Abstract
Purpose of Review
Psoriasis is a chronic inflammatory skin condition affecting greater than 7 million adults in the USA. There are a multitude of treatment options available for psoriasis management. This review provides an overview of the current use of phototherapy in the treatment of psoriasis, and an update on recent phototherapy advances, either as a single modality or in conjunction with other agents.
Recent Findings
Advances in pharmacotherapy and an increased understanding of the pathogenesis of psoriasis have led to the development of new systemic treatments for psoriasis. A small number of recent studies have evaluated the use of phototherapy in comparison to or in combination with emerging and traditional treatment modalities.
Summary
Psoriasis is a chronic skin condition with a high disease burden among patients. More than half of patients are dissatisfied with their therapy, and many are not receiving adequate levels of treatment. Phototherapy remains an effective modality for the treatment of psoriasis with minimal risk. While some studies have evaluated the use of phototherapy with systemic options, more studies are needed to make definitive conclusions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
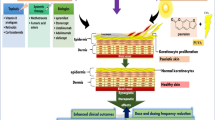
Psoriasis is a T cell-mediated disease that increases morbidity and mortality, reduces quality of life, and has a profound impact on patients’ psychosocial well-being [1]. Psoriasis is categorized, based on the percentage of body surface area (BSA) involvement, as either mild (< 3% BSA), moderate (3–10% BSA), or severe (> 10% BSA). The impact upon the individual is also a consideration when evaluating psoriasis [2]. Treatment planning is developed based on these severity classifications and the impact of psoriasis on the individual (Fig. 1).
Topical agents and localized phototherapy are ideal for mild psoriasis, while moderate-to-severe psoriasis is treated with topical therapy plus phototherapy and/or systemic therapy [3]. Topical therapy options include corticosteroids, vitamin D analogues, retinoids, anthralin, coal tar, and salicylic acid [4,5,6,7]. Systemic therapy options include non-biologics and biologics [8•].
The use of phototherapy for the treatment of skin conditions dates back thousands of years. Its utility in the treatment of psoriasis was established with the advent of broadband ultraviolet B (BB-UVB), narrowband ultraviolet B (NB-UVB), psoralen plus ultraviolet A (PUVA), and laser treatment modalities [9]. Phototherapy is considered a first-line therapy in the treatment of psoriasis due to its efficacy and limited contraindications [10]. We aim to provide an overview of the current use of phototherapy in the treatment of psoriasis and highlight recent updates in the field.
Methods
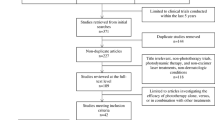
A PubMed search of clinical trials from May 1995 to November 2017, using the terms psoriasis, phototherapy, UVB, PUVA, UVA, excimer laser, and light-emitting diodes (LED), was conducted. This search generated articles pertaining to the use of phototherapy versus, or in combination with, topical, systemic, or psychological treatments (Table 1). Articles published within the last 5 years reporting patient-centered outcomes were selected for inclusion in this review. A separate PubMed search using the same terms was conducted without the use of a 5-year filter in order to generate additional information.
Phototherapy Overview
Mechanism of Action
Psoriasis is a T cell-mediated disease driven by T helper 1 (Th1) and T helper 17 (Th17) pathways. Keratinocyte hyperproliferation and inflammation occurs via a milieu of cytokines including tumor necrosis factor (TNF)-α, interleukin (IL)-17, and IL-23 [12••]. There are several mechanisms by which phototherapy may impact the pathogenesis of psoriasis [13]. Phototherapy shifts cytokine production in the direction of the counter-regulatory T helper 2 (Th2) immune response both locally and systemically [14, 15]. Keratinocyte apoptosis and upregulation of p53, a tumor suppressor gene, are induced by ultraviolet radiation [16, 17]. Phototherapy induces the migration of histiocytes out of the epidermis [18]. The cumulative effects of these mechanisms improve psoriasis [19].
Indications, Contraindications, and Adverse Reactions (See Table 2)
Phototherapy, either as a targeted or whole body treatment, improves mild or moderate-to-severe psoriasis, with NB-UVB constituting the most widely administered form [10, 20]. As with any treatment option, use should be tailored to the individual characteristics of the patient.
There are relatively few absolute contraindications to commencing UVB phototherapy or PUVA. Diagnoses of photosensitive dermatoses such as systemic lupus erythematous or xeroderma pigementosum are contraindications to phototherapy use [21]. Relative contraindications to phototherapy include personal history of melanoma or non-melanoma skin cancer, family history of melanoma, Fitzpatrick skin type 1/2, immune suppression due to solid organ transplant, emotional or physical limitation to treatment, or previous arsenic or ionizing radiation treatment. Pregnancy, lactation, liver disease, or a history of cyclosporine or methotrexate use are relative contraindications to the use of PUVA [11•, 21].
