Abstract
Self-cross linked poly(4,4′-diaminodiphenylmethane) was prepared by an oxidative polymerization. Also a molecular imprinted polymer was prepared by employing 4,4′-diaminodiphenylmethane as the functional monomer, ammonium persulfate and dibenzothiophene as the oxidizing agent and template, respectively. It is suggested that the π–π stacking effect between 4,4′-methylenedianiline and dibenzothiophene induced the compact imprinted polymer and the formation of more imprinted sites. Field emission scanning electron microscopy, X-ray diffraction, differential scanning calorimetry and FTIR spectroscopy were used for the characterization of the synthesized polymers. The removal of template molecules leads to the preparation of molecularly imprinted polymer (MIP), which was subsequently used as an electrode material for the preparation of an electrochemical sensor for the detection of dibenzothiophene. Electrochemical behavior of dibenzothiophene on the sensors' construction based upon the application of imprinted (MIP) and non-imprinted (NIP) polymers was investigated in 0.1 mol L−1 of LiClO4 (as the supporting electrolyte), by means of cyclic voltammetry. The obtained cyclic voltammograms of the MIP electrode showed one irreversible anodic peak at ~ 1.5 V. This peak was attributed to the electrochemical oxidation of dibenzothiophene to dibenzothiophene sulfoxide. The current variation acquired through the developed imprinted sensors as a function of dibenzothiophene concentration was linear in the concentration range of 60–150 mg L−1. The corresponding limit of detection was calculated as 11 mg L−1. The imprinted sensor showed a significantly improved sensitivity towards the investigated analyte rather than that observed using a non-imprinted polymer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sulfur-containing compounds of fuels are considered as the major sources of environmental hazards such as acid rain and health problems [1]. Consequently, desulfurization of fuels becomes an urgent task. Thiophene and its derivatives are among the main sulfur compounds in petro-fuels with the boiling points higher than 100 °C. These compounds contain usually up to 50–80% of the total sulfur content in diesel. It is estimated that the commercial gasoline and diesel contain 300–500 mg kg−1 sulfur compounds, which depend on the oil source and refinery process [2,3,4]. Different methods have been developed for the selective measurement of the sulfur compounds [5,6,7]. Aromatic sulfur compounds are the main materials which cannot be quantitatively removed by the hydrodesulfurization process. Determination of the concentration of thiophene and its derivatives over the other aromatic and olefin compounds present in the hydrocarbon fuel in different kinds of diesel oils can be used for improving the methods used for desulfurization.
Dibenzothiophene (DBT) and their isomers can be identified by gas chromatography–flame photometric detection, gas chromatography–mass spectrometry [8], X-ray fluorescence spectrometry (XRF) [9], and electrochemical methods [10]. The general application of electrochemical methods for the detection of DBT comprises of polarography with mercury electrodes [11]. High-cost skilled analytical methods such as GC and GC–MS, and toxicity of mercury limit the usage of mercury electrodes in the analytical practice, so the application of these methods is limited. Therefore, the development of new sensing systems for the detection of DBT remains a necessity.
Molecularly imprinted polymer (MIP) technology is a method which offers the possibility to develop synthetic receptors for target molecules [12]. This method has been used in various applications such as chemical sensors [13]. Particular advantages of the MIP-based sensors are their low production cost and their expanded area of function under various environmental conditions with respect to temperature, pH, ionic strength, and complex matrices. Molecular imprinting of conductive polymer is generally used for the production of electrochemical sensors [14]. These polymers are able to bind target molecules with various size, shape, and chemical functionalities. By extraction of the target molecule (i.e., imprinting step) they will be acting as a selective host for the analyte.
Polyaniline and its derivatives are conductive polymers which have been employed in the construction of sensors [15]. 4,4′-Methylenedianiline (MDA), known as diaminodiphenyl methane, is an aromatic amine that has been used as a raw material or intermediate for the synthesis of industrial chemicals. It is also well known as a curing agent for resins [16, 17].
Oxidative polymerization has been reported as a powerful technique for the design, synthesis, and architecture of novel multifunctional polymers from several types of aromatic diamines under facile reaction conditions [18,19,20,21]. This potential approach motivates us to apply this method for the preparation of poly(4,4′-methylenedianiline) (PMDA). This study also bears the results obtained by the application of the imprinted polymer poly(4,4′-methylenedianiline) (MIP/PMDA) as a modifier for fabricating an electrochemical sensor for the detection of DBT.
Experimental
Materials
MDA functional monomer and ammonium persulfate (NH4)2S2O8 were purchased from Sigma-Aldrich (Darmstadt, Germany). Acetonitrile and DBT were purchased from Sigma-Aldrich (Mumbai, India). All other chemicals were of analytical grade, commercially available and used without further purification.
