Abstract
A macroporous temperature-responsive poly(N,N-diethylacrylamide) (PDEA) hydrogel was synthesized and optimized through free radical polymerization. The optimized hydrogel was achieved by evaluating the swelling characteristics, physical stability and mechanical strength through altering the components namely concentration of N,N-diethylacrylamide (monomer), ammonium peroxodisulfate (initiator), N,N′-methylbisacrylamide (cross-linker) and N,N,N′,N′-tetramethylethylenediamine (accelerator). The equilibrium swelling behavior was performed gravimetrically, and the PDEA hydrogel synthesized at 36 °C exhibited a maximum swelling of 18.332 g.g−1. Also, the LCST of the prepared PDEA hydrogel was found to be around 29 °C. However, the results of time-controlled swelling and deswelling kinetics indicated that hydrogels are temperature sensitive. Further, characterization of the hydrogel was performed using scanning electron microscopy, differential scanning calorimetry, thermal gravimetric analysis, and Fourier transform infrared spectroscopy. The hydrogel was assessed for its cytotoxicity in MDA-MB-231 cell line by MTT assay. The release behavior of anticancer drug doxorubicin (DOX), a hydroxyl derivative of anthracycline, was studied at above and below the LCST temperature. It was found that the DOX release from the DOX-loaded hydrogels was significantly improved when the surrounding temperature of the release media was increased near to physiological temperature. The cumulative release profile of hydrogel at different temperatures was fitted to different kinetic model equations and non-Fickian diffusion release mechanism was revealed. These results suggest that PDEA has a potential application as an intelligent drug carrier.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrogels are 3D polymeric cross-linked networks comprising hydrophilic segments that are capable of absorbing an immense amount of water, biological fluid or drug [1]. The absorption capacity of a hydrogel is owing to the presence of hydrophilic pendant moieties such as -SO3H, -OH, -CONH2-COOH, -CONH-, etc., in the polymeric backbone [2, 3]. The 3D network of a hydrogel is constituted either by physical/reversible bonds or by covalent bonds. Suppose, the cross-links are fabricated by physical bonds, namely hydrogen, ionic, or van der Waal’s bonds, the response of hydrogel to the external stimuli is traditionally reversible [4, 5]. To retain the 3D structure of the hydrogel, polymer chains are physically or chemically cross-linked. Stimuli-responsive polymers (hydrogels) have received substantial attention by researchers due to their vast applications in major areas like medicine, biotechnology and electronics [6,7,8]. Different forms of hydrogels are used in a broad range of applications including: (a) pressed powder matrices (e.g., pills or capsules for oral, nasal, ocular, rectal, vaginal, epidermal and subcutaneous applications), (b) microparticles (e.g., as bio-adhesive carriers to insulin, as drug delivery vector in stomach, liver, colon, intestinal, brain, blood, nervous system, and as wound-dressing materials [9, 10] and tumor-targeted delivery vectors), (c) membranes or sheets (e.g., as a reservoir in a transdermal drug delivery of allergens in skin testing [11], as wound dressing material, and as 2D electrophoresis gels), (d) coatings (e.g., as implants or catheters [12], as pills or capsules, and as coatings on the inside capillary wall in capillary electrophoresis), (e) encapsulated solids (e.g., in osmotic pumps), (g) liquids (e.g., as temperature-maintaining mediums, as cellular regenerators, and in proliferation for growth of new extra cellular matrix [13]), and (f) solid molded forms (e.g., soft contact lenses [14]).
Stimuli-responsive polymers exhibit phase or volume transitions by trivial variations in environmental changes such as pH, temperature, light, ionic strength, electric and magnetic fields [15]. Among these, temperature-responsive polymers have gained much importance in the drug delivery domain due to the significant change in the human body temperature under various conditions [16, 17]. Much attention has been given towards the synthesis of temperature-responsive hydrogels, due to their specific properties like high water content, porous structure, response to the stimuli, controlled release of the loaded drug, biodegradability and biocompatibility [18, 19]. In the temperature-responsive hydrogels, temperature has a surprising effect on the hydrophobic and hydrophilic interactions between the water molecules and polymeric chains. Thus, minute variations in the temperature can interrupt the equilibrium in the hydrogel and induce sol–gel transition [2]. The limitations of thermo-responsive hydrogels include toxicity, carcinogenic nature, non-biodegradability and non-biocompatibility [12]. On the other hand, high water holding capacity and larger pore size of thermo-responsive hydrogels often result in a relatively rapid drug release [20]. The above-mentioned drawbacks of temperature-responsive hydrogels can be overcome using natural polymers [21].
PDEA hydrogel has lower critical solution temperature (LCST) at about 25 °C–36 °C and exhibits phase separation in aqueous solution depending on the concentration of the polymer, tacticity and molecular weight [22, 23]. Below LCST, PDEA acts as a hydrophilic hydrogel and can form an extended coiled structure of polymer chains surrounded by ordered water molecules, whereas above LCST, polymer chains follow a sharp coil-to-globule transition leading to a hydrophobic aggregation that precipitates out of solution [23]. Many researchers have reported the increase in LCST of PDEA by grafting the polymers with natural polymers like pectin [24], chitosan [25, 26], by cross-linking with laponite clay [27] and also by polymerization with synthetic monomers such as N,N-dimethyl-α-(hydroxymethyl) acrylamide [28], polyacrylamide [29], and poly(vinyl alcohol) [23].