Although phototherapy is generally well tolerated, there are some short-term and long-term factors that may influence adherence and the phototherapy modality employed. Short-term complications include erythema, blistering, and itching. Long-term complications include photoaging and increased risk of developing non-melanoma skin cancer in those with higher cumulative doses of oral PUVA [11•, 22, 23]. Studies have not confirmed an increased risk of squamous or basal cell carcinoma with the use of UVB therapy alone [24].
Special Considerations
Pediatric and pregnant patients represent population subsets that often generate extra scrutiny prior to the administration of phototherapy. Pregnancy is not considered a contraindication to UVB phototherapy [25]. UVB phototherapy should be considered a primary treatment for pregnant patients with plaque or guttate psoriasis [26]. Phototherapy is a safe and effective second-line treatment option for pediatric patients who are unresponsive to topical therapy [27].
Home Phototherapy
The time commitment and potential cost of office-based phototherapy can be prohibitive for many patients. The potential success of a phototherapy regimen may, in part, depend on the location of treatment [28]. Home UVB is as effective as office-based UVB and is associated with a greater degree of patient satisfaction [29]. In appropriate patients, home UVB may be an ideal option for the treatment of psoriasis [8•].
Clinical Use
Prior to initiating treatment with phototherapy, psoriasis disease severity should be assessed. Objective assessments include psoriasis area and severity index (PASI), BSA, and physician global assessment (PGA) [19]. Phototherapy protocols are typically based on the minimal erythema dose (MED) or the Fitzpatrick skin type [11•]. The MED is the lowest dose of UVB that generates trace erythema 24 h after commencing UVB therapy [30]. BB-UVB, NB-UVB, excimer laser, and PUVA protocols recommended by the American Academy of Dermatology are available online [31,32,33,34].
Broadband Ultraviolet B, Narrowband Ultraviolet B, and Excimer Laser
NB-UVB (311–313 nm) is a first-line treatment for patients with moderate-to-severe psoriasis and a second-line treatment for patients with mild disease which has been unresponsive to topical therapy. BB-UVB (290–320 nm) treatments begin with an initial 20- to 60-mJ/cm2 dose based on the Fitzpatrick skin type, or a dose equivalent to 50% of MED, and are typically administered three to five times per week [11•].
NB-UVB has largely replaced the use of BB-UVB due to decreased time to the achievement of plaque clearance, and an increased duration of remission [35]. The side effects of BB-UVB and NB-UVB are similar. Since efficacy of NB-UVB is achieved at a lower total cumulative dose of UVB radiation, the long-term complications of UVB phototherapy may be reduced when using NB-UVB [36, 37]. NB-UVB treatments begin with an initial 130- to 400-mJ/cm2 dose based on the Fitzpatrick skin type, or a dose equivalent to 50% of MED, and are typically administered three to five times per week [11•].
Excimer, or “excited dimer,” laser therapy is a targeted phototherapy treatment which uses 308 nm UVB light, and has emerged over the last two decades as an effective option for localized disease unresponsive to topical steroids [38]. Excimer treatment, and other forms of localized UV treatment, has the benefit of targeting only involved skin, while sparing uninvolved areas, allowing for a reduction in the number of treatments and the long-term adverse effects of phototherapy [39, 40]. Localized UV is also useful in treating recalcitrant areas and locations which are difficult to reach with generalized phototherapy [41]. Excimer therapy is initiated based on skin type and plaque thickness, at a dose range of 400 to 900 mJ/cm2; treatment is typically administered two to three times per week [11•].
Psoralen and Ultraviolet A
PUVA is an established and efficacious form of phototherapy and is particularly useful in the treatment of palmoplantar psoriasis. However, concerns over increased long-term risk of carcinogenicity have led to reduction in its use at present [42]. UVA (340–400 nm) penetrates deeper into the skin than UVB and modifies processes deeper in the dermal layer by effecting blood vessels, endothelial cells, dermal fibroblasts, and dendritic cells in addition to reducing keratinocyte proliferation and promoting histiocyte migration out of the epidermis [43].
UVA therapy can be combined with oral or topical psoralen to produce a greater degree of immune suppression than observed with UVA alone [44]. Standard therapy in the USA is to administer oral 8-methoxypsoralen which intercalates between DNA base pairs. When subjected to UVA, 8-methoxypsoralen inhibits DNA replication by forming crosslinks. The activation of psoralen by UVA also promotes membrane and mitochondrial damage by promoting the formation of reactive oxygen species [45]. 8-Methoxypsoralen is administered 1 to 2 h prior to UVA treatment at a dose of 0.4–0.6 mg/kg. Treatment is typically administered two to three times per week [11•].
Current Trends and Recent Updates
NB-UVB
NB-UVB in Combination with Behavioral Therapy
There is some evidence to support the idea that psychological stress may reduce the rate of clearance in psoriasis patients undergoing treatment with phototherapy [46, 47]. A study evaluating response to NB-UVB in 40 subjects randomized to either NB-UVB or NB-UVB plus cognitive behavioral therapy (CBT) noted that the PASI75 rate at 8 weeks in the NB-UVB plus the CBT group was higher than the NB-UVB group alone (65 vs. 15%; p = 0.007) [48].
