice and can cause protracted courses of therapy or, in some cases, non-resolving infections [9]. Recurrent ESI, peritonitis treated with broad-spectrum antibiotics and immunosuppression are often predisposing factors for ESI infection caused by NTM [4]. Warm weather and humidity are favorable conditions for developing these infections [11]. Recent reports have suggested that routine care of ESI with topical agents may be associated with an increase in the proportions of ESI related to atypical organisms, including NTM infections [12]. Our patient with an active lifestyle (sailing) in favorable weather conditions and the use of topical antibiotics could have played a role, but no definitive source of infection could be found.
Few investigators have recently chronicled their experience with PD-associated M. abscessus infections [6, 7, 13]. In a recent mini review, Yoshimura et al. compiled a total of 28 reported cases of M. abscessus-associated PD infections, including peritonitis and ESI [5]. The majority of the patients required conversion to HD and surgical debridement of the wound infection. Only 4 cases of attempted PD catheter salvage with antimicrobial therapy have been reported to date with M. abscessus ESI. Among these, 2 died with infectious complications of failed treatment, and 2 were reported as successful eradication of the M. abscessus ESI or tunnel infection (TI). However, one of these cases was treated for only 2 months, raising concerns for true infection vs. colonization [6, 7, 11]. Based on the experience published in the available medical literature, we initially recommended that our patient undergo PD catheter removal at the time of ESI diagnosis. However, his social needs and lifestyle necessitated conservative management, and, indeed, he showed excellent early clinical response to treatment. The Infectious Diseases Society of America (IDSA) guidelines recommend a susceptibility-based combination antimicrobial therapeutic regimen for serious M. abscessus infections. The therapeutic regimens usually combine clarithromycin or azithromycin with an additional parenteral drug such as amikacin and cefoxitin, for a period of 4–6 months for severe infections [14]. We managed our patient with a prolonged oral and intraperitoneal regimen with susceptibility-guided antimicrobial agents for a total duration of 9 months to achieve the clinical resolution of the ESI. Despite this robust approach, the patient developed a quick relapse of M. abscessus ESI on discontinuation of the therapy and developed concurrent peritonitis with a different organism.
When considered together with the prior published reports, our report underscores several important points. First, among the various NTM species that cause clinical infection in humans, M. abscessus remains among the most difficult to treat because of its well-recognized underlying virulence and resistance to available anti-mycobacterial agents. Second, a conservative approach towards the management of M. abscessus ESI is ineffective, and early PD catheter removal is likely more appropriate for these patients, as there remains a possibility of residual infection capable of causing relapse and failure of therapy even among those clinically responding to the antimicrobials. Additionally, prior reports have shown that M. abscessus ESI/TI can spread through the para-catheter route to cause peritonitis. Ono et al. reported that 60% of ESI with M. abscessus eventually progressed to M. abscessus peritonitis [15]. Though our patient did not develop M. abscessus peritonitis, the high morbidity and mortality associated with peritonitis caused by M. abscessus or secondary fungal infection should serve to deter the maintenance of clinical state favorable to such spread. Third, the course of therapy with anti-mycobacterial agents remains prolonged even with catheter removal, and patients need close follow-up with careful examination of exit site and tunnel areas and ascertainment of peritonitis. While one may argue for a simultaneous PD catheter removal and reinsertion in these PD catheter-associated ESI or TI, the available literature does not support this approach. In our review of the literature, we found a total of 3 previously published case reports of M. abscessus ESI or TI without peritonitis wherein PD catheter was simultaneously removed and reinserted. All patients developed peritonitis with such an approach [4, 5, 16]. Thus, in our patient, we abandoned this approach in favor of catheter removal. Finally, the choice of drugs is mandated by susceptibility testing and may require combinations that have not been evaluated or tested (in our case, we sought the advice of researchers at NIH).
To conclude, we believe that the ESI/TI with M. abscessus are associated with poor outcomes with conservative management. We suggest early consideration of PD catheter removal with at least temporary transfer to hemodialysis, without simultaneous PD catheter reinsertion. Considering the rising trends for the NTM ESI, we believe that these considerations should shape the next iterations of the ISPD infection guidelines.
References
Shen JI, Mitani AA, Saxena AB, Goldstein BA, Winkelmayer WC. Determinants of peritoneal dialysis technique failure in incident US patients. Perit Dial Int. 2013;33(2):155–66. https://doi.org/10.3747/pdi.2011.00233.
Szeto CC, Li PKT, Johnson DW, Bernardini J, Dong J, Figueiredo AE, et al. ISPD catheter-related infection recommendations: 2017 update. Perit Dial Int. 2017;37(2):141–54. https://doi.org/10.3747/pdi.2016.00120.