The motive of this study is to unveil the effects of process variables (monomer, cross-linker, initiator, temperature, etc.) on the preparation of stable PDEA hydrogel to achieve better swelling property. PDEA hydrogels were prepared by free radical polymerization using ammonium peroxodisulfate (APS) as initiator, N,N-diethylacrylamide (DEA) as monomer, and N,N′-methylenebisacrylamide (MBA) as the cross-linker. The process was optimized by varying DEA, MBA, APS and synthesis temperature. The synthesized hydrogels were characterized by FTIR, SEM, TGA and DSC. A model drug DOX was used to analyze the loading and release characteristics of the hydrogel. The release characteristics were studied at different temperatures like 20 °C, 30 °C, 37 °C and 40 °C. The release pattern of the model drug was analyzed by fitting the release data in different mathematical models.
Experimental
Materials
N,N-Diethylacrylamide (DEA, 98%) was procured from Tokyo Chemical Industry Co., Ltd. (TCI), Tokyo, Japan. Ammonium peroxodisulfate (APS, 98%) was purchased from Merck Specialities Pvt. Ltd., Mumbai, India. N,N′-Methylbisacrylamide (MBA, 99%) was procured from Loba Chemie, Mumbai, India. N,N,N′,N′-Tetramethylethylenediamine (TEMED, 99%) was procured from HiMedia Laboratories Pvt. Ltd., Mumbai, India. Doxorubicin hydrochloride pharmaceutical secondary standard (DOX) was procured from Sigma-Aldrich. For all the experiments, Milli-Q water was used.
Synthesis of poly (N,N-diethylacrylamide) (PDEA) hydrogel
The poly (N,N-diethylacrylamide) (PDEA) hydrogel was synthesized by free radical polymerization [30]. Briefly, the known amount of monomer DEA (2.179–15.2 mmol), cross-linker MBA (129.7 µmol), initiator APS (87.67 µmol), milli-Q water (5 mL) were stirred vigorously in a beaker, and then TEMED (50 µL) was added dropwise to accelerate the reaction. The polymerization reaction was performed at ambient room temperature (28 °C) for 4 h. After polymerization reaction, the synthesized hydrogels were immersed in milli-Q water for 48 h to eliminate the residual unreacted molecules and the water was replaced with fresh water for every 4 h. The synthesized polymeric hydrogels were examined visually for their physical strength, rigidity and mechanical stability. Further, maximum swelling ability of the synthesized hydrogel was measured using water and the water content in the swollen hydrogel was calculated by Eq. (1) as follows:
where WW is the weight of equilibrated hydrogel at room temperature and WD is the dry weight of the hydrogel.
The hydrogel which absorbed a large amount of water was considered for further optimization by varying the cross-linker MBA (38.91–324.3 µmol), initiator APS (21.91–175.3 µmol), TEMED (20–100 µL) and temperature (25–40 °C) using ‘one-variable-at-a-time’ method (Table 1).
Characterization of PDEA hydrogel
FTIR analysis (Bruker, Germany) was carried out between 4000 and 500 cm−1 to confirm the chemical properties of the hydrogel. Elemental analysis was done using an ElementarVario EL III (Germany) analyzer. The weight change in the lyophilized hydrogel was measured under the nitrogen atmosphere at a flow rate of 50 mL.min−1 using a thermogravimetric differential thermal analyzer (Exstar, 6000 TG-DTA 6300, USA). The temperature was raised from room temperature to 700 °C at a heating rate of 10 °C.min−1. DSC analysis of the samples was performed utilizing TA instruments, Model Q150 at a heating rate of 10 °C.min−1.
The interior morphology of the hydrogel was analyzed by a scanning electron microscope (JEOL, JSM-6360 LV analytical SEM, USA). Prior to the analysis, the hydrogel samples were sliced into required dimensions, sputtered with gold and then examined. UV–Vis absorption analysis was carried out using a UV/Vis/NIR spectrophotometer (HITACHI, U-2900, Japan).
Temperature-dependent equilibrium swelling ratio
To evaluate the equilibrium swelling ratio (ESR) of the hydrogel, a gravimetric protocol was used between the temperature range of 20–60 °C. The hydrogel was immersed in milli-Q water for 30 min to attain equilibrium at a fixed temperature. Equilibrated hydrogel sample surface was wiped with wet filter paper to remove excess water and then weighed. Eventually, the hydrogel was stabilized at the temperatures as mentioned above. The samples were dried under vacuum at room temperature and weighed. The equilibrium swelling ratio (ESR) was calculated by Eq. (2) as follows:
where Wi is the weight of equilibrated hydrogel at a particular temperature and Wr is the dry weight of the hydrogel.
Time-controlled swelling ratio
The method mentioned in the above section (ESR) was followed to document the swelling ratio (SR) of hydrogel at ambient room temperature (28 °C). The lyophilized gels were placed in milli-Q water and the SR was measured using the gravimetric method at every 30 min. At time t, the swelling ratio (SR) was calculated by Eq. (3) as follows:
where We is the weight of equilibrated hydrogel at time t and Wd is the dry weight of the hydrogel.
Deswelling kinetics
Formed hydrogels were equilibrated in milli-Q water and the surface was wiped with a wet filter paper, and immediately transferred from room temperature to 50 °C. The water retention (WR) was calculated by Eq. (4) as follows:
where Wr is the dry weight of the hydrogel, Wq is the weight of equilibrated swollen hydrogel at 24 °C and Wt is the weight of the hydrogel at a regular time interval.
