Abstract
This study includes the synthesis of amino acid graft copolymer from N- Acryloyl-L-phenylalanine amino acid and Guar gum polymer through free radical polymerization. Then the dual responsive (pH & temperature) hydrogels are synthesized by employing free radical cross linking polymerization using graft copolymer, N-isopropyl acrylamide and 2-(dimethylamine) ethylmethacrylate using N,N′-methylenebisacrylamide as a cross-linker and ammonium per sulfate as initiator. The structural properties of the hydrogels are evaluated by the determination of various network properties such as mesh size, crosslink density, Flory-Huggins interaction parameter, volume fraction in the swollen state and the average molecular weight of the polymer chain between two neighboring cross links. Dynamic and equilibrium swelling studies of hydrogels are performed in distilled water, pH 1.20 and pH 7.40 solutions at 25 °C and 37o ± 5 °C, swelling capacity in various salt solutions and corresponding swelling kinetic parameters are also calculated. Grafting, cross linking and structural changes are studied by fourier transform infrared and scanning electron microscope, distribution of imatinib mesylate drug in hydrogel is confirmed by X-ray diffraction, differential scanning calorimetry, thermo gravimetric analysis. Drug loading and encapsulation efficiency of Imatinib mesylate in various formulations of the hydrogels are also studied. The in-vitro drug release studies are performed in pH 1.20 and 7.40 phosphate buffer solutions at different amounts of cross linker, monomer and graft copolymer and temperature. The mode of drug release mechanism is analyzed by various kinetic models such as zero order, First order, Higuchi Square root, Hixson-Crowell cube root and Koresmeyer-Peppas equations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, researchers have been paid more attention on development of potential Controlled Drug Delivery System (CDDS) for sustained drug delivery applications. The CDDS delivers the drug safely and effectively into the body while maintaining the optimum level of dosage in the blood for a long period of time. Dosage of drug released through CDDS is sufficient for delivering optimum therapeutic concentration of drug. The efficacy of CDDS can be seen in its ability to deliver the drug molecule to the targeted cell and stimulate the localized action [1]. The CDDS overcomes patient compliances, reduce the side effects of drug, and minimize the unwanted side effects while lowering drug concentration accumulated by chronic dosing. Thus, the CDDS offers effective treatment by balancing the drug levels in the blood. The development of the stimuli responsive drug delivery networks under physiological conditions is essential for controlled drug delivery applications.
Hydrogels are three dimensional interpenetrated networks of physical or chemical cross-linked polymers. They have the hydrophilic behavior and the networks absorb huge amount of solvents or water or other cellular fluids resulting in swelling behavior, due to which they resemble the natural tissues and also have numerous additional features like biocompatibility, non-toxicity and biodegradability [2]. Hydrogels have the functional groups that can able to change structural properties in response to environmental stimuli such as pH [3], temperature [4]. Carbohydrates are widely used in the synthesis of pH responsive hydrogels due to presence of both acidic and basic responsive functional groups [5]. Among these, pH stimuli hydrogels are effective drug delivery carriers due to pH modulated swelling and release behavior in stomach (pH = 1.20) and intestinal (pH = 7.40) conditions.
Hydrogel’s thermo-responsive behavior is caused by the existence of hydrophobic or a combination of hydrophilic and hydrophobic groups in them. The familiar thermo-responsive polymers are poly (N-isopropyl acrylamide) (PIPAM) [Lower Critical Solution Temperature (LCST) = 32 °C], [poly(N-vinyl caprolactum) (PNVCL) (LCST = 32–34 °C)] and [poly (2-dimethylamino) ethylmethacrylate (PDMAEMA) (LCST = 38 °C)]. The majority of LCST having thermo-sensitive polymers are used in biomedical applications [6]. As both these pH and thermo-responsive hydrogels factors are easily controllable and can be used to perform both in-vitro and in-vivo calculations, they find their extensive usefulness in CDDS [7]. Imatinib mesylate (IMS) is used as an anti-Cancer drug, which is a specific site drug that has been used for the treatment of mainly chronic myeloid leukemia (CML), gastrointestinal stromal tumors (GISTs), chronic myelomonocytic leukemia [8]. Imatinib mesylate retards the growth of the Cancer cells by inhibiting enzymatic action of tyrosine kinase, along with inhibiting and damaging any other non-specific quickly dividing cells without harming the healthy cells [9].
Thermo and pH responsive three dimensional GG-g-PNPA-cl-(PIPAM-co-PDMAEMA) [GGND] hydrogel networks are prepared from GG-g-PNPA graft copolymer, N-isopropyl Acryiamide (IPAM) and 2-(dimethylamine) ethylmethacrylate (DMAEMA) using N,N′-methylenebisacrylamide (MBA) as a cross-linker through single pot-free radical polymerization reaction. The structural and drug delivery properties of GGND hydrogels are evaluated by determining various network properties such as mesh size (ξ), cross-link density (ve), Flory-Huggins interaction parameter (χ), volume fraction (ɸ) and the average molecular weight between two neighboring cross-links (M̅c) with effect of cross-linker, graft copolymer and monomer. The structure of newly synthesized GGND hydrogels is characterized by FTIR spectroscopy, X-RD, DSC, and SEM studies.
To determine swelling kinetic parameters of pH-responsive GGND hydrogels, the swelling experiments are conducted with distilled water, pH 1.20 and pH 7.40 solutions at 25 °C and 37 °C ± 5 °C. The swelling and deswelling capacity of GGND hydrogels in various salt solutions are also estimated. Surface charge of the GGND hydrogels is determined by point of zero charge (PZC) analysis. Drug loading, encapsulation of IMS molecules into GGND hydrogels and in-vitro drug delivery studies are performed in pH 1.20 and pH 7.40 solutions at 25 °C and 37 °C ± 5 °C. To understand the drug delivery profile of the GGND hydrogels, drug release data is applied to different kinetic models.
Materials and methods
Materials
Guar gum (GG), acryloyl chloride, ammonium persulfate (APS) and sodium hydroxide are purchased from Merck limited Mumbai (India). IPAM, DMAEAM and L-phenylalanine are purchased from Aldrich Chemicals (USA). MBA and N,N,N’N′ tetramethylethylenediamine (TEMED) are purchased from s.d fine chem limited Mumbai (India). IMS is received from Suven Life Sciences, Hyderabad as a gift sample. All chemicals are used as it is without any purification. Double distilled water (DDW) is used throughout the experiment.