Instruments
Cyclic voltammograms were recorded on a Metrohm voltammeter (model 797 VA computrace, Switzerland) using a three-electrode system. Saturated calomel electrode (SCE) and a platinum wire were used as reference and counter electrodes, respectively. Fourier transform infrared spectra were recorded on a FTIR instrument (Brucker-Vector 22 spectrophotometer, Germany). Scanning electron microscope (MIRA3TESCAN, USA), X-ray diffractometer (Bruker D8 Advance, Germany) and differential scanning calorimeter (DSC) (Mettler FP90) were used to study the properties of PMDA.
Synthesis of DBT-imprinted (MIP) and non-imprinted (NIP) polymers
The MIP/PMDA was prepared by the following method. Half a gram MDA and 0.1 g DBT were first dissolved in 42 mL acetonitrile/water (70/30 v/v%), by stirring the solution magnetically for 10 min. To this solution 1.2 g of ammonium persulfate was added as the oxidant. The obtained mixtures were ultrasonically irradiated for 30 min at 0 °C. Finally, the mixtures were filtered and washed with water and acetonitrile. Thereby the PMDA polymer was obtained, which was inserted with the DBT templates. The obtained polymer was washed with water and acetonitrile. For the removal of templates, the obtained product was washed three times, with 10 mL acetonitrile under ultrasonic irradiation for 90 min. The non-imprinted polymer (NIP) was also prepared by following the same polymerization process except elimination of the template during the applied process.
Fabrication of modified electrode
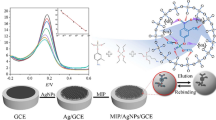
For the preparation of modified carbon paste electrode, 0.020 g graphite powder and 0.005 g MIP/PMDA were mixed. Then, 0.010 g liquid paraffin was added to this mixture and the mixture was further mixed until a homogeneous paste was formed. This paste was then used for the fabrication of the modified carbon paste working electrode.
Electrochemical characterization and measurement
A solution of LiClO4 (0.1 mol L−1) in acetonitrile was used as the electrolyte. All the electrochemical experiments were conducted by scanning the potential with 100 mVs−1 at room temperature.
Results and discussion
The monomer MDA possesses two functional aniline groups. The aniline moieties can primarily be polymerized to form an organic conductive polymer. Additionally, in the polymerization of MDA, the network structure of the polymer is produced in the absence of a cross-linker. The lack of cross-linker in the production of the network structure is an advantage for the molecular imprinting of PMDA. The schematic representation of the fabrication process for the polymerization of MDA and molecular imprinting of PMDA is shown in Scheme 1.
During the process of self-assembly of MDA, DBT with aromatic rings can be entrapped by the polymer through π–π interactions. The π–π stacking interaction, a type of non-covalent interaction, refers to interactions involving aromatic groups containing π bonds. The MDA with high π-electron density groups and aromatic rings would increase π–π interaction forces and DBT loading capacity. Although, it is worth noting that, other non-covalent mechanisms can also contribute as driving forces.
Structural characterization
The molecular structures of DBT molecularly imprinted and non-imprinted PMDA (MIP and NIP) were characterized by FTIR spectroscopy (Fig. 1). The band observed at 3373.16 cm−1 is due to N–H stretching. The absorption bands at 2918.74 and 2850.27 cm−1 are due to asymmetric C–H stretching and symmetric C–H stretching vibrations. The absorption peaks observed at 1614.12 and 1512.07 cm−1 were attributed to the C = C stretching in the aromatic nuclei. The peaks observed at 1411, 1384, 1330, 1210, 1177, and 1078 cm−1 represent the branch-like structure of PMDA. Also, the peaks found at about 810, 858 and 1098 cm−1 indicate the in-plane C–H bending in the quinoid and benzenoid units and the out-of-plane C–H bending in the aromatic ring [22]. The absorption peaks of MIP/PMDA and NIP/PMDA do not show any significant difference for the MIP/PMDA and NIP/PMDA polymers.
The SEM microscope images of PMDA are displayed in Fig. 2. A type of spherical like particles with uniform distribution was revealed for PMDA. This type of uniform morphology can be interpreted by electrostatic interactions regarding the PMDA chain π − π stacking.
Spectrophotometric studies on the intermolecular interactions between the functional monomers and template prior to polymerization could be useful for the suggestion on the mechanism of binding the template and the imprinted polymer. Figure 3 shows the UV spectra of MDA, DBT and their mixture in the ratio of 2:1 with respect to MDA and DBT. As it is evident, in the mixture of MDA and DBT a new absorption peak with a relatively high absorptivity at 288 nm is seen. This can be considered as an indication for the presence of π–π interactions between these components (i.e. MDA and DBT) [18, 23,24,25]. This well-bonded interface interaction may also result in the reinforcement of the electrochemical performance of the nanocomposite as concluded in the electrochemical properties.