Cytocompatibility of hydrogels
Cytocompatibilty of the hydrogels was evaluated by performing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The hydrogel samples were sterilized and exposed to the DMEM medium for 48 h. After sufficient exposure, the supernatant was taken and filtered through a syringe filter to remove undissolved hydrogel debris. This filtrate was used as the test sample. For the cytotoxicity test, exponentially growing MDA-MB-231 (Human mammary gland, Adenocarcinoma) cells were harvested and the cell count was adjusted to 10,000 cells mL−1 of medium. 100 µL of this suspension was seeded to each well of a 96 well plate. The plate was incubated at 37 °C in an atmosphere consisting 5% CO2 for 24 h for cell adhesion. After 24 h, the medium was replaced with different concentrations of the test samples. Cells were further incubated for a period of 48 h in the same conditions. After the incubation, the medium in each well was replaced with 50 µL of MTT reagent (2 mg mL−1 MTT reagent in PBS buffer). The cells were incubated for another 4 h at 37 °C in dark. On completion of incubation, the MTT reagent was removed and the formazan crystals formed were dissolved in 100 µL dimethyl sulfoxide (DMSO) solution. The optical density (OD) was measured at 540 nm with a microplate reader [14, 30]. The cell viability rate was calculated by Eq. (5) as follows:
Analysis of in vitro drug loading and drug release
The loading of drug (DOX) in the synthesized PDEA was done through equilibrium partitioning in presence of the drug. The hydrogels (0.1 g each) were submerged in a 30 ppm DOX solution and kept at 4 °C for 24 h for sufficient absorption of the drug, and furthermore the samples were isolated from drug solution after imbibitions. The drug loaded in the hydrogel was estimated by measuring the difference in the mass of hydrogel before and after loading. An accurately weighed amount of DOX-encapsulated hydrogel was suspended in 10 mL of freshly prepared PBS and the drug release experiment was performed at 20 °C, 30 °C, 37 °C and 40 °C. At regular time intervals, the solution was analyzed using UV–Vis spectroscopy at 480 nm to record the amount of DOX released. The withdrawn solution was replaced to maintain constant volume. The outcomes are represented as percentage cumulative release as a function of time which was calculated by Eq. (6) as follows:
where M∞ is the estimated amount of drug loaded into the hydrogel and Mt is the amount of drug released from the hydrogel at time t.
Drug encapsulation efficiency (EE) and loading capacity (LC) were evaluated using the initial drug concentration before suspending the dry hydrogel. In addition, the final drug concentration was measured after 24 h of incubation. EE and LC were calculated using Eqs. (7 and 8):
where MDE is the mass of drug encapsulated within the hydrogels (MDE = MDI − MFD), MFD is the mass of final drug present in the supernatant after drug loading, MDI is the initial mass of drug present in the solution and MN is the initial mass of the lyophilized hydrogels.
Analysis of drug release pattern
The release of solute from the cross-linked structure is based on the swelling behavior of the polymers and rate of diffusion. To deduce the release mechanism, drug release from the cross-linked networks of PDEA hydrogels was analyzed using zero-order, first-order, Higuchi, and Korsmeyer–Peppas models. The following equations are the model equations used to analyze drug release mechanism [31, 32]:
Zero-order kinetic model:
where K0 is the zero-order rate constant, Ct is the amount of drug released at time t and C0 is the initial concentration of drug (at t = 0).
First-order kinetic model:
where K1 is the first-order rate constant, Ct is the amount of drug released at time t and C0 is the initial concentration of drug (at t = 0).
Higuchi model:
where K2 is the Higuchi constant and Q is the cumulative release of drug in time t.
Korsmeyer–Peppas model:
where K3 is the release rate constant, n is the release exponent and \(\frac{{{M_t}}}{{{M_\infty }}}\) is the fraction of drug release at time t.
Results and discussion
Synthesis of PDEA hydrogel
The PDEA hydrogel was synthesized by free radical polymerization and the reaction mechanism is illustrated in Scheme 1. DEA in an aqueous solution was polymerized using MBA and APS/TEMED at ambient room temperature (28 °C). The polymerization mechanism occurs in two stages. In the first stage, TEMED facilitates the decomposition of APS into free radicals, and in the second stage, the free radical initiator attacks the monomer to initiate polymerization. The free radical does attack one of carbon double bonds (vinyl group) of the monomer molecule to form a new chemical bond between the monomer and initiator fragment. This process repeats and polymer chains become chemically cross-linked through the random incorporation of MBA until the exhaustion of monomer.
Effect of process parameters
The feed compositions were optimized at ambient room temperature (28 °C) to achieve better swelling ratio and stability. The stability was quantified by visual observation and swelling ratio was analyzed using gravimetric analysis in the presence of water. The composition of monomer, cross-linker, initiator and accelerator was optimized by varying one variable at a time and the effects are shown in Fig. 1a–d.
Effect of monomer
To understand the effect of monomer (DEA) on the swelling capability of the PDEA hydrogels, some experiments were conducted with different monomer concentrations (2.179, 4.358, 6.538, 8.717, 10.8, 13 and 15.2 mmol) keeping the other variables constant (MBA—129.7 µmol; APS—87.65 µmol and TEMED—50 µL). Figure 1a, shows the influence of monomer concentration on the absorption capacity of water. It is evident from Fig. 1a that water absorption improves with increase in monomer concentration upto 13 mmol, after that there is a slight decrease in water absorption.
It was also visually observed that under 8.717 mmol of monomer concentration, the gel had a very poor structural stability. Above 8.717 mmol of monomer concentration, gels were mechanically stable under swollen state and had a brittle nature due to increased intermolecular cross-linking. To form a stable gel, the monomer content had to be diluted to improve the intermolecular cross-linking, otherwise there will be a formation of undesirable intermolecular cross-linking structure [33]. Therefore, the optimal monomer concentration was found to be 8.717 mmol (i.e., in 5 mL of water 1.2 g of DEA) which was used in further studies.
Effect of cross-linker
For a stable hydrogel, better cross-linking is required to prevent dissolution of the hydrophilic polymer chains in an aqueous environment. Capability to swell or uptake water is an essential trait of a temperature-responsive hydrogel, therefore, the optimal cross-linker concentration was determined based on the amount of water absorbed by the hydrogel. PDEA hydrogel was prepared by varying the concentration of MBA (38.91, 103.7, 129.7, 194.5 259.4 and 324.3 µmol) while keeping the other variables constant (DEA—8.717 mmol; APS—87.65 µmol and TEMED—50 µL). Hydrogels prepared with less than 129.7 µmol cross-linker were sticky and had weaker network formation. Hydrogels prepared with more than 129.7 µmol cross-linker retained their structural stability and was easy to handle.