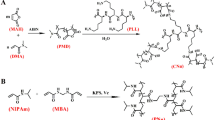
Synthesis of N-Acryloyl -L- phenyl alanine (NPA) monomer and guar gum –g- poly (N-Acryloyl – L - phenyl alanine) [GG-g-PNPA] graft copolymer
The new monomer N-Acryloyl-L-Phenyl Alanine (NPA), which is prepared by the reaction between acryloyl chloride and L-Phenyl Alanine (essential amino acid) according to the method previously reported [10]. The graft copolymerization reaction is carried out between guar gum (GG) polymer and NPA monomer by free radical polymerization reaction in the presence of APS initiator as per the method reported in the literature [11].
Synthesis of GGND hydrogels
GGND hydrogels are synthesized through the free radical polymerization reaction using definite concentrations of GG-g-PNPA, IPAM, DMAEMA, cross-linker (MBA), initiator (APS) and TEMED as an accelerator [Table −1].
The reactants are taken into a vessel and stirred continuously for two hours at 25 °C to get homogeneous mixture. A steam of pure N2 gas is purged into reaction mixture for one hour to create deoxygenated atmosphere. The polymerization is carried out under a continuous flow of pure nitrogen gas. The polymerization reaction is commenced by rising reaction mixture temperature and allowing the propagation process to perform at 60 °C for another three hours to get hydrogel networks. The synthesis of GGND hydrogels is presented in scheme −1. After the reaction, hydrogels are allowed to cool up to the room temperature and are cut into disks. The photographic images of fresh, swollen and dry GGND hydrogels are presented in the Figure −1.
Characterisation
The FTIR spectra of GGND plain hydrogel, IMS loaded GGND (IMS-GGND) hydrogels and pristine IMS are obtained using Perkin Elmer Spectrometer (Spectrum two model, UK). XRD diffractograms of placebo and IMS loaded GGND hydrogels and pristine IMS are recorded using X- ray diffactometer (D8 advance BRUKER Germany). Thermographs of placebo and IMS loaded GGND hydrogels and pristine IMS are obtained using a differential scanning calorimeter (SDT Q600 V20.9, Build 20). Images of GGND hydrogels are obtained using Scanning Electron microscope (JEOL model JSM-6390 LV). SEM photographs of placebo GGND hydrogels surface at different magnifications recorded.
Swelling studies of GGAH hydrogels
The dynamic swelling studies are performed in distilled water as well as in different pH conditions (1.20, 7.40) and at different thermal conditions (at 25 °C and 37 °C). The amount of fluid imbibed with respect to time is calculated gravimetrically. Then, a predetermined amount of dry gels is immersed in test bottles containing 50 mL of swelling medium. Now, the swollen hydrogels are removed from the swelling medium at regular time intervals and the surface adhered solution is blotted with a paper tissue. Finally, the hydrogels’ weights are taken on an electronic balance (Sarto-rius, BSA-224S-C.W, CHINA-). Percentage of dynamic swelling ratio [%DSR] and the percentage of equilibrium swelling ratio [% ESR] are determined from the following formulae (1) and (2) respectively [12].
Where, Wd, is dry gel weight, Wt is swollen gel weight at a time and We is swollen gel weight at equilibrium. The swelling experiments are performed in duplicate.
Swelling kinetics of GGND hydrogels
Dynamic swelling studies of GGND hydrogels have been studied to evaluate the amount of fluid absorbed at various time intervals. The swelling data substituted in the following Korsmeyer-Peppas kinetic equation.
Wt is Weight of the fluid absorbed at time‘t’, W∝ is weight of the fluid absorbed at equilibrium, K is swelling kinetic constant of hydrogel and n is the diffusion exponent. K, n values were calculated from intercept and slope values of the graph respectively, which are obtained from logarithmic form Eq. 3. The swelling kinetic parameter, diffusion co-efficient (D) was calculated from the following equation [13].
l is the thickness of the xerogel.
Effect of salts on swelling measurements
To study the effect of salts on swelling of GGND hydrogels, the equilibrium swelling experiments are performed in different salts (variable valence) such as NaCl, CaCl2, and AlCl3. Different concentrations of salt solutions are prepared (0.05, 0.1, 0.15 and 0.2 M) and GGND hydrogels are allowed to swell in the salt solutions for 72 h, and then % ESR is calculated.
Determination of network parameters of GGND hydrogels
The network parameters of GGND hydrogels such as the average molecular weight of the polymer chain between two neighboring cross links (M̅c), polymer volume fraction in the swollen state (ɸ), polymer and solvent interaction parameter (χ), the effective cross linking density (ve) and mean pore size (ξ) are determined. The network parameters are calculated as per the literature reported [3, 14,15,16].
Point of zero charge (PZC) studies
As per the method depicted in the literature [17], the point of zero charges (PZC) of pure GGND hydrogels and IMS loaded GGND hydrogels are measured. In this experiment, different pH solutions (1.00, 3.00, 5.00, 7.00, 9.00, 11.00 and 13.00) are prepared by taking 40 mL of 0.1 N KNO3 solution into each 100 mL conical flask, 0.1 N HNO3 or 0.1 N NaOH solution added to adjust the initial pH (pHi) of the solution. To the series of different pH solutions, around 0.1 g of hydrogel (GGND- 3) samples of both drug free and drug loaded are added and securely capped. The gels containing conical flasks were kept for 48 h to reach equilibrium condition with occasional shaking. After that, final pH values (pHf) are measured using pH meter. The PZC of different hydrogels are calculated by taking into consideration the pH difference (ΔpH = pHi - pHf) and the graph is plotted against pHi and pHi – pHf.