NB-UVB in Combination with Vitamin D Analogues
Vitamin D analogues, such as calcipotriene, when used concurrently with UVB phototherapy decrease the time to symptomatic relief and lower the overall cumulative UVB dose that patients receive [49]. A 12-week, open-label, prospective right-left, intra-individual clinical trial assessed the efficacy and safety of topical tacalcitol in combination with NB-UVB versus NB-UVB alone in 30 subjects with psoriasis. Target lesions on one side of the subjects were treated with tacalcitol ointment; no treatment was applied to target lesions on the opposite side. NB-UVB was administered three times weekly. Tacalcitol plus NB-UVB use led to earlier plaque clearance and better maintenance of response than NB-UVB alone [50]. In comparing the efficacy of calcipotriol versus tacalcitol in combination with NB-UVB, 30 subjects with stable plaque psoriasis were enrolled in 12-week clinical trial. Target lesions on the subject’s left side were treated with tacalcitol ointment once daily while target lesions on the subject’s right side were treated with calcipotriol ointment twice daily. NB-UVB was administered three times weekly [51]. The calcipotriol group achieved lower mean target plaque scores throughout the study (p < 0.05). At 12 weeks, 90.0% of subjects in the calcipotriol group versus 76.67% of subjects in the tacalcitol group achieved or maintained target plaque clearance (p < 0.05). Adverse events (AEs) were mild, consisting mainly of pruritus and hyperpigmentation, and were similar among the two groups [51].
NB-UVB in Combination with Non-biologic Systemic Therapies
In a recent study, the efficacy of methotrexate (MTX) plus NB-UVB versus either MTX or NB-UVB alone was evaluated in 113 subjects with psoriasis. The MTX plus NB-UVB group achieved PASI90 at 6.11 ± 1.28 versus 20.87 ± 4.21 weeks in the MTX alone group, and 11.42 ± 2.36 in the NB-UVB alone group (p < 0.0001). The cumulative dose of NB-UVB was 12.13 ± 4.02 J/cm2 in the MTX plus NB-UVB group versus 34.48 ± 13.13 J/cm2 in the NB-UVB alone group (p < 0.0001). The cumulative dose of MTX was 116.04 ± 20.47 mg in the MTX + NB-UVB group versus 298.63 ± 60.26 in the MTX alone group (p < 0.0001). No significant differences in AEs were noted between groups [52].
NB-UVB in Combination with Biologic Therapies
A prospective clinical trial evaluated the efficacy of etanercept (ETN) in combination with NB-UVB in the treatment of psoriasis. In the cohort, NB-UVB had been administered as a first-line treatment to 322 subjects with moderate-to-severe psoriasis. Subjects who were unresponsive to NB-UVB (defined as failing to achieve a PASI75 at 8 weeks) were evaluated for treatment with conventional systemic therapy (MTX, cyclosporine, acitretin, PUVA). If subjects were ineligible for conventional therapies or failed to respond, they were treated with ETN 50 mg twice weekly. If subjects subsequently failed to achieve PASI75 in 12 weeks on ETN alone, they were treated with NB-UVB plus ETN. A total of eight patients met these criteria. Of the eight that received NB-UVB plus ETN, all achieved a PASI75 after 14.6 ± 3.3 exposures to NB-UVB. No significant AEs were observed in patients receiving this combination [53].
In a study assessing the efficacy of ETN plus NB-UVB, 99 subjects received a 12-week course of ETN. Seventy-five subjects did not achieve PASI90 after 12 weeks of ETN alone and were randomized to receive etanercept 50 mg once weekly monotherapy or in combination with NB-UVB three times weekly over a 4-week time period. Of the subjects with high adherence to NB-UVB, PASI90 was achieved at week 16 in 42.9% of subjects in the ETN plus NB-UVB group compared to 3.4% in the ETN monotherapy group (p = 0.018). There were no significant differences in AEs in either group [54].
The combination of NB-UVB + ETN versus ETN alone was evaluated in 30 subjects with a BMI > 30. All subjects initially received 50 mg subcutaneous ETN twice weekly for 12 weeks. Subjects were then randomized to receive either a maintenance dose of 50 mg ETN weekly or NB-UVB plus 50 mg ETN maintenance dose. The NB-UVB plus ETN group had similar PASI75 scores at 24 weeks when compared to the ETN monotherapy group (53.3 vs. 46.7%) [55].
Targeted UVB
A recent study evaluated the use of a single dose of targeted UVB at ten times the MED (MED10) in 18 subjects with plaque psoriasis, excluding the face, hands, feet, scalp, or intertriginous areas. Subjects were evaluated 4 and 8 weeks after receiving a single MED10 dose. PASI scores were reduced from a baseline of 16.2 at treatment start, to 8.0 and 7.2 at 4 and 8 weeks, respectively (p = 0.005). AEs typically resolved within 3 days, were reported as mild, and included erythema, edema, and clear exudate at treated sites 1 to 2 days post treatment in 5 of the 18 subjects [56].