Influence of the cross-linker concentration on H2O holding capacity of the PDEA hydrogel is shown in Fig. 1b. The hydrogel prepared with 129.7 µmol cross-linker can hold more water after equilibrating it with water. As the concentration of cross-linker is increased, the degree of swelling tends to decrease and exhibits the formation of hydrogel with a denser network which affected the water uptake. Water uptake depicts the migration of H2O molecules into pores of the polymeric structure. A denser structure of hydrogel reduces the accessibility of H2O molecules to hydrophilic segments of the polymer, therefore, it limits the amount of water penetrating into the hydrogel structure. According to Flory’s theory, excessive concentration of cross-linker will impel the generation of immoderate cross-link points, which have a tendency to augment the cross-linking density [34, 35]. As a result, the network voids for holding water are reduced and the water absorption decreases. In 2010, Wang et al. reported similar observations [36].
Effect of initiator and accelerator
The effect of initiator concentration was also studied as mentioned above. PDEA hydrogel was prepared by varying the concentration of APS (21.91, 43.82, 87.65, 131.5 and 175.3 µmol) while keeping the other variables constant (DEA—8.717 mmol; MBA—129.7 µmol and TEMED—50 µL). The initiator contributes a salient role in the polymerization, primarily in the nucleation stage. The mechanical stability of the hydrogel was improved with increase in the initiator concentration. The inset in Fig. 1c shows the digital images of a hydrogel prepared using different initiator concentrations. From the inset Fig. 1c, it is understood that the hydrogel with 21.91 µmol of initiator concentration is transparent, which make it evident that the cross-linking structure is homogeneous, but as the concentration of initiator is increased (above 21.91 µmol), an opaque hydrogel is formed, which it may imply formation of a heterogeneous hydrogel structure. It is also found that the absorption of water in the hydrogel increases by increasing APS concentration, which perhaps can be attributed to the escalation of active free radicals. The maximum water content in the hydrogel is obtained at 175.3 µmol (0.04 g) of APS.
Similarly, the accelerator concentration was also optimized by varying the TEMED (20, 40, 60, 80 and 100 µL) while keeping the other variables constant (DEA—8.717 mmol; MBA—129.7 µmol and APS—175.3 µmol). The time of polymerization reaction was decreased with increase in accelerator concentration and also water holding capacity within the hydrogel was enhanced (Fig. 1d). In this work, APS–TEMED was the redox initiator used to initiate the radical polymerization. To acquire a fast polymerization and high initiation efficiency, the combination of APS and TEMED has been vastly utilized [37].
From the above discussion, we can conclude that the optimum composition to produce PDEA hydrogel with good mechanical stability and high water absorption capacity are DEA = 8.717 mmol; water = 5 mL; MBA = 129.7 µmol; APS = 175.3 µmol and TEMED = 100 µL. This optimized composition was further used to prepare hydrogels at various temperatures (25–40 °C) and its temperature dependencies were further studied.
Effect of temperature on equilibrium swelling ration
ESR is an essential parameter for evaluating the LCST behavior of the hydrogel. The ESR of PDEA was determined for the hydrogel prepared at varying temperatures from 25 to 40 °C in distilled water. The hydrogels were in a swollen state by absorbing water below LCST and shrank gradually by desorbing water above LCST. The process behind the absorption and desorption behavior of hydrogel was caused by the hydrophilic (–CONR2) and hydrophobic (–CH2CH3) regions of polymeric network which could undergo many interactions (polymer–polymer hydrophobic interactions, hydrogen bond, etc), resulting in dehydration–hydration change in the hydrogel in response to the surrounding temperature. Further, from Fig. 2, it is obvious that the ESR value decreases with increase in the temperature from 20 to 60 °C. The hydrogel synthesized at 36 °C shows the maximum ESR compared to the hydrogels synthesized at other temperatures.
At lower temperatures, the hydrophilic regions of the hydrogel bind with water molecules forming hydrogen bonds, and a firm coating around the hydrophobic segments is formed, and hence, these regions exhibit a greater water uptake and their appearance changes from transparent to translucent. The structure of the PDEA polymer with hydrophilic and hydrophobic groups is shown in Scheme 2. At lower temperatures, strong hydrogen bonds are formed between the amide groups and water molecules compensate the unfavorable free energy related to the exposure of hydrophobic groups to water molecules, leading to good solubility of the polymer in water.
Despite the difference in swelling capacities, a change in transparency is also an indication of a change in LCST. As the temperature increases, the appearance of the hydrogel becomes opaque owing to the collapse of hydrogen bonds, and the hydrophobic interactions in the polymer network are increased, and consequently, the hydrogel shrinks by releasing the entrapped water molecules and phase separation occurs. ESR decreases instantly with an increase in the surrounding temperature of the hydrogel [30].
Time-dependent swelling ratio of hydrogels
Water uptake capability of hydrogels differs due to the difference in their inter-connected pore structures and uptake capacity leading to difference in their swelling rate because of the change in the interaction of water molecules inside the hydrogel with the external aqueous phase [38]. Initially, the dried hydrogel rapidly absorbs water molecules by hydrating the hydrophilic regions that expand the polymeric hydrogel inter-connected network. As the hydrophilic regions attain saturation, the polymeric network then exchanges the free water molecules with the external water molecules [39]. The swelling behavior of the lyophilized hydrogel was measured gravimetrically in milli-Q water at ambient room temperature. It is observed in Fig. 3 that the swelling rate of PDEA for T36 increases to 6.37 g.g−1 in 30 min and it reaches its maximum within 450 min, whereas, the swelling rate of T25 increases to 1.30 g.g−1 and it reaches its maximum within 270 min. The diffusion of H2O molecules and the porosity of the gel are responsible for the swelling behavior of the polymeric hydrogels. A decrease in SR is because of the less-packed polymeric structure, which favors the hydrophobic interaction in the PDEA.