Determination of drug loading and encapsulation efficiency
The Imatinib mesylate (IMS) is loaded into the GGND hydrogels by swelling of hydrogels in the drug solution at equilibrium condition. Approximately 500 mg of hydrogel samples are taken into a beaker individually and predetermined concentration of imatinib mesylate solution is added. The drug is diffused into hydrogels when the hydrogels are swollen in drug solution for 48 h at 37 °C. The drug loaded hydrogels are taken out carefully from the solution and cleaned with same drug solution to separate free drug on the surface of the hydrogel. The remaining drug solution is filtered, and the assay is estimated by using UV-visible spectrophotometer at 265 nm λmax. The drug loaded hydrogels are dried for 48 h, and then placed in vaccum oven at 40 °C until weights of hydrogels are not changed. Approximately 20 mg of dried drug loaded hydrogels are powdered with an agate mortar. The hydrogel powder is added to 50 mL of 7.40 pH buffer solution and stirred for 24 h. The resulting solution is centrifuged and filtered with Whatman filter paper to remove hydrogel fragments. The drug content present in supernatant solution is analyzed by UV-visible spectro photometer at λmax 265 nm. The % of IMS loading in the hydrogels and % Encapsulation efficiency of IMS are determined by the following formulae 5 & 6.
In-vitro drug release studies of GGND hydrogels
In-vitro release studies of IMS-GGND hydrogels are carried out in pH 1.20 (stomach pH) and pH 7.40 (intestinal /colon pH) phosphate buffer solutions at 37 °C and 25 °C ± 0.5. Dissolution experiment is carried out by using a tablet dissolution tester (Dissolution Apparaturs, DS-8000, LAB INDIA, Mumbai, India) equipped with 8 baskets. Known weights of each IMS loaded GGND hydrogels (200 mg approximately) are dipped in a basket stirrer having 500 mL of buffer medium at 37 °C or 25 °C ± 0.5 °C, with constant speed of 100 rpm. At a fixed time intervals 5 mL of buffer medium is withdrawn from each basket with a syringe and 5 mL of fresh buffer is refilled into each basket to maintain constant medium volume throughout the experiment. The amount of IMS released from GGND hydrogels corresponding to the time is estimated by UV-Visible spectrophotometer at λmax 265 nm.
In –vitro drug release kinetic studies of GGAH hydrogels
The in-vitro drug release data is fitted to various kinetic mathematical models such as zero order, first order, Higuchi square root equation, Hixson-Crowell cube root equation and Korsmeyer-Peppas kinetic equation to calculate kinetic release parameters such as regression coefficient (R2) drug release rate constant (K) and diffusion exponent (n) to evaluate the best model among different kinetic models [18, 19].
Results and discussion
Characterization
FTIR studies of GGND hydrogels
The FTIR spectrum of pristine IMS is given in Fig. 2(a). The peaks identified at 3425 cm−1 and 1660 cm−1 are the characteristic absorption bands of –N–H vibrational stretching and –C=O vibrational stretching in the amide functional group respectively.
These bands confirm the presence of amide functional group in IMS. The IMS shows characteristic absorption bands at 3056 cm−1, 1576 cm−1 and 808 cm−1 corresponding to –C–H aromatic stretching, –C=C– aromatic stretching and –C–H aromatic bending respectively. These bands also strongly confirm the presence of benzene rings in IMS. The absorption peaks at 3261 cm−1, 1260 cm−1 and 1042 cm−1 are attributed to –N–H vibrational stretching of 2o amines, –C–N aromatic and –C–N aliphatic vibrational stretchings respectively. These are characteristic bands of 2o amine group and aromatic nitrogen contained by the IMS. The characteristic absorption bands at 2973 cm−1 and 1445 cm−1 are ascribed to –C–H vibrational stretching and existence of SO2 group in IMS respectively.
IR spectrum of placebo GGND hydrogels is presented in Fig. 2(b). The amide linkages present in the GGND hydrogel network confirm with characteristic absorption bands appeared at 1641 cm−1 and 1586 cm−1, indicating –C=O vibrational stretching frequencies of amide-I and amide-II linkages. From the IR spectrum, it is observed that a broad peak at 3368 cm−1 is the characteristic absorption peak for the –O–H vibrational stretching and a small peak at 2912 cm−1 is for –C–H vibrational stretching. Other characteristic peaks of GGND hydrogel occur at 1650 cm−1, 1488 cm−1 and 1007 cm−1 correspond to –O–H bending, –C–H bending and –C–O–C– vibrational stretching bands respectively. The absorption bands present in placebo GGND hydrogels confirm the IPN hydrogel structure. From the FTIR spectra data, IMS-GGND hydrogels spectrum is compared with pure GGND hydrogels [Fig. 2(b) & 2(c)]. Here, no additional characteristic absorption peaks are identified. From these FTIR spectral studies, it is found that the IMS drug molecules are entrapped in the GGND hydrogel network only by the physical forces and not by any chemical interactions.
SEM studies of GGND hydrogels
In order to investigate the surface and swelling characteristics of GGND hydrogels, these hydrogels are examined under SEM at different magnifications such as 3000X, 6000X, 10,000X and 15,000X. The SEM images of GGND-5 hydrogels at different magnifications are depicted in Fig. 3.
The SEM images show that the GGND hydrogels’ morphological surface is highly porous in the form of smooth homogeneous surface. The pores present in the hydrogel network are responsible for diffusion and shrinking of water molecules, which causes swelling and deswelling. These pores play a crucial role in the drug charging and controlled molecular drug release in the GGND hydrogel network.
DSC studies of GGND hydrogels
To investigate thermal stableness of pristine IMS, placebo GGND hydrogels and IMS-GGND hydrogels, the DSC studies are conducted. For pristine IMS [Fig. 4a(i)], a sharp peak is appeared at 226.05 °C indicating the melting point of IMS. For placebo GGND hydrogels [Fig. 4a(ii)], two endothermic peaks have appeared, one at 147.00 °C with a fusion heat of 8.86 J/g and the other at 170.59 °C with a fusion heat of 203.00 J/g. As shown in [Fig. 4a(iii)], for IMS-GGND hydrogels mainly two endothermic peaks are recorded – one at 148.91 °C with a fusion heat of 10.60 J/g and another at 169.67 °C with a fusion heat of 182.00 J/g, which may be the result of decomposition of polymer chains. IMS-GGND hydrogels also show the curves, similar to that of the placebo GGND hydrogels. However, the identified peaks of IMS are not observed, indicating that the IMS drug is molecularly loaded into the GGND hydrogel matrix.