Excimer Laser
Excimer Laser Monotherapy
In a separate study evaluating the efficacy and safety of a 308-nm excimer laser in the treatment of scalp and palmoplantar psoriasis, 41 subjects with resistant scalp or palmoplantar psoriasis were recruited. Twice-weekly treatment was initiated at three times the MED (MED3) and increased at intervals no greater than 20% for each subsequent visit. Single treatments were skipped if subjects exhibited sunburn-like reactions from the previous treatment cycle. Subjects with scalp psoriasis were evaluated at baseline using the Psoriasis Severity Scalp Index (PSSI) and re-evaluated at 2-week intervals until week 12. At 12 weeks, 13 subjects achieved at least a 75% reduction in PSSI, and 8 subjects achieved at least a 50% reduction in PSSI. In the palmoplantar psoriasis group, 15 subjects were evaluated at baseline using the Psoriasis Severity Index (PSI). PSI scores at baseline were 7.4 and were reduced to 1.3 after 12 weeks of laser treatment. Clinical response was observed in as few as one or two treatments. Treatment was well tolerated; side effects generally consisted of sunburn-like reactions such as hyperpigmentation, erythema, crusting, blistering, or itching [57].
Excimer Laser in Combination with Topical Therapies
A 12-week, open-label, pilot study assessed the efficacy of calcitriol 3 μg/g ointment and clobetasol dipropionate 0.05% spray in combination with the XTRAC® 308 nm excimer laser in the treatment of psoriasis. Twenty-one subjects with ≤ 10% BSA plaque psoriasis were recruited. All subjects were treated with excimer laser twice weekly for the first 6 weeks. Subjects who did not achieve PASI75 after week 6 were treated with the excimer laser twice weekly as needed for weeks 7 through 12. Dosing was based on induration of the target lesion. Topical treatment was rotated during the 12-week study period in combination with excimer laser. During weeks 1 through 4, subjects were treated with clobetasol spray twice daily. During weeks 5 through 8, subjects were treated with calcitriol ointment twice daily. During weeks 9 through 12, subjects were treated with clobetasol spray and calcitriol ointment twice daily. At week 12, 76% of subjects achieved PASI75, and 52% of subjects achieved a PGA score of clear or almost clear. AEs were mild and consisted of short-term phototoxic reactions [58].
UVA and PUVA
PUVA treatment can be administered using oral 8-methoxypsoralen, or via warm bath administration in which psoralen derivatives have been dissolved. A two-armed multicenter trial evaluating the two approaches was undertaken in 74 subjects with moderate-to-severe psoriasis. No significant difference in efficacy was observed in the two treatment groups. Bath PUVA may be an effective option for patients with contraindications to receiving oral PUVA [59].
In a study comparing the efficacy of BB-UVA and PUVA in the treatment of psoriasis, 61 subjects with > 30% BSA involvement were enrolled. Thirty subjects received PUVA, 16 received BB-UVA at 10 J/cm2, and 15 received BB-UVA at 15 J/cm2. The BB-UVA group achieved similar results until session 24, but then plateaued, while the PUVA group continued to achieve improvement. The study concluded at 48 sessions. Phototoxic reactions were the most common side effect and occurred more frequently and with greater severity in the BB-UVA 15-J/cm2 group [60].
The efficacy of UVA1, which uses non-erythemogenic wavelengths between 340 and 400 nm, was evaluated in the treatment of 62 subjects with palmoplantar pustulosis (PPP). Subjects were evaluated using the palmoplantar pustular psoriasis area and severity index (PPPASI) score. Subjects with a baseline score of 9.4 were treated for up to 30 sessions with UVA1 light three times per week with a wavelength range of 340 to 400 nm (peak emission at 365 nm). A 75% reduction in PPPASI (1.7 ± 1.9) score was achieved in 72.6% of subjects by session 30. AEs included hyperpigmentation, pruritus, and sunburn-like reactions [61].
Recent studies have evaluated the efficacy of oral psoralen plus sunlight (PUVAsol) [62, 63]. In a randomized prospective clinical trial comparing the clinical and cost effectiveness of PUVAsol versus PUVA therapy in the management of psoriasis, subjects were randomized to receive either PUVA treatment or PUVAsol treatment. Thirty-six subjects completed the study. The reduction in PASI score was greater in the PUVA group at 2 and 4 weeks compared to the PUVAsol group (p = 0.034). At 8 weeks, 12 weeks, and endpoint, treatment response was comparable in both groups [62]. In a study assessing the efficacy of PUVAsol versus the combination of oral isotretinoin and PUVAsol, 40 subjects with psoriasis were randomized to receive PUVAsol alone or PUVAsol plus oral isotretinoin. Subjects who received PUVAsol plus oral isotretinoin achieved PASI75 in a mean of 8 weeks while subjects who received PUVAsol alone achieved PASI75 in a mean of 10 weeks [63]. Variability in the dose of UV light is inevitable with use of PUVAsol, but PUVAsol has a similar efficacy and side effect profile as traditional PUVA and may be beneficial in resource-limited countries.