Deswelling kinetics or water retention (WR) at 50 °C
Response rate of a temperature-responsive hydrogel is one of the important parameters in determining its application. The deswelling kinetics of the hydrogel prepared at different temperatures were initially equilibrated at ambient temperature (28 °C) and then immediately transferred to a water bath maintained at 50 °C. Figure 4 illustrates that the deswelling rate of PDEA prepared at higher temperature is faster than those hydrogels prepared at lower temperatures. Samples T36, T38 and T40 hydrogels have lost nearly 80% of H2O molecules within 20 min from their interconnected polymeric structure, while T25 and T28 have lost about 50% of H2O molecules. The deswelling behavior of the hydrogel is ascribed to the alteration of hydrophobic–hydrophilic segments of the polymer network. As the hydrogel was immersed in H2O and maintained at 50 °C, a rapid shrinkage was observed, because the outermost region of hydrogel would be first affected, then the hydrophilic interactions among the hydrophobic groups become stronger, leading to the formation of a dense layer on the outer surface. Once the dense layer was formed, the diffusion of H2O molecules from the surface of the hydrogel was obstructed, which resulted in a low deswelling ratio [40,41,42].
From all the above swelling and deswelling analyses, it was concluded that T36 was the best and it was used for further characterization tests.
Structure and composition analysis
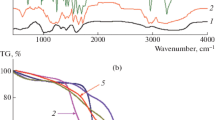
The molecular structure of the hydrogel was investigated in detail using ATR–FTIR as shown in Fig. 5. The absorption peak at 979 cm−1 for the monomer is resulted from the vinyl group [30]. The peaks at 2975 cm−1 and 2934 cm−1 are related to the C–H stretching vibrations of –CH3 and –CH2– in the DEA side groups [36, 41]. The significant peak at 1640 cm−1 (amide I) can be attributed to the characteristic peak of the carbonyl group (C=O) stretching vibration [43,44,45]. The peaks at 1433 cm−1 and 1362 cm−1 are assigned to the symmetrical C–H bending vibrations. The stretching of C–N (amide III) bond in the monomer has absorption at 1262 cm−1 [46]. The disappearance of the characteristic peaks at 1640 cm−1 and 979 cm−1 in the spectrum of polymer PDEA confirms the successful polymerization of the monomer DEA.
Further, elemental analysis was performed to determine the hydrogen (H), carbon (C), nitrogen (N) and oxygen (O) content in PDEA. The weight percentage of components was as follows: C = 50.62%, H = 10.08%, O = 29.14% and N = 10.16%. The molar ratio of N to C in the synthesized PDEA was determined to be 1:4.94. The polymerization temperature may cause the degradation of the amide as discussed above.
Thermal analysis
Thermal behavior of the synthesized hydrogel was determined using TGA and the DSC is provided in the supplementary image (Fig. 6). Thermogravimetric analysis of PDEA hydrogel was performed as a function of temperature versus weight loss percentage. Figure 6 shows three weight loss regions at temperatures 68.37 °C, 459.17 °C and 727.89 °C. The initial weight loss of 6.39% between 25 and 68.37 °C can be related to the dehydration of the polymer [47]. The second weight loss of 84.99% between 322.20 and 459.17 °C is due to the cleavage of the side chain (–N(CH2CH3)) from PDEA, and also the decomposition of oxygen-related functional groups and amides from the polymer matrix [23]. The final weight loss of 3.24% is ascribed to the decomposition of the polymer backbones in PDEA. The polymer shows 50% of weight loss at 417.22 °C, which reveals that the polymer is thermally more stable. The thermal resistance of PDEA is due to a combination of more covalent bonds through MBA as a cross-linker [47].
The thermodynamic parameters of the hydrogels were obtained by the DSC thermograms. The major and minor shifts in the thermogram observed in Fig. 6 have corresponded to the glass transition temperatures (Tg) at 69 °C, 90 °C and 97 °C. With an increase in the synthesis temperature of PDEA hydrogel, there is an increase in the chain segment activity, then the number of interconnected pores also increases, and thereby the Tg of the hydrogels decreases [36].
Polymeric branches of PDEA cause PDEA hydrogels to decompose at a high temperature. These hydrogels have a large number of C=O, C–N and –CH2CH3 bonds than –OH bonds [16]. Below Tg, the polymer chains are frozen and they just vibrate slightly, however, above Tg, parts of polymer chains can move or rotate. The glass transition temperature changes with the cooling rate as well as the incorporation of additives and structural change in the molecules. The SEM images have further confirmed this result.
Internal structure morphology analysis of the hydrogel
The internal structure of the hydrogel was analyzed using SEM analysis. The SEM micrographs of the swollen freeze-dried hydrogel of PDEA samples are shown in Fig. 7. As the synthesis temperature increases, the porosity of the hydrogel also increases and a change in interior morphology is observed. The surfaces of gels T25 and T30 have many independent and unconnected large pores. T36 sample shows a higher number of uniformly interconnected pores with decreased pore size. It has been found that with further increase in the synthesis temperature (above 36 °C), a more interconnected porous structure is observed in the hydrogel network. This is due to the crystallization of water molecules in the swollen hydrogel that act as pore-forming agent [45]. The changes in the surface morphology of PDEA can be attributed to the variation in the synthesis temperature.