X-ray diffraction studies of GGND hydrogels
X-RD graphs of placebo GGND hydrogel, IMS-GGND hydrogel and pristine IMS drug are shown in Fig. 4(b). The X-RD studies are performed to analyze the drug’s polymorphism in the hydrogel network after encapsulation of drug molecules. The X-RD curves of imatinib mesylate (IMS) have peaks of 2θ angles at 10o, 15o, 18o, 21o, 25o, and 28o. A more intense peak is observed at 2θ of 18o; these peaks indicate crystalline structure of IMS drug. However, such kinds of peaks are not identified in IMS-GGND hydrogels. The peaks observed in placebo GGND hydrogels are very similar to the peaks of IMS-GGND hydrogels. From these X-RD studies, it is confirmed that IMS drug molecules are dispersed at molecular level into the GGND hydrogel matrix.
Swelling studies of GGND hydrogels
To examine the swelling limit of GGND hydrogels regarding to pH, temperature stimuli conditions and feed compositions of hydrogels, the dynamic and equilibrium swelling tests are done in different pH conditions (1.20, 7.40 and distilled water) and at 25 °C and 37 °C temperatures. The swelling kinetic parameters are determined and detailed in Table −2.
Effect of cross-linker on swelling capacity of GGND hydrogels
The effect of cross-linker concentration on the GGND hydrogel swelling capacity is determined by employing various amounts of MBA and all other reactants are kept constant. For formulations GGND-6, GGND-2 and GGND-7, concentration of cross-linker are 2.61 × 10−2 mol/L, 4.32 × 10−2 mol/L and 8. 64 × 10−2 mol/L respectively. The equilibrium swelling ratio of hydrogels is decreased rapidly (30.4891 g/g to 9.4597 g/g) with a corresponding increase in cross-linker concentration. At lower concentration of cross-linker, higher swelling of GGND-6 hydrogel is observed. It is mainly because of the loosening of network structure caused by wide hydrodynamic free space, which accommodates more swelling molecules leading to an increase in the equilibrium swelling. Higher the cross-linker concentration, lower is the swelling ratio, which is mainly due to more number of cross-links make the hydrogel denser and results in the decrease of mesh size making it difficult for solvent molecules to permeate [20]. With an increase in cross-link density, the hydrogel’ glass transition temperature (Tg) is also increased and thus the glassy nature of the matrix did not permit the solvent molecules to permeate, which results in lower equilibrium swelling. For formulations GGNA-6, GGND-2, GGND-7 synthesized from different amounts of cross-linker, the diffusion exponent ‘n’ value is more than 0.5. This shows that these hydrogels followed the non-Fickian diffusion method and the swelling of these hydrogels is mainly because of dispersion of solvent molecules as well as polymer relaxation.
Effect of pH on swelling capacity of GGND hydrogels
The swelling properties of pH-responsive hydrogels show significant impact on controlled drug delivery applications. The pH-responsive hydrogels demonstrate varied swelling behaviour under various pH conditions. The influence of pH on the GGND swelling ratio is understood by examining the swelling of GGND hydrogels in pH 1.20 solutions, water and pH 7.40 solutions at 27 °C. The swelling kinetic parameters for GGND hydrogels in different pH conditions are computed and tabulated [Table −2]. Swelling ratio of hydrogels is noted to be higher in pH 7.40 (19.8605 g/g of gel) as compared to that of water (18.4475 g/g of gel) and that of pH 1.20 (17.5339 g/g of gel). At pH 7.40, dissociation of –COOH groups takes place and converted into anionic groups. Due to strong intra anionic repulsions, the pores in hydrogels matrix expand, resulting in an increase in the water uptake capacity [21]. At pH 1.20, the dissociation of –COOH groups is low. These acid groups involves in hydrogen bonding between the polymer chains, which reduce the pore size in the polymer network. Therefore, the degree of GGND hydrogel swelling is decreased at pH 1.20 [22, 23]. The diffusion exponent (n) values are found to be greater than 0.5 for hydrogel in pH 1.20 and pH 7.40 solutions and also in distilled water. So, swelling of hydrogels is found mainly due to the dispersion of solvent molecules and relaxation of polymer network, hence, thereby, the process followed the non- Fickian mechanism.
Effect of temperature on swelling capacity of GGND hydrogels
Swelling medium temperature is a significant property that also influences the hydrogel swelling behavior. In the present work, the influence of temperature on the GGND hydrogel swelling ratio is investigated by conducting swelling experiments at 25 °C and 37 °C at pH 7.40. The GGND hydrogels’ swelling ratio is declined with a rise in temperature of the swelling medium from 25 °C to 37 °C. It is well known fact that IPAM is a thermo-responsive monomer with LCST 32 °C. At 25 °C (below LCST), the thermo-responsive PIPAM contains hydrophilic groups and diffuses many solvent molecules into the hydrogel network, thus, resulting in higher swelling ratio [24]. When the swelling medium temperature is raised above the LCST (37 °C), the thermo-responsive PIPAM undergoes a phase separation due to collapse of hydrogel matrix resulting in the formation of hydrophobic aggregates. Above the LCST, hydrophobic interactions dominate to make the solvent molecules pass out from the hydrogel network, thereby, decreasing the equilibrium swelling ratio [25].
Effect of salts on swelling studies GGND hydrogels
Hydrogel’ swelling studies are conducted with solutions having various concentrations of salts assume significance in the biomedical fields like controlled drug release applications and elimination of metal ions from waste water [26]. In the present study, the swelling experiments of GGND hydrogels are performed in various concentrations (0.05 M, 0.10 M, 0.15 M and 0.20 M) of different salt solutions (NaCl, CaCl2 and AlCl3) and presented in Fig. 5. Basically, swelling capacities for all “anionic” hydrogels in the salt solution are expected to be reduced. The swelling capacities of GGND hydrogels are reduced with a corresponding increase in the concentration of salt solution and it is attributed to more domination of ion-ion interactions than ion-water interactions. In case of hydrogel swelling in salt solution, the difference in osmotic pressure between hydrogel network and salt solution reduces, while the metal ions enhance the screening effect corresponding to an increase in concentration of salt solution [27]. By increasing the charge of the metal ion (M+ < M+2 < M+3), the degree of cross-linking is increased leading to a decrease in swelling capacity of GGND hydrogels.
Determination of GGND hydrogel network parameters
Swelling nature and drug release characteristics of the GGND hydrogels depend upon the network of the hydrogel matrix. Important network parameters (ɸ, M̅c, χ, ve,dp, & ξ) are determined to explain the network structure of the hydrogel. Variations of the GGND hydrogel network parameters with respect to the MBA, IPAM and GG-g-PNPA are evaluated and tabulated [Table −3].