The X-ray diffraction pattern of the samples is illustrated in Fig. 4. This pattern shows a semi-crystalline nature with the peaks at 2θ 16°, 19° and 46°. These peaks are interpreted by considering a good molecular alignment through the chains created by the π − π stacking in the polymer.
The differential scanning calorimetry (DSC) analysis was applied to study the thermal transitions of PMDA (Fig. 5). In the DSC curve of PMDA, the peak was attributed to the glass transition temperature appearing at 112 °C, and the peak at 212 °C can be ascribed to the crystalline temperature of PMDA. This crystalline temperature is due to the presence of chain π–π stacking in polymer that verifies the crystalline morphology obtained by the XRD pattern. The PMDA indicated a peak at 257 °C relative to the decomposition of the polymer.
Cyclic voltammetry analysis
The electrochemical behavior of DBT in acetonitrile was investigated with modified NIP/PMDA and MIP/PMDA electrodes in 0.1 mol L−1 of LiClO4, by means of cyclic voltammetry in the potential range 0.0–2.0 V and the scan rate 100 mVs−1. The modified MIP/PMDA and NIP/PMDA electrodes in the absence of DBT did not show any peaks. As can be seen in Fig. 6, by addition of DBT (60 ppm) to the sample solution, the electrode modified by MIP/ PMDA gave a significant current as a chemically irreversible anodic peak at ~ 1.5 V. This peak can be attributed to the electrochemical oxidation of DBT to dibenzothiophene sulfoxide.
The CVs of various concentrations of DBT (i.e., 60, 100, 120 and 150 ppm) are given in Fig. 7. The experimental results from cyclic voltammetry studies demonstrated that MIP/PMDA electrode responds to the addition of different concentrations of DBT. The results indicate that the oxidation current increases linearly with the DBT concentration in the range 60–150 ppm. It is noteworthy that the corresponding correlation coefficient and limit of detection (LOD) were found to be 0.9913 and 11 mg L−1. The LOD was calculated using Eqs. 1 and 2:
where, Sm, sb, Cm and m are the signal of the limit of detection, average current of repeated determinations of blank solution, standard deviation of the blank, limit of detection and slope of the calibration curve, respectively. These values were evaluated by four replicate determinations of the blank signals.
To appraise the selective attitude and the developed sensor, the response of the sensor towards DBT in the presence of an organosulfur family benzothiophene (BT) with a slight difference in the molecular size and structure was followed. To this end, the MIP electrodes were immersed in the solutions containing 60 ppm of BT. The sensor did not present any observable peak current. In contrast, by addition of DBT (60 ppm) to the sample solution, the cyclic voltammogram showed an irreversible anodic peak (Fig. 8). This showed an excellent selectivity of the prepared sensor.
Conclusion
In this study, NIP/PMDA and MIP/PMDA were synthesized by an oxidative emulsion polymerization method and characterized by FTIR, XRD, SEM, EDX, DSC analyses. The FTIR spectra confirmed the successful formation of polymer. The XRD results showed a semi-crystalline structure for PMDA. This crystalline nature can be due to the chain π–π stacking in molecular alignment in PMDA. In the DSC curves, a crystalline temperature was detected for PMDA which proves the crystalline peak obtained from the XRD results. MIP/PMDA was used for the modification of carbon paste electrode to fabricate a selective DBT electrochemical sensor. The calibration curve for DBT determination was obtained by applying the investigated sensor using the working electrode in cyclic voltammetry. A suitable linear range and short time required for DBT analysis, good selectivity and accuracy, low cost and simple preparation process are among the advantages of the proposed sensor. It was shown that the fabricated electrode can be successfully used for determination of DBT.