Cytotoxicity
Cytotoxicity is an indispensable aspect of a hydrogel acting as a drug carrier and can be assessed by MTT assay in MDA-MB-231 cell lines. The capability of the cells to live in an unsafe environment has been a fundamental to cytotoxicity assays. The cell viability was compared with the control group and it is shown in Fig. 8a. The figure shows the viability of T36 and T36 loaded with DOX hydrogel. The exposure of cells at varying concentrations (62.5–1000 µg/mL) of T36 and T36-DOX has no apparent effect on cell viability. After 24 h, the samples were examined by the microscope (Fig. 8b). The microscopic images of control and other samples also show that the cells are overwhelmingly viable, regardless of the concentration of hydrogels. These cytotoxicity data have shown that IC50 value was not attained in none of the cases, which confirms the non-toxic behavior of the hydrogel.
In vitro drug release kinetics of the hydrogel
The release of drug from a polymer matrix is related to various aspects, such as drug affinity, swelling/ deswelling capability of the polymer, mode of drug release, and drug solubility [48, 49]. DOX has an effective chemotherapeutic activity against solid tumors, such as breast cancer, small cell lung cancer and ovarian carcinoma. DOX has the risk of cardiotoxicity. Recently, it has been reported that encapsulation of DOX in polymeric carriers can noticeably decrease the cardiac damage [50]. Temperature-responsive polymeric carriers can control the drug release at the tumor site. Hence, the effect of different temperatures, i.e., 20 °C, 30 °C, 37 °C and 40 °C on the release of drug from polymer matrix was analyzed and shown in Fig. 9. The cumulative release of DOX (30 ppm) from sample T36 shows higher ESR, SR and DSR. When the dry samples (0.1 g) were immersed in 10 mL of 30 ppm drug solution, their final equilibrium weight was noted to be 0.3 ± 0.05 g (concentration of the drug loaded in the hydrogel was 19.69 ppm).
The difference in the initial and final concentration of drug shows an interesting behavior of the hydrogel, where the hydrogel has a capacity to encapsulate drug molecules. The T36 hydrogel encapsulation efficiency was found to be 34.365% and loading capacity was found to be 10.309%. The swollen hydrogel was then placed in 10 mL PBS solution at 20 °C, 30 °C, 37 °C and 40 °C to study its release characteristics. From Fig. 9, it is noticed that the drug released by hydrogels at 20 °C, 30 °C, 37 °C and 40 °C is found to be 13%, 20%, 30% and 38% of the total drug absorbed. With increase in the surrounding temperature of the hydrogel, there is an increase in the drug release percentage.
When these gels were transferred to the temperature above the gel collapse point, we could observe a fast contraction of the polymer matrix. The increased temperature resulted in a faster relaxation time of the polymer network due to the increased kinetic energy, which aids in water sorption process. As a result, initially the gel collapsed, more loss of drug molecules was observed, followed by the slower release of drug molecules from shrunken and physically compacted gels. It was then contemplated that the hydrogels took 8 h to release the absorbed DOX molecules at three different temperatures, i.e., the controlled and sustained release was due to the release of drug molecules from the center of hydrogels to the surrounding PBS medium.
Further, the data were fitted in zero-order, first-order, Higuchi and Korsmeyer–Peppas models (Fig. 10) to evaluate the DOX release pattern which are tabulated in Tables 2 and 3. The appropriate model was selected on the criterion of ideal fit by regression coefficient (R) values. The R values for zero-order, first-order, Higuchi and Korsmeyer–Peppas at different release temperatures are given in Table 2. The values of R were found to be lower for zero-order release rate constant than those of first-order release rate constant. Thus, it imputed the fact that DOX release from the sample at different temperatures obeys the first-order release. While in the Higuchi model, the R value at different temperatures suggests the diffusion-controlled drug release mechanism.
DOX release kinetics studied for PDEA hydrogel at different temperatures: a percentage cumulative release versus time (zero-order kinetics), b percentage log cumulative drug remaining versus time (first-order kinetics), c percentage cumulative release versus sqrt of time (Higuchi model), and d ln Mt/M∞ versus ln T (Korsmeyer–Peppas model)
In the Korsmeyer–Peppas model, the general classification of the diffusion from the polymeric matrix is as follows: if n is 0.5, then the drug release is due to the Fickian diffusion model (drug diffusion is dominant); if n is 1, then the drug release is due to the other predominant factors like external stimuli; and if 0.5 < n < 1, the non-Fickian or anomalous release is predominant (a synergistic effect of drug diffusion and external stimuli) [30, 33, 51]. The effect of surrounding temperature on the DOX release exponent (n) values are given in Table 3 and all the samples show the non-Fickian behavior.
In Table 4, the LCST values of the PDEA obtained in this study are compared with those reported in literature. From the table, it is clear that the present work shows better LCST with simple free radical polymerization without addition of other monomers, and clay or natural polymer.
Conclusion
Temperature-sensitive PDEA hydrogel was successfully prepared by free radical polymerization. The composition of cross-linker, monomer, accelerator and initiator for the preparation of stable hydrogel was optimized by varying one variable at a time and the effect was studied on the basis of swelling behavior of the hydrogel. Further, the temperature of polymerization reaction was optimized, which was found to be 36 °C and the maximum ESR observed was 18.332 g.g−1. From the ESR studies, LCST of the synthesized PDEA hydrogel was found to be around 29 °C. The FTIR results confirmed the polymerization of DEA by the disappearance of characteristic peaks of carbonyl group at 1640 cm−1 and a vinyl group at 979 cm−1 in the spectrum of PDEA. The SEM analysis confirmed the formation of interconnected porous structure within the hydrogel. It was also observed that with an increase in the hydrogel synthesis temperature, the network porosity increased.