Effect of feed cross-linker on network parameters of GGND hydrogels
To examine the influence of cross-linker (MBA) on GGND hydrogel network parameters, cross-linker concentrations are varied as per the Table −3. The values of ξ and M̅c of GGND hydrogel matrix are decreased with increasing cross-linker concentration from 2.1621 × 10−2 mol/L to 8.6484 × 10−2 mol/L. Consequently, the cross-linking density (ve) of GGND hydrogels networks is also increased from 0.0860 × 10−7 mol/cm3 to 1.3272 × 10−7 mol/cm3 [28]. The mesh size (ξ) and cross-linking density (ve) are important parameters for evaluating swelling, mechanical strength, degradability and drug release properties. These parameters can be controlled by modifying the cross-linker concentration. With an increase in cross-linker concentration, the Flory-Huggins interaction parameter (χ) values are also increased correspondingly, which indicates deceased polymer-solvent interactions and increased polymer-polymer chains interactions. These types of results might be expected because of the fact that there is an increase in number of cross-links between polymer chains in the hydrogel matrix with corresponding increase in the amount of cross-linker, which prompts decline in pore size of voids.
Effect of feed monomer on network parameters of GGND hydrogels
To investigate the influence of feed monomer on the network structure of GGND hydrogels, different network parameters such as ɸ, M̅c, χ, ve,dp, and ξ are determined as a function of feed IPAM and tabulated [Table −3]. The mesh size (ξ) is decreased from 183 nm to 55 nm, while molecular weight between two cross-links (M̅c) is decreased from 15 × 104 g/mol to 10 × 104 g/mol with the increase of IPAM amount from 0.25 g to 1.00 g. The cross linking density (ve) (from 0.1436 × 10−7 mol/cm3 to 1.4513 × 10−7 mol/cm3), volume fraction (ɸ) (from 0.0405 to 0.0664) and Flory-Huggins interaction parameter (χ) (from 0.5152 to 0.5305) – are increased with an increase in feed IPAM concentration. These out comes can be attributed to the facts that as monomer concentration increases cross links of polymer chain also increases and leads to more cross- linking density to decrease mesh size (ξ), the average molecular weight between cross links (M̅c) and increase crosslink density (ve), volume fraction in the swollen state (ɸ). The Flory-Huggins interaction parameter (χ) increased with a corresponding increase of IPAM content. It is because of the hydrophobic moieties present in the hydrogel network, which increase the polymer-polymer interactions while decreasing polymer-water interactions [29].
Effect of feed GG-g-PNPA on network parameters of GGND hydrogels
The feed amount of GG-g-PNPA (2%) is changed from 1 mL to 3 mL for formulations, GGND-1, GGND-2 and GGND-3 to understand the influence of graft copolymer content on network parameters. The obtained results are tabulated [Table −3]. It is observed that there is an increase in values of ξ and M̅c, and a decrease in values of cross link density (νe) and volume fraction (ɸ) with a corresponding increase in the amount of GG-g-PNPA content. This trend of results is caused by the increasing of more number of hydrophilic side chains on the hydrogel network [30]. Flory-Huggins interaction parameter (χ) values are decreased from 0.5308 to 0.5146 with increasing content of GG-g-PNPA in the hydrogel matrix. The decreasing values of (χ) indicate that the polymer–water interactions are decreased and polymer-polymer interactions are increased [31]. These results are strongly supported by swelling trends of GGND hydrogels with variation of graft copolymer content.
Point of zero charge studies of GGND hydrogels
The swelling and drug delivery properties of functional hydrogels depend strongly on the swelling medium’s pH. In the presence of a hydrogel, the swelling medium’s initial pH (pHi) transforms to a dissimilar pH (pHf). The pH point at which the difference between initial pH and final pH (ΔpH = pHi – pHf) of the solution is zero, it is known as the point of zero charge (pHPZC). The pHPZC depends on the net charge accumulated on surface of the hydrogel. In case of neutral hydrogels, the pH of the solution is equal to pHPZC; for negative charged hydrogels, the solution pH is less than pHPZC, and for positive charged hydrogels, the pH of hydrogels is greater than pHPZC [30]. The (pHi – pHf) of the solution is plotted against pHi for placebo hydrogels and IMS loaded GGND (IMS-GGND) hydrogels, which are depicted in Fig. 6(a) & 6(b). Figure 6 shows pHPZC of placebo and IMS–GGND hydrogels around 6.65.
Drug loading and encapsulation efficiency of GGND hydrogels
The percentage drug loading (% DL) and percentage encapsulation efficiency (% EE) of IMS-GGND hydrogels are evaluated and reported in Table −4. The IMS is loaded into GGND hydrogels via equilibrium swelling method and % DL of IMS in GGND hydrogels is found to lie in between the range from 23.14 to 48.68. The results of % EE of different formulations of GGND hydrogels stand in between the range from 38.35 to 69.54. % EE depends on drug concentration, composition of hydrogel network and cross-linking density [32]. The amount of cross-linker showed a consequential effect on % EE; i.e. hydrogels cross-linked with 0.0216 mol/L, 0.0432 mol/L and 0.0864 mol/L of MBA (GGND-6, GGND-2 and GGND-7 respectively), % EE are 38.35, 52.82, 69.54 respectively. This trend is the result of an increase in rigidity of hydrogels, caused by increasing cross-linking density, making IMS leach out in lesser amounts from the hydrogel network. Hence, the % EE is increased.
The % EE decreased with a corresponding increase in the amount of GG-g-PNPA in the hydrogels. For formulations GGND-1, GGND-2 and GGND-3 containing 1 mL, 2 mL and 3 mL (2% w/v) of GG-g-PNPA, the % EE values stood at 59.85, 52.82 and 47.25, respectively. It happens because of the increasing number of –COOH groups with a corresponding increase in graft copolymer (GG-g-PNPA). This further leads to an increase in hydrophilicity and percolation of the drug molecules from loose hydrogel network. The % EE increased with a corresponding increase in the amount of IPAM in hydrogel network. For the hydrogels GGND-4, GGND-2 and GGND-5 synthesized with 0.25 g, 0.5 g and 1.0 g of IPAM, the % EE values are derived at 45.42, 52.82 and 64.24 respectively. This type of outcome may be because of the stiffness of GGND hydrogels resulting from more amount of IPAM.