Singh H, Saha T. Exit-site infection due to nontubercular Mycobacteria in an immunocompromised peritoneal dialysis patient. Dialysis & Transplantation. 2008;37(10):401–9. https://doi.org/10.1002/dat.20225.
Mooren V, Bleeker MWP, van Ingen J, Hermans MHA, Wever PC. Disseminated Mycobacterium abscessus infection in a peritoneal dialysis patient. IDCases. 2017;9:6–7. https://doi.org/10.1016/j.idcr.2017.05.001.
Yoshimura R, Kawanishi M, Fujii S, Yamauchi A, Takase K, Yoshikane K, et al. Peritoneal dialysis-associated infection caused by Mycobacterium abscessus: a case report. BMC Nephrol. 2018;19(1):341. https://doi.org/10.1186/s12882-018-1148-2.
Renaud CJ, Subramanian S, Tambyah PA, Lee EJ. The clinical course of rapidly growing nontuberculous Mycobacterial peritoneal dialysis infections in Asians: a case series and literature review. Nephrology (Carlton). 2011;16(2):174–9. https://doi.org/10.1111/j.1440-1797.2010.01370.x.
Lo MW, Mak SK, Wong YY, Lo KC, Chan SF, Tong GM, et al. Atypical Mycobacterial exit-site infection and peritonitis in peritoneal dialysis patients on prophylactic exit-site gentamicin cream. Perit Dial Int. 2013;33(3):267–72. https://doi.org/10.3747/pdi.2011.00184.
Bernardini J, Bender F, Florio T, Sloand J, Palmmontalbano L, Fried L, et al. Randomized, double-blind trial of antibiotic exit site cream for prevention of exit site infection in peritoneal dialysis patients. J Am Soc Nephrol. 2005;16(2):539–45. https://doi.org/10.1681/ASN.2004090773.
Petrini B. Mycobacterium abscessus: an emerging rapid-growing potential pathogen. APMIS. 2006;114(5):319–28. https://doi.org/10.1111/j.1600-0463.2006.apm_390.x.
Macheras E, Roux A-L, Bastian S, Leão SC, Palaci M, Sivadon-Tardy V, et al. Multilocus sequence analysis and rpoB sequencing of Mycobacterium abscessus (sensu lato) strains. J Clin Microbiol. 2011;49(2):491–9. https://doi.org/10.1128/JCM.01274-10.
Tsai S-F. Catheter related infection due to Mycobacterium abscessus in a patient under peritoneal dialysis. Therapeutic Apheresis and Dialysis. 2013;17(3):349–50. https://doi.org/10.1111/1744-9987.12005.
Tse KC, Lui SL, Cheng VC, Yip TP, Lo WK. A cluster of rapidly growing Mycobacterial peritoneal dialysis catheter exit-site infections. Am J Kidney Dis. 2007;50(1):e1–5. https://doi.org/10.1053/j.ajkd.2007.04.017.
Jiang SH, Roberts DM, Clayton PA, Jardine M. Non-tuberculous Mycobacterial PD peritonitis in Australia. Int Urol Nephrol. 2013;45(5):1423–8. https://doi.org/10.1007/s11255-012-0328-4.
Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous Mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. https://doi.org/10.1164/rccm.200604-571ST.
Ono E, Uchino E, Mori KP, Yokoi H, Toda N, Koga K, et al. Peritonitis due to Mycobacterium abscessus in peritoneal dialysis patients: case presentation and mini-review. Ren Replace Ther. 2018;4(1):52. https://doi.org/10.1186/s41100-018-0192-5.
Kameyama H, Mori Y, Kimura T, Sugishita C, Adachi T, Sonomura K, et al. A case report of Mycobacterium abscessus peritonitis in a peritoneal dialysis patient. Ther Apher Dial. 2007;11(6):449–51. https://doi.org/10.1111/j.1744-9987.2007.00526.x.
Acknowledgements
We would like to thank Dr. Kevin Fennelly (Senior Research Clinician, Laboratory of Chronic Airway Infection, Pulmonary Branch, Division of Intramural Research. National Institutes of Health) for his input on M. abscessus infection management.
Funding
A. M. Shukla reports the ongoing grant support from the Department of Veterans Affairs. The grant support is unrelated to and has no conflicts with the work published here.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest exists.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individuals participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Chamarthi, G., Kamboj, M., Archibald, L.K. et al. Mycobacterium abscessus exit-site infection in peritoneal dialysis patients: should we ever aim to salvage the catheter?. CEN Case Rep 10, 12–16 (2021). https://doi.org/10.1007/s13730-020-00506-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13730-020-00506-5