LED Light
The use of LED light therapy in the treatment of psoriasis has emerged as a potential treatment option [64]. LED blue light (LED-BL) with peak wavelengths ranging from 420 to 453 nm is efficacious and associated with minor side effects such as hyperpigmentation [65, 66].
The efficacy of 630-nm peak wavelength LED red light (LED-RL) compared to 430-nm peak LED-BL was evaluated in 20 subjects with psoriasis. Clinical improvement was noted in both treatment groups. Improved erythema of psoriatic plaques was noted throughout the study period in those who receive LED-BL, while those who received LED-RL had no improvement in erythema beyond six illuminations. Patient-reported hyperpigmentation was less in subjects who received LED-RL [67].
The efficacy of LEDs emitting in the UVB spectrum (UVB-LED) was evaluated in a prospective, right-left comparative, open study. Twenty subjects with psoriasis were enrolled; symmetrical psoriasis lesions on the extremities or trunk were chosen. One lesion was treated with UVB-LED with aggressive dose escalation or slow dose escalation; the other lesion was left untreated and served as control. Overall improvement at the end of treatment was 93% in the aggressive dose escalation group and 84% in the slow dose escalation group. Results were similar to NB-UVB delivery by traditional bulbs, providing evidence that a conversion to more cost-effective LED lighting may be an option pending the availability of more evidence to support this switch [68].
Conclusions
Barriers to treatment with phototherapy exist and compliance may be a challenge for patients [3]. The use of phototherapy may be impeded by geographic accessibility of phototherapy units, cost of travel, and personal and professional conflicts that interfere with consistent treatment [69••]. Home phototherapy options may help address these hurdles. Phototherapy remains a safe and efficacious treatment modality in psoriasis patients with optimal treatment adherence.
There has been a rapid expansion in the available treatment options for psoriasis. New pharmacologic therapies which target specific immune pathways have emerged. However, clinical trials assessing the role of phototherapy in combination with emerging biologic therapies are lacking. Limited data suggests that simultaneous use of phototherapy modalities and biologic therapies may be safe and beneficial in select patients.
Approximately 10–15% of patients with psoriasis receive some form of light therapy annually [70••]. Phototherapy should continue to be a first-line treatment for patients with moderate-to-severe disease or in those who are unresponsive to topical treatment. Future studies should evaluate the use of phototherapy in comparison to and in conjunction with recent and emerging treatment options.
References
Papers of particular interest, published recently, have been highlighted as: •Of importance ••Of major importance
Fowler JF, Duh MS, Rovba L, Buteau S, Pinheiro L, Lobo F, et al. The impact of psoriasis on health care costs and patient work loss. J Am Acad Dermatol. 2008;59(5):772–80. https://doi.org/10.1016/j.jaad.2008.06.043.
National Psoriasis Foundation. Learn about plaque psoriasis, guttate psoriasis, inverse psoriasis, and pustular psoriasis. https://www.psoriasis.org/about-psoriasis. Accessed November 6, 2017.
Smith J, Cline A, Feldman SR. Advances in psoriasis. South Med J. 2017;110(1):65–75. https://doi.org/10.14423/SMJ.0000000000000596.
Ozdemir M, Engin B, Baysal I, Mevlitoglu I. A randomized comparison of acitretin-narrow-band TL-01 phototherapy and acitretin-psoralen plus ultraviolet A for psoriasis. Acta Derm Venereol. 2008;88(6):589–93. https://doi.org/10.2340/00015555-0529.
Juzeniene A, Grigalavicius M, Juraleviciute M, Grant WB. Phototherapy and vitamin D. Clin Dermatol. 2016;34(5):548–55. https://doi.org/10.1016/j.clindermatol.2016.05.004.
van de Kerkhof PCM, Barker J, Griffiths CEM, Kragballe K, Mason J, Menter A, et al. Psoriasis: consensus on topical therapies. J Eur Acad Dermatol Venereol. 2008;22(7):859–70. https://doi.org/10.1111/j.1468-3083.2007.02534.x.
Feldman SR, Goffe B, Rice G, et al. The challenge of managing psoriasis: unmet medical needs and stakeholder perspectives. Am Health Drug Benefits. 2016;9(9):504–13.
• Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61(3):451–85. https://doi.org/10.1016/j.jaad.2009.03.027. Discusses general systemic treatment options in the management of psoriasis.
Jarrett P, Scragg R. A short history of phototherapy, vitamin D and skin disease. Photochem Photobiol Sci. 2017;16(3):283–90. https://doi.org/10.1039/c6pp00406g.