In-vitro drug release studies of GGND hydrogels
The drug release profile of Imatinib mesylate (IMS) derived from IMS-GGND hydrogels is evaluated with respect to the influence of pH, cross-linker, graft copolymer and temperature.
Impact of pH on in-vitro release of GGND hydrogels
In order to study in-vitro delivery of IMS from GGND hydrogels, in-vitro release experiments are conducted with pH = 1.20 (gastric conditions) and pH = 7.40 (colon conditions) at 37 °C ± 0.5 °C. Higher percentage cumulative release of IMS is observed for all formulations of GGND hydrogels at pH 7.40 than at pH 1.20 (Fig. 7a). At acidic condition (pH 1.20), –COOH functional groups formed hydrogen bonds in the hydrogel network that hinder the release of IMS from GGND hydrogels, which in turn results in less % cumulative release. At pH 7.40, deprotination of acidic groups produces inter-anionic repulsion that leads to enlargement of mesh size and increased leaching out of IMS drug molecules from hydrogels [33]. The void size of hydrogels has an impact on the diffusion of captured drug molecules from hydrogel matrix. The pore size of GGND hydrogel is less in case of pH 1.20 solutions than that of pH 7.40 solutions, which results in lesser seepage of captured drug in pH 1.20 solutions. Drug discharge from the pH-responsive hydrogels is increased with a corresponding increase in the swelling capacity and pore size [34].
Impact of graft copolymer on in-vitro release of GGND hydrogels
To observe the influence of GG-g-PNPA on in-vitro diffusion of IMS from GGND-1, GGND-2 and GGND-3 hydrogels containing 1 mL, 2 mL and 3 mL graft copolymer [2% (w/v)] respectively, the in-vitro cumulative release studies of GGND hydrogels are conducted. Since GGND-3 has a higher content of GG-g-PNPA than GGND-2 or GGND-1, it is observed that GGND-3 had higher % cumulative release (88%) than that of GGND-1 (74%) and GGND-2 (83%) at pH 7.40, extended up to 36 h. These results are depicted in Fig. 7b. These results may be attributed to more hydrophilic graft copolymers contained in GGND-3, which increases its swelling capacity to the extent more than GGND-2 and GGND-1. This helps in release of more amount of IMS compared to GGND-1 and GGND-2 hydrogels. It is observed that the swelling capacity of hydrogels is directly proportional to the drug delivery rate of hydrogels. The drug release is increased by a corresponding increase in the swelling ability of hydrogels. More is the swelling ability, more will be the diffusion of water into the hydrogels that increases osmotic pressure inside GGND hydrogels making them to swell and release more amounts of drug [35].
Impact of cross-linker on in-vitro release of GGND hydrogels
To analyze the impact of cross-linker on drug release, GGND hydrogels synthesized with different concentrations of MBA are studied. For GGND-7 containing 8.64 × 10−2 mol/L of MBA has less % cumulative release (68%), while GGND-6 hydrogels with 2.16 × 10−2 mol/L of MBA have higher % cumulative release (91%) and GGND-2 hydrogels with 4.32 × 10−2 mol/L of MBA have moderate % cumulative release (88%) in pH 1.20 buffer solutions. Similar trend of results are observed in pH 7.40 solution also and graphically presented in Fig. 8a. These results can be explained based on the fact that swelling capacity of GGND hydrogels decreases with increasing cross-linker (MBA) content leading to the retardation of water molecules diffusion into hydrogel matrix. The rate of swelling of hydrogels is determined by their water uptake capability which depends upon the pore size and the availability of hydrophilic functional groups. At lower cross-linker concentration, drug is released at higher rate, because the hydrogel network has more hydrodynamic free space to keep many solvent molecules, resulting in extra swelling of hydrogel matrix [36].
Impact of temperature on in-vitro release of GGND hydrogels
Modern researchers are keen on developing temperature-responsive drug delivery systems that stimulate controlled molecular drug release in response to the variations in body temperature, thus acting as self-regulating systems [37]. In order to evaluate the effect of temperature on IMS drug release, the in-vitro drug release experiments are conducted at 25 °C and 37 °C in pH 7.40 buffer solutions. In-vitro drug release results of GGND-2 hydrogels shown in Fig. 8b depict that a rise in temperature from 25 °C to 37 °C results in higher percentage of cumulative drug release. This outcome can be ascribed to the free volume theory and PIPAM aggregates formed above LCST that triggers release of drug [37]. Free volume theory advocates that the thermal expansion of polymer chains produces free volume in the hydrogel network [38]. At a higher temperature, solvent molecules penetrate into these free spaces, thus, amplifying the pace of IMS molecular release. Aggregation theory advocates that a rise in temperature above the LCST results in the precipitation of the PIPAM chains and generation of more dominant hydrophobic interactions. Because of this, the hydrogel matrix begins to condense, squeezing out the drug molecules from hydrogel network [39].
In-vitro drug release kinetic analysis of GGND hydrogels
Many factors affect the drug release kinetics of hydrogels such as swelling of hydrogel network, erosion of hydrogel network, mode of diffusion of drug into the matrix, drug concentration in hydrogel matrix and geometry of the hydrogel [40]. To understand in–vitro kinetic drug release mechanism of IMS-GGND hydrogels, the drug release data is substituted into different empirical kinetic models. The Regression coefficient (R2) and rate-constant values of each kinetic equation for different formulations of IMS-GGND hydrogels are calculated in pH 1.20 and 7.40 media at 37 °C and tabulated respectively [Tables 5 & 6]. The regression coefficients (R2) values in pH 7.40 buffer media for formulations GGND-1, GGND-5 and GGND-7 release kinetic results that best fit to Higuchi Square root model. This model states that to begin with, the molecular drug release is initiated in the surface layer and once it is fully depleted of the drug molecules, the drug release starts from the second layer forcing the dispersion of drug molecules into the external solution through the network. In this manner, the dispersed drug molecules are moved into the dissolution medium from hydrogel network [41]. The Higuchi Square root equation indicates that drug release from hydrogel network follows a controlled diffusion mechanism and there is a direct relationship between rate and time of drug release. The drug release rate is directly proportional to the square root of drug release time. From Table 5, highest values of regression coefficients (R2) for hydrogels formulations GGND-1 and GGND-5 in pH 1.20 are best suited to the first order equation. In the first order model, the rate constant of drug release is directly proportional to the initial drug concentration and nature of polymer.