Cytotoxicity test confirmed that the prepared hydrogel is not toxic in nature. Further, the release studies of DOX-loaded hydrogel were found to be 13% at 20 °C, 20% at 30 °C, 30% at 37 °C and 38% at 40 °C. The drug release mechanism was studied using different models (zero-order, first-order, Higuchi and Korsmeyer–Peppas models), and found to follow the non-Fickian diffusion. Conclusively, we can establish the prime role of PDEA hydrogel in the drug delivery system, particularly in the presence of a variable thermal environment, i.e., homologous to disease-infected biological systems. Many diseases that are fatal in nature like cancer are accompanied by hyperthermic conditions, which can be exploited by PDEA hydrogel for effective treatment.
References
Chai Q, Jiao Y, Yu X (2017) Hydrogels for biomedical applications: their characteristics and the mechanisms behind them. Gels 3:6–21
Bajpai AK, Sandeep KS, Smitha B, Sanjana K (2008) Responsive polymers in controlled drug delivery. Prog Polym Sci 33:1088–1118
Pinar I, Ozgur O (2017) Novel stimuli-responsive hydrogels derived from morpholine:synthesis, characterization and absorption uptake of textile azo dye. Iran Polym J 26:391–404
Kamath K, Park K (1993) Biodegradable hydrogels in drug delivery. Adv Drug Deliv Rev 11:59–84
Park K, Shalaby WSW, Park H (1993) Biodegradable hydrogel for drug delivery. Technomic Publishing Co., Inc. Lancaster,
Liu F, Tao GL, Zhuo RX (1993) Synthesis of thermal phase-separating reactive polymers and their applications in immobilized enzymes. Polym J 25:561–567
Kim JJ, Park K (1999) Smart hydrogels for bioseparation. Bioseparation 7:177–184
Chen JK, Chang CJ (2014) Fabrications and applications of stimulus-responsive polymer films and patterns on surfaces: a review. Materials 7:805–875
Hoffman AS (2012) Hydrogels for biomedical applications. Adv Drug Deliv Rev 64:18–23
Sosnik A, Seremeta KP (2017) Polymeric hydrogels as technology platform for drug delivery applications. Gels 3:25–47
Caló E, Khutoryanskiy VV (2015) Biomedical applications of hydrogels: a review of patents and commercial products. Eur Polym J 65:252–267
Ullah F, Othman MB, Javed F, Ahmad Z, MdAkil H (2015) Classification, processing and application of hydrogels: a review. Mater Sci Eng C 57:414–433
Kondiah PJ, Choonara YE, Kondiah PPD, Marimuthu T, Kumar P, du Toit LC, Pillay V (2016) A review of injectable polymeric hydrogel systems for application in bone tissue engineering. Molecules 21:1580–1611
Bahram M, Mohseni N, Moghtader M (2016) In: Majee SB (ed) An introduction to hydrogels and some recent applications. IntechOpen London
Strandman S, Zhu XX (2015) Thermo-responsive block copolymers with multiple phasetransition temperatures in aqueous solutions. Prog Polym Sci 42:154–176
Xiao XC (2007) Effect of the initiator on thermosensitive rate of poly(N-isopropylacrylamide) hydrogels. Express Polym Lett 1:232–235
Karimi M, Sahandi PZ, Ghasemi A, Amiri M, Bahrami M (2016) Temperature-responsive smart nanocarriers for delivery of therapeutic agents: applications and recent advances. ACS Appl Mater Interfaces 8:21107–21133
Patil JS, Gurav PB, Mandave SV, Jadhav SM, Kulkarni RG (2014) Hydrogel system, a ‘smart’ and ‘intelligent’ drug delivery device: a systematic and concise review. Ind J Nov Drug Deliv 6:93–105
Rizwan M, Yahya R, Hassan A, Yar M, Azzahari AD, Selvanathan V, Sonsudin F, Abouloula CN (2017) pH sensitive hydrogels in drug delivery: brief history, properties, swelling, and release mechanism, material selection and applications. Polymers 9:137–174
Simões S, Figueiras A, Veiga F (2012) Modular hydrogels for drug delivery. J Biomater Nanobiotechnol 3:185–199
Wang L, Li B, Xu F, Xu Z, Wei D, Feng Y, Wang Y, Jia D, Zhou Y (2017) UV-crosslinkable and thermo-responsive chitosan hybrid hydrogel for NIR-triggered localized on-demand drug delivery. Carbohydr Polym 174:904–914
Wei W, Qi X, Li J, Zuo G, Sheng W, Zhang J, Dong W (2016) Smart macroporous salecan/poly (N,N-diethylacrylamide) semi-IPN hydrogel for anti-inflammatory drug delivery. ACS Biomater Sci Eng 2:1386–1394
Işıklan N, Kazan H (2018) Thermoresponsive and biocompatible poly(vinyl alcohol)-graft poly(N,N-diethylacrylamide) copolymer: microwave-assisted synthesis, characterization, and swelling behavior. J Appl Polym Sci 135:45969
Işıklan N, Ş Tokmak (2018) Microwave based synthesis and spectral characterization of thermo-sensitive poly(N,N-diethylacrylamide) grafted pectin copolymer. Int J Biol Macromol 113:669–680
Ngadaonye JI, Geever LM, Killion J, Higginbotham CL (2013) Development of novel chitosan-poly(N,N-diethylacrylamide) IPN films for potential wound dressing and biomedical applications. J Polym Res 20:161–174
Ngadaonye JI, Geever LM, McEvoy KE, Killion J, Brady DB, Higginbotham CL (2014) Evaluation of novel antibiotic-eluting thermoresponsive chitosan-PDEAAm based wound dressings. Int J Polym Mater Po 63:873–883