Lapolla W, Yentzer BA, Bagel J, Halvorson CR, Feldman SR. A review of phototherapy protocols for psoriasis treatment. J Am Acad Dermatol. 2011;64(5):936–49. https://doi.org/10.1016/j.jaad.2009.12.054.
• Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62(1):114–35. https://doi.org/10.1016/j.jaad.2009.08.026. Provides an overview of phototherapy and photochemotherapy in the treatment of psoriasis.
•• Nakamura M, Farahnik B, Bhutani T. Recent advances in phototherapy for psoriasis. F1000Res. 2016;5:F1000. https://doi.org/10.12688/f1000research.8846.1. Highlights the recent updates pertaining to phototherapy use in psoriasis.
Hemne PS, Kunghatkar RG, Dhoble SJ, Moharil SV, Singh V. Phosphor for phototherapy: review on psoriasis. Luminescence. 2017;32(3):260–70. https://doi.org/10.1002/bio.3266.
Enk CD, Sredni D, Blauvelt A, Katz SI. Induction of IL-10 gene expression in human keratinocytes by UVB exposure in vivo and in vitro. J Immunol. 1995;154(9):4851–6.
Coimbra S, Oliveira H, Reis F, Belo L, Rocha S, Quintanilha A, et al. Interleukin (IL)-22, IL-17, IL-23, IL-8, vascular endothelial growth factor and tumour necrosis factor-alpha levels in patients with psoriasis before, during and after psoralen-ultraviolet A and narrowband ultraviolet B therapy. Br J Dermatol. 2010;163(6):1282–90. https://doi.org/10.1111/j.1365-2133.2010.09992.x.
Weatherhead SC, Farr PM, Jamieson D, Hallinan JS, Lloyd JJ, Wipat A, et al. Keratinocyte apoptosis in epidermal remodeling and clearance of psoriasis induced by UV radiation. J Invest Dermatol. 2011;131(9):1916–26. https://doi.org/10.1038/jid.2011.134.
Zhang D, Chen Y, Chen L, Yang R, Wang L, Liu W, et al. Ultraviolet irradiation promotes FOXP3 transcription via p53 in psoriasis. Exp Dermatol. 2016;25(7):513–8. https://doi.org/10.1111/exd.12942.
DeSilva B, McKenzie RC, Hunter JAA, Norval M. Local effects of TL01 phototherapy in psoriasis. Photodermatol Photoimmunol Photomed. 2008;24(5):268–9. https://doi.org/10.1111/j.1600-0781.2008.00366.x.
Matos TR, Ling TC, Sheth V. Ultraviolet B radiation therapy for psoriasis: pursuing the optimal regime. Clin Dermatol. 2016;34(5):587–93. https://doi.org/10.1016/j.clindermatol.2016.05.008.
Wong T, Hsu L, Liao W. Phototherapy in psoriasis: a review of mechanisms of action. J Cutan Med Surg. 2013;17(1):6–12. https://doi.org/10.2310/7750.2012.11124.
Vassantachart JM, Soleymani T, Wu JJ. Comparison of phototherapy guidelines for psoriasis: a critical appraisal and comprehensive review. J Drugs Dermatol. 2016;15(8):995–1000.
Pathirana D, Ormerod AD, Saiag P, Smith C, Spuls PI, Nast A, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23(Suppl 2):1–70. https://doi.org/10.1111/j.1468-3083.2009.03389.x.
Vangipuram R, Feldman SR. Ultraviolet phototherapy for cutaneous diseases: a concise review. Oral Dis. 2016;22(4):253–9. https://doi.org/10.1111/odi.12366.
Walker D, Jacobe H. Phototherapy in the age of biologics. Semin Cutan Med Surg. 2011;30(4):190–8. https://doi.org/10.1016/j.sder.2011.08.004.
Tauscher AE, Fleischer ABJ, Phelps KC, Feldman SR. Psoriasis and pregnancy. J Cutan Med Surg. 2002;6(6):561–70. https://doi.org/10.1177/120347540200600608.
Bangsgaard N, Rørbye C, Skov L. Treating psoriasis during pregnancy: safety and efficacy of treatments. Am J Clin Dermatol. 2015;16(5):389–98. https://doi.org/10.1007/s40257-015-0137-5.
Eustace K, Dolman S, Alsharqi A, Sharpe G, Parslew R. Use of phototherapy in children. Pediatr Dermatol. 2017;34(2):150–5. https://doi.org/10.1111/pde.13072.
Schaarschmidt M-L, Schmieder A, Umar N, Terris D, Goebeler M, Goerdt S, et al. Patient preferences for psoriasis treatments: process characteristics can outweigh outcome attributes. Arch Dermatol. 2011;147(11):1285–94. https://doi.org/10.1001/archdermatol.2011.309.
Koek MBG, Buskens E, van Weelden H, Steegmans PHA, Bruijnzeel-Koomen CAFM, Sigurdsson V. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338(may07 2):b1542. https://doi.org/10.1136/bmj.b1542.