It has been observed that (R2) values for formulations GGND-2, GGND-3, GGND-4 GGND-6 and GGND-7 in pH 1.20 solution and for formulations GGND-2, GGND-3, GGND-4, and GGND-6 in pH 7.40 media are best suited for Koresmeyer-Peppas equation with highest values of regression coefficients (R2) greater than 0.98. The results calculated from Koresmeyer-Peppas equation show that the n values for all formulations in both pH 1.20 & 7.40 are >0.45 and < 0.89. It indicates that the process of IMS molecular drug release from GGND hydrogels followed the non-Fickian diffusion mechanism.
Conclusion
In present research work, the pH- and thermo-responsive GGND hydrogels are developed by free radical polymerization of GG-g-PNPA graft copolymer, IPAM and DMAEMA using MBA as a cross-linker. The GGND hydrogel network parameters are calculated from swelling data. It is observed that the mesh size (ξ) and the average molecular weight between two adjacent cross-links (M̅c,) of GGND hydrogel is decreased, while the cross-link density (ve) and Flory-Huggins interaction (χ) of gels is increased with the concentrations of MBA and PIPAM. The reverse results are also observed with graft copolymer content. The swelling capacity of GGND hydrogels changes significantly with cross-linker content, pH and temperature of swelling medium. The GGND hydrogel swelling capacity is observed to be maximum in pH 7.40 than in pH 1.20 and distilled water. This swelling capacity is decreased with a corresponding increase in MBA concentration and increasing temperature from 25 °C to 37 °C. The GGND hydrogel networks are characterized to confirm grafting, structural, surface morphology, drug interaction and thermal properties by FTIR, SEM, X-RD, and DSC studies respectively. The GGND hydrogel swelling ability is notably decreased with a rise in salt concentration and enhanced metal ion valence. The surface charges of the pure and IMS loaded GGND hydrogels are analyzed by point of zero charge. The IMS is loaded into GGND hydrogels by using equilibrium swelling process and the maximum values of % DL (49%) for GGND-6 and % EE (69%) for GGND-7 formulations are obtained. The controlled release of IMS from GGND hydrogels is found to be depended upon the content of cross-linker, graft copolymer, pH and temperature. The maximum release of IMS is observed in GGND-6 hydrogels (94%) in pH of 7.40 at 37 °C. In-vitro kinetic release of IMS is determined by empirical kinetic equations and the values are found to be best suited with Koresmeyer-Peppas equations, Higuchi Square root model and the first order reaction, which follow non-Fickian transport mechanism. These results recommend that the GGND hydrogels can be used as a pH- and temperature-responsive drug transport carriers in the biomedical applications.
References
Narasimharao R, Anushareddy M, Swethareddy N, Divyasagar P, Keerthana K (2011). Int J Res Pharmaceut Biomed Sci 2:1118–1133
Sharpe LA, Daily AM, Horava SD (2014) Peppas NA. Therapeutic applications of hydrogels in oral drug delivery Expert Opin Drug Deliv 11:901–915
Krishnarao KSV, Ha C-S (2009) pH sensitive hydrogels based on acryl amides and their swelling and diffusion characteristics with drug delivery behavior Polym bull 62:167–181
Muniz EC, Geuskens G (2001) Compressive elastic modulus of polyacrylamide hydrogels and semi-IPNs with poly(N-isopropylacrylamide). Macromolecules 34:4480–4484
KrishnaRao KSV, Naidu BVK, Subha MCS, Sairam M (2002) Aminabhavi TM. Novel chitosan-based pH-sensitive interpenetrating network microgels for the controlled release of cefadroxil Carbohyd Polym 66:333–344
Pratikkumar P, Abhirup M, Vrinda G, Dhananjay P, Ashim KM (2019) Thermosensitive hydrogel-based drug delivery system for sustained drug release. J Polym Res 26:131–141
Guo BL, Gao QY (2007) Preparation and properties of a pH/temperature-responsive carboxy methyl chitosan/poly(N-isopropyl acrylamide)semi-IPN hydrogel for oral delivery of drugs. Carbohydr Res 342:2416–2422
David GS, Richard MS, John MGB (1997) Clinical features at diagnosis in 430 patients with chronic myeloid leukemia seen at referral Centre over a 16 year period Br. J Haematol 96:111–116
Nagesh RS, Senthilkumar KL, Senthil PS (2012) Design and evaluation of imatinib mesylate microspheres using chitosan & HPMC- K100. Int J Pharm Pharm Sci 4:4749–4758
Du J, O’Reilly RK (2010) pH-responsive vesicles from a schizophrenic Di block copolymer Macromo. Chem Phys 211:1530–1537
Jalababu R, Veni SS, Suresh Reddy KVN (2018) Synthesis and characterization of dual responsive sodium alginate-g-acryloyl phenylalanine-poly N-isopropyl acrylamide smart hydrogels for the controlled release of anticancer drug. J Drug Deliv Sci Technol 44:190–204
Gulen OA, Mehlika P (2020) Controlled release behavior of zinc-loaded carboxymethyl cellulose and carrageenan hydrogels and their effects on wheatgrass growth. J Polym Res 27:6–16
Babic MM, Antic KM, Jovasˇevic JS, Bojan D, Bozˇic V, Davidovic SZ, Filipovic JM, Tomic SL (2015) Oxaprozin/poly(2-hydroxyethyl acrylate/itaconic acid) hydrogels:morphological, thermal, swelling, drug release and antibacterial properties. J Mater Sci 50:906–922
Madhusudana rao K, Mallikarjuna B, KSV K, Sudhakar K, Chowdojirao K, MCS S (2013) Synthesis and characterization of pH sensitive poly (Hydroxy ethyl methacrylate-co-acrylamidoglycolic acid) based hydrogels for controlled release studies of 5-fluorouracil. Int J Polym Mater 62:565–571