Li H, Wu R, Zhu J, Guo P, Ren W, Xu S, Wang J (2015) pH/temperature double responsive behaviors and mechanical strength of laponite-crosslinked poly(DEA-co-DMAEMA) nanocomposite hydrogels. J Polym Sci B 53:876–884
Kohsaka Y, Tanimoto Y (2016) Synthesis of thermo-responsive polymer via radical (co)polymerization of N,N-dimethyl-α-(hydroxymethyl)acrylamide with N,N-diethyl acrylamide. Polymers 8:374–381
Hanyková L, Spěváček J, Radecki M, Zhigunov A, Kouřilová H, Sedláková Z (2016) Phase transition in hydrogels of thermoresponsive semi-interpenetrating and interpenetrating networks of poly(N,N-diethylacrylamide) and polyacrylamide. Eur Polym J 85:1–13
Maheswari B, Babu PEJ, Agarwal M (2014) Role of N-vinyl-2-pyrrolidinone on the thermoresponsive behavior of PNIPAm hydrogel and its release kinetics using dye and vitamin-B12 as a model drug. J Biomater Sci Polym Ed 25:269–286
Qi X, Wei W, Li J, Liu Y, Hu X, Zhang J, Bi L, Dong W (2015) Fabrication and characterization of a novel anticancer drug delivery system: salecan/poly(methacrylic acid) semi-interpenetrating polymer network hydrogel. ACS Biomater Sci Eng 1:1287–1299
Dash S, Murthy PN, Nath L, Chowdhury P (2010) Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm 67:217–223
Siegel RA, Rathbone MJ (2012) In: Siepmann J, Siegel RA, Rathbone MJ (eds) Fundamentals and applications of controlled release drug delivery. Springer, New York
Šponarov ÁD, Horák D (2008) Poly (N,N-diethyl acrylamide) microspheres by dispersion polymerization. J Poly Sci Part A Pol Chem 46:6263–6271
Flory PJ (1953) Principles of polymer chemistry. Cornell University Press, New York
Barron V, Killion JA, Pilkington L, Burke G, Geever LM, Lyons JG, McCullagh E, Higginbotham CL (2016) Development of chemically cross-linked hydrophilic–hydrophobic hydrogels for drug delivery applications. Eur Polym J 75:25–35
Wang WB, Wang AQ (2010) Preparation, swelling and water-retention properties of cross-linked superabsorbent hydrogels based on guar gum. Adv Mater Res 96:177–182
Zhang N, Liu M, Shen Y, Chen J, Dai L, Gao C (2011) Preparation, properties, and drug release of thermo- and pH-sensitive poly(2-dimethylamino)ethyl methacrylate)/poly(N,N-diethylacrylamide) semi-IPN hydrogels. J Mater Sci 46:1523–1534
Yin L, Fei L, Cui F, Tang C, Yin C (2007) Superporous hydrogels containing poly(acrylic acid-co-acrylamide)/O-carboxymethyl chitosan interpenetrating polymer networks. Biomaterials 28:1258–1266
Wang ZC, Xu XD, Chen CS, Wang GR, Wang B, Zhang XZ, Zhuo RX (2008) Study of novel hydrogels based on thermosensitive PNIPAAm with pH-sensitive PDMAEMA grafts. Colloids Surf B 67:245–252
Babu PEJ, Kumar RS, Maheswari B (2011) Synthesis and characterization of temperature sensitive P-NIPAM macro/micro hydrogels. Colloids Surf A 384:466–472
Chen J, Liu M, Chen W, Zhang N, Zhu S, Zhang S, Xiong Y (2011) Synthesis, swelling and drug-release behavior of a poly(N,N-diethyl acrylamide-co-(2-dimethylamino) ethyl methacrylate) hydrogel. J Biomater Sci Polym Ed 22:1049–1068
Chen J, Liu M, Liu H, Ma L, Gao C, Zhu S, Zhang S (2010) Synthesis and properties of thermo- and pH-sensitive poly(diallyldimethylammonium chloride)/poly(N,N-diethylacrylamide) semi-IPN hydrogel. Chem Eng J 159:247–256
Ngadaonye JI, Geever LM, Cloonan MO, Higginbotham CL (2012) Photopolymerised thermo-responsive poly(N,N-diethyl acrylamide)-based copolymer hydrogels for potential drug delivery applications. J Polym Res 19:9822–9837
Qi X, Wei W, Li J, Zuo G, Hu X, Zhang J, Dong W (2016) Development of novel hydrogels based on Salecan and poly(N-isopropylacrylamide-co-methacrylic acid) for controlled doxorubicin release. RSC Adv 6:69869–69881
Chen J, Liu M, Liu H, Ma L (2009) Synthesis, swelling and drug release behavior of poly(N,N-diethylacrylamide-co-N-hydroxymethyl acrylamide) hydrogel. Mater Sci Eng C 29:2116–2123
Akın A, Işıklan N (2016) Microwave-assisted synthesis and characterization of sodium alginate-graft-poly (N,N′-dimethylacrylamide). Int J Biol Macromol 82:530–540
Brazel CS, Peppas NA (1999) Mechanisms of solute and drug transport in relaxing, swellablehydrophilic glassy polymers. Polymer 40:3383–3398
Hoffman AS (2002) Hydrogels for biomedical applications. Adv Drug Deliv Rev 54:3–12
Zhao L, Zhang X, Liu X, Li J, Luan Y (2017) pH-responsive poly(ethylene glycol)-poly(ɛ-caprolactone)-poly(glutamic acid) polymersome as an efficient doxorubicin carrier for cancer therapy. Polym Int 66:1579–1586
Fariba G, Ebrahim VF (2009) Hydrogels in controlled drug delivery systems. Iran Polym J 18:63–88
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Havanur, S., Farheenand, V. & JagadeeshBabu, P.E. Synthesis and optimization of poly (N,N-diethylacrylamide) hydrogel and evaluation of its anticancer drug doxorubicin’s release behavior. Iran Polym J 28, 99–112 (2019). https://doi.org/10.1007/s13726-018-0680-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-018-0680-z