Moseley H, Allan D, Amatiello H, Coleman A, du Peloux Menagé H, Edwards C, et al. Guidelines on the measurement of ultraviolet radiation levels in ultraviolet phototherapy: report issued by the British Association of Dermatologists and British Photodermatology Group 2015. Br J Dermatol. 2015;173(2):333–50. https://doi.org/10.1111/bjd.13937.
American Academy of Dermatology. Psoriasis: general principles for phototherapy. https://www.aad.org/practicecenter/quality/clinical-guidelines/psoriasis/phototherapy-and-photochemotherapy. Accessed November 6, 2017.
American Academy of Dermatology. Psoriasis: recommendations for excimer laser therapy. https://www.aad.org/practicecenter/quality/clinical-guidelines/psoriasis/phototherapy-and-photochemotherapy/excimer-laser-therapy. Accessed November 21, 2017.
American Academy of Dermatology. Psoriasis: recommendations for broadband and narrowband UVB therapy. https://www.aad.org/practicecenter/quality/clinical-guidelines/psoriasis/phototherapy-and-photochemotherapy/uvb-therapy. Accessed November 21, 2017.
American Academy of Dermatology. Psoriasis: recommendations for oral PUVA photochemotherapy. https://www.aad.org/practicecenter/quality/clinical-guidelines/psoriasis/phototherapy-and-photochemotherapy/oral-puva-photochemotherapy. Accessed November 21, 2017.
Coven TR, Burack LH, Gilleaudeau R, Keogh M, Ozawa M, Krueger JG. Narrowband UV-B produces superior clinical and histopathological resolution of moderate-to-severe psoriasis in patients compared with broadband UV-B. Arch Dermatol. 1997;133(12):1514–22. https://doi.org/10.1001/archderm.1997.03890480034005.
Man I, Crombie IK, Dawe RS, Ibbotson SH, Ferguson J. The photocarcinogenic risk of narrowband UVB (TL-01) phototherapy: early follow-up data. Br J Dermatol. 2005;152(4):755–7. https://doi.org/10.1111/j.1365-2133.2005.06537.x.
Maiorino A, De Simone C, Perino F, Caldarola G, Peris K. Melanoma and non-melanoma skin cancer in psoriatic patients treated with high-dose phototherapy. J Dermatol Treat. 2016;27(5):443–7. https://doi.org/10.3109/09546634.2015.1133882.
Bonis B, Kemeny L, Dobozy A, Bor Z, Szabo G, Ignacz F. 308 nm UVB excimer laser for psoriasis. Lancet (London, England). 1997;350(9090):1522. https://doi.org/10.1016/S0140-6736(05)63945-1.
Gerber W, Arheilger B, Ha TA, Hermann J, Ockenfels HM. Ultraviolet B 308-nm excimer laser treatment of psoriasis: a new phototherapeutic approach. Br J Dermatol. 2003;149(6):1250–8. https://doi.org/10.1111/j.1365-2133.2003.05709.x.
Mudigonda T, Dabade TS, Feldman SR. A review of targeted ultraviolet B phototherapy for psoriasis. J Am Acad Dermatol. 2012;66(4):664–72. https://doi.org/10.1016/j.jaad.2011.07.011.
Mysore V. Targeted phototherapy. Indian J Dermatol Venereol Leprol. 2009;75(2):119–25. https://doi.org/10.4103/0378-6323.48655.
Zhang P, Wu MX. A clinical review of phototherapy for psoriasis. Lasers Med Sci. October 2017;33(1):173–80. https://doi.org/10.1007/s10103-017-2360-1.
Bulat V, Situm M, Dediol I, Ljubicic I, Bradic L. The mechanisms of action of phototherapy in the treatment of the most common dermatoses. Coll Antropol. 2011;35(Suppl 2):147–51.
Shenoi SD, Prabhu S. Photochemotherapy (PUVA) in psoriasis and vitiligo. Indian J Dermatol Venereol Leprol. 2014;80(6):497–504. https://doi.org/10.4103/0378-6323.144143.
Zanolli M. Phototherapy treatment of psoriasis today. J Am Acad Dermatol. 2003;49(2 Suppl):S78–86.
Hunter HJA, Griffiths CEM, Kleyn CE. Does psychosocial stress play a role in the exacerbation of psoriasis? Br J Dermatol. 2013;169(5):965–74. https://doi.org/10.1111/bjd.12478.
Fortune DG, Richards HL, Kirby B, McElhone K, Markham T, Rogers S, et al. Psychological distress impairs clearance of psoriasis in patients treated with photochemotherapy. Arch Dermatol. 2003;139(6):752–6. https://doi.org/10.1001/archderm.139.6.752.
Piaserico S, Marinello E, Dessi A, Linder MD, Coccarielli D, Peserico A. Efficacy of biofeedback and cognitive-behavioural therapy in psoriatic patients: a single-blind, randomized and controlled study with added narrow-band ultraviolet B therapy. Acta Derm Venereol. 2016;96(217):91–5. https://doi.org/10.2340/00015555-2428.