References
Claxton LD (2015) The history, genotoxicity, and carcinogenicity of carbon-based fuels and their emissions: part 5. Summary, comparisons, and conclusions. Mutat Res-Rev Mutat 763:103–147
Mguni L, Yao Y, Liu X, Yuan Z, Hildebrandt D (2019) Ultra-deep desulfurization of both model and commercial diesel fuels by adsorption method. J Environ Eng Chem 7:102957
Millo F, Rafigh M, Andreata M, Vlachos T, Arya P, Miceli P (2017) Impact of high sulphur fuel and de-sulfation process on a close-coupled diesel oxidation catalyst and diesel particulate filter. Fuel 198:58–67
Zhang K, Hu J, Gao S, Liu Y, Huang X, Bao X (2010) Sulfur content of gasoline and diesel fuels in northern China. Energ Policy 38:2934–2940
Han Y, Zhang Y, Xu C, Hsu CS (2018) Molecular characterization of sulfur-containing compounds in petroleum. Fuel 221:144–158
Wei W, Zhang K, Qiao Z, Jiang J (2019) Functional Uio-66 for the removal of sulfur-containing compounds in gas and liquid mixtures: a molecular simulation study. Chem Eng J356:737–745
Djokic MR, Ristic ND, Olahova N, Marin GB, Van Geem KM (2017) Quantitative on-line analysis of sulfur compounds in complex hydrocarbon matrices. J Chromatogr A1509:102–113
Panda SK, Alawani NA, Lajami AR, Al-Qunaysi TA, Muller H (2019) Characterization of aromatic hydrocarbons and sulphur heterocycles in Saudi Arabian heavy crude oil by gel permeation chromatography and ultrahigh resolution mass spectrometry. Fuel 235:1420–1426
Marguí E, Resano M, Queralt I (2019) A sustainable and simple energy dispersive X-ray fluorescence method for sulphur determination at trace levels in biodiesel samples via formation of biodiesel spots on a suitable solid support. Spectrochim Acta B 156:7–12
Gebicki J (2016) Application of electrochemical sensors and sensor matrixes for measurement of odorous chemical compounds. Tracc-Trend Anal Chem 77:1–13
Da S, Domingos G, de Carvalho LM, MontoyaDomenech-Carbó NA (2017) Solid state electrochemical behavior of organosulfur compounds. J Electroanal Chem 806:180–190
Hillberg A, Brain K, Allender C (2005) Molecular imprinted polymer sensors: implications for therapeutics. Adv Drug Deliv Rev 57:1875–1889
Tokonami S, Shiigi H, Nagaoka T (2009) Review: micro- and nanosized molecularly imprinted polymers for high-throughput analytical applications. Anal Chim Acta 641:7–13
Naveen MH, Ganesh Gurudatt N, Shim YB (2017) Applications of conducting polymer composites to electrochemical sensors: a review. Appl Mater Today 9:419–433
Naseri M, Fotouhi L, Ehsani A (2018) Recent progress in the development of conducting polymer-based nanocomposites for electrochemical biosensors applications: a mini-review. Chem Rec 18:599–618
Nikkhah S, Tahermansouri H, Chekin F (2018) Synthesis, characterization, and electrochemical properties of the modified graphene oxide with 4,4′-methylenedianiline. Mater Lett 211:323–327
AbouEl-Enein SA, Emam SM, Polis MW, Emara EM (2015) Synthesis and characterization of some metal complexes derived from azo compound of 4, 4′-methylenedianiline and antipyrine: evaluation of their biological activity on some land snail species. J Mol Struct 1099:567–578
Li XG, Huang MR, Duan W, Yang YL (2002) Novel multifunctional polymers from aromatic diamines by oxidative polymerizations. Chem Rev 102:2925–3030
Huang MR, Peng QY, Li XG (2006) Rapid and effective adsorption of lead ions on fine poly(phenylenediamine) microparticles. Chem Eur J 12:4341–4350
Huang MR, Ding YB, Li XG, Liu Y, Xi K, Gao CL, Kumar RV (2014) Synthesis of semiconducting polymer microparticles as solid ionophore with abundant complexing sites for long-life Pb (II) sensors. ACS Appl Mater Inter 6:22096–22107
Li XG, Liu R, Huang MR (2005) Facile synthesis and highly reactive silver ion adsorption of novel microparticles of sulfodiphenylamine and diaminonaphthalene copolymers. Chem Mater 17:5411–5419
Dines TJ, MacGregor LD, Rochester CH (2001) The Surface acidity of oxides probed by IR spectroscopy of adsorbed diazines. Phys Chem Chem Phys 3:2676–2685
Li XG, Ma XL, Sun J, Huang MR (2009) Powerful reactive sorption of silver (I) and mercury (II) onto poly(o-phenylenediamine) microparticles. Langmuir 25:1675–1684
Huang MR, Lu HJ, Li XG (2012) Synthesis and strong heavy-metal ion sorption of copolymer microparticles from phenylenediamine and its sulfonate. J Mater Chem 22:17685–17699
Li XG, Wang HY, Huang MR (2007) Synthesis, film-forming, and electronic properties of o-phenylenediamine copolymers displaying an uncommon tricolor. Macromolecules 40:1489–1496
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohseni, E., Yaftian, M.R., Shayani-jam, H. et al. Molecularly imprinted poly(4,4′-methylenedianiline) for selective electrochemical detection of dibenzothiophene. Iran Polym J 29, 403–409 (2020). https://doi.org/10.1007/s13726-020-00804-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-020-00804-w