Chung JT, Vlugt-Wensink KDF, Hennink WE (2005) Zhang Z. Effect of polymerization conditions on the network properties of dex-HEMA microspheres and macro-hydrogels Int J Pharm 288:51–61
Aithal US, Aminabhavi TM (1990) Interactions of organic halides with a polyurethane elastomer. J Membr Sci 50:225–241
Mall ID, Srivastava VC, Kumar GVA (2006) Mishra IM. Characterization and utilization of mesoporous fertilizer plant waste carbon for adsorptive removal of dyes from aqueous solution Colloids Surf A Physicochem Eng Aspects 278:175–187
Dash S, Murthy PN, Nath L (2010) Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems Acta Pol Pharm 67:217–223
Angadi SC, Manjeshwar LS, Aminabhavi TM (2011). Stearic Acid-Coated Chitosan-Based Interpenetrating Polymer Network Microspheres:Controlled Release Characteristics Ind Eng Chem Res 50:4504–4514
Peng G, Shimei X, Peng Y, Wang J (2008) Zheng L. A new amphoteric superabsorbent hydrogel based on sodium starch sulfate Bioresour Technol 99:444–447
Krishna B, Dhiraj B, Veena K, Veena C (2001) Interpenetrating polymer networks based on poly-(acrylicacid) and gelatin I Swelling & Thermal Behavior. J Appl Polym Sci 82:217–227
Gao Qi Z, Liu Sheng Z, Mei Hua Z, Jing Hong M, Bo Run L (2005) Responsive semi-interpenetrating polymer network hydrogels based on linear sodium alginate and cross-linked poly (N-isopropyl acrylamide). J Appl Polym Sci 97:1931–1940
Xue B, Xinghui S, Xiaoya D, Lijie D, Chunsheng X (2019) pH-responsive hydrogels based on the self-assembly of short polypeptides for controlled release of peptide and protein drugs. J Polym Res 26:278–287
Rama subbareddy P, Madhusudana Rao K, Krishna Rao KSV, Shchipunov Y, Chang-Sik H (2014) Synthesis of alginate based silver nano-composite hydrogels for biomedical applications. Macromol Res 22:832–842
Sivagangi Reddy N, Krishna Rao KSV, Eswaramma S (2016) Madhusudana Rao K. Development of temperature responsive semi-IPN hydrogels from PVA/PNVC/PAm for controlled release of anti-cancer agent Soft Mater 14:96–106
Clara I, Lavanya R, Natchimuthu N (2016) pH and temperature responsive hydrogels of poly-(2-acrylamido-2-methyl-1-propanesulfonicacid-co-methacrylicacid) synthesis and swelling characteristics. J Macromol Sci A 53:492–499
Wenbo W, Qin W (2011) Aiqin W (2011) pH-responsive Carboxy methyl cellulose-g-poly (sodiumacrylate)/poly vinyl pyrrolidone semi-IPN hydrogels with enhanced responsive and swelling properties. Macromol Res 19:57–65
Xiuyu L, Wenhui W, Jianquan W, Yufeng D (2006) The swelling behavior and network parameters of guargum/poly-(acrylicacid) semi interpenetrating polymer network hydrogels. Carbohydr Polym 66:473–479
Ali E, Ebrahim VF, Mohammad I (2007) Swelling behavior, mechanical properties and network parameters of pH-& temperature sensitive hydrogels of poly(2- dimethylamino) ethyl methacrylate-co-butyl methacrylate. Eur Polym J 43:1986–1995
Bidyadhar M, Samit Kumar R (2014) Swelling, diffusion, network parameters and adsorption properties of IPN hydrogel of chitosan and acrylic co-polymer mater. Sci Eng C 44:132–143
Baljit S, Chauhan N, Vikrant S (2011). Design of Molecular Imprinted Hydrogels for Controlled Release of Cisplatin Evaluation of Network Density of Hydrogels Ind Eng Chem Res 50:13742–13751
Praveen BK, Manjeshwar LS, Aminabhavi TM (2011) Novel inter-penetrating polymer network hydrogel microspheres of chitosan and poly (acryl amide)-grafted Guargum for controlled release of ciprofloxacin Ind. Eng Chem Res 50:13280–13287
Ali L, Ahmad M, Usman M (2014) Yousuf M. Controlled release of highly water soluble anti-depressant from hybrid co-polymer polyvinylalcohol hydrogels Polym Bull 71:31–46
Viswanatha Reddy G, Sivagangi Reddy N, Nagaraja K, Krishna Rao KSV (2018) Synthesis of pH-responsive hydrogel matrices from Guargum and poly (acrylamide-cl-acrylamidoglycolicacid) for anti cancer drug delivery. J Appl Pharm Sci 8:84–91
Hussain T, Ansari M, Ranjha NM, Khan IU, Shahzaad Y (2013) Chemically cross linked poly (acrylic-co-vinyl sulfonic) acid hydrogel for the delivery of isosorbide mononitrate. Sci World J 1:1–9
Kumaresh SS (2002) Aminabhavi TM. Water transport & drug release study from cross linked poly acrylamide grafted guargum hydrogel microspheres for the controlled release application Eur J Pharm Biopharm 53:87–98
Chu LY, Park SH, Yamaguchi T, Nakao S (2001) Preparation of thermo responsive core shell microcapsules with a porous membrane and poly (isopropyl acrylamide) gates. J Membr Sci 192:27–39
Mark JE, Eisenberg A, Grataessly WW, Maldelkern L (1984) Koening JL. Physical Properties of Polymers American Chemical Society, Washington
Zhang JT, Cheng SX, Huang SW, Zhuo RX (2003) Temperature sensitive poly (N-isopropyl acrylamide) hydrogels with macro-porous structure and fast response rate. Macromol Rapid Commun 24:447–451
Dadsetan M, Taylor KE, Yong C, Bajzer Z, Lu L (2013) Yaszemski MJ. Controlled release of doxorubicin from pH-responsive microgels Acta Biomater 9:5438–5446
Rakesh KM, Mahesh D, Ajit KB (2008) Synthesis & Characterization of pectin-PVP hydrogel membranes for drug delivery system. AAPS Pharm Sci Tech 9:395–403
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jalababu, R., Rao, K.K., Rao, B.S. et al. Dual responsive GG-g-PNPA/PIPAM based novel hydrogels for the controlled release of anti- cancer agent and their swelling and release kinetics. J Polym Res 27, 83 (2020). https://doi.org/10.1007/s10965-020-02061-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-020-02061-0