Kragballe K. Vitamin D and UVB radiation therapy. Cutis. 2002;70(5 Suppl):9–12.
Aggarwal P, Aggarwal K, Jain VK. Tacalcitol: a useful adjunct to narrow band ultraviolet B phototherapy in psoriasis. J Dermatolog Treat. 2016;27(6):546–51. https://doi.org/10.3109/09546634.2016.1163318.
Dua I, Aggarwal K, Jain VK. Comparative evaluation of efficacy and safety of calcipotriol versus tacalcitol ointment, both in combination with NBUVB phototherapy in the treatment of stable plaque psoriasis. Photodermatol Photoimmunol Photomed. 2017;33(5):275–81. https://doi.org/10.1111/phpp.12324.
Al-Hamamy HR, Al-Mashhadani SA, Mustafa IN. Comparative study of the effect of narrowband ultraviolet B phototherapy plus methotrexate vs. narrowband ultraviolet B alone and methotrexate alone in the treatment of plaque-type psoriasis. Int J Dermatol. 2014;53(12):1531–5. https://doi.org/10.1111/ijd.12444.
Calzavara-Pinton PG, Sala R, Arisi M, Rossi MT, Venturini M, Ortel B. Synergism between narrowband ultraviolet B phototherapy and etanercept for the treatment of plaque-type psoriasis. Br J Dermatol. 2013;169(1):130–6. https://doi.org/10.1111/bjd.12277.
Lynde CW, Gupta AK, Guenther L, Poulin Y, Levesque A, Bissonnette R. A randomized study comparing the combination of nbUVB and etanercept to etanercept monotherapy in patients with psoriasis who do not exhibit an excellent response after 12 weeks of etanercept. J Dermatol Treat. 2012;23(4):261–7. https://doi.org/10.3109/09546634.2011.607795.
Park KK, Wu JJ, Koo J. A randomized, “head-to-head” pilot study comparing the effects of etanercept monotherapy vs. etanercept and narrowband ultraviolet B (NB-UVB) phototherapy in obese psoriasis patients. J Eur Acad Dermatol Venereol. 2013;27(7):899–906. https://doi.org/10.1111/j.1468-3083.2012.04611.x.
Kagen M, Cao LY, Oyetakin-White P, Tacastacas JD, Yan C, McCormick TS, et al. Single administration of lesion-limited high-dose (TURBO) ultraviolet B using the excimer laser: clinical clearing in association with apoptosis of epidermal and dermal T cell subsets in psoriasis. Photodermatol Photoimmunol Photomed. 2012;28(6):293–8. https://doi.org/10.1111/j.1600-0781.2012.00692.x.
Al-Mutairi N, Al-Haddad A. Targeted phototherapy using 308 nm Xecl monochromatic excimer laser for psoriasis at difficult to treat sites. Lasers Med Sci. 2013;28(4):1119–24. https://doi.org/10.1007/s10103-012-1210-4.
Levin E, Debbaneh M, Malakouti M, Brown G, Wang E, Gupta R, et al. Supraerythemogenic excimer laser in combination with clobetasol spray and calcitriol ointment for the treatment of generalized plaque psoriasis: interim results of an open label pilot study. J Dermatol Treat. 2015;26(1):16–8. https://doi.org/10.3109/09546634.2013.860210.
Berneburg M, Herzinger T, Rampf J, Hoetzenecker W, Guenova E, Meisner C, et al. Efficacy of bath psoralen plus ultraviolet A (PUVA) vs. system PUVA in psoriasis: a prospective, open, randomized, multicentre study. Br J Dermatol. 2013;169(3):704–8. https://doi.org/10.1111/bjd.12466.
El-Mofty M, Mostafa WZ, Yousef R, Abdel Halim MRE, el Hawary M, Abdel Kader H, et al. Broadband ultraviolet A in the treatment of psoriasis vulgaris: a randomized controlled trial. Int J Dermatol. 2014;53(9):1157–64. https://doi.org/10.1111/ijd.12317.
Su L-N, Xu X, Tang L, Yu N, Ding Y-F. UVA1 phototherapy in the treatment of palmoplantar pustulosis: a pilot prospective study. Lasers Med Sci. 2016;31(8):1641–3. https://doi.org/10.1007/s10103-016-2031-7.
Aggarwal K, Khandpur S, Khanna N, Sharma VK, Pandav CS. Comparison of clinical and cost-effectiveness of psoralen + ultraviolet A versus psoralen + sunlight in the treatment of chronic plaque psoriasis in a developing economy. Int J Dermatol. 2013;52(4):478–85. https://doi.org/10.1111/j.1365-4632.2012.05692.x.
Gahalaut P, Soodan PS, Mishra N, Rastogi MK, Soodan HS, Chauhan S. Clinical efficacy of psoralen + sunlight vs. combination of isotretinoin and psoralen + sunlight for the treatment of chronic plaque-type psoriasis vulgaris: a randomized hospital-based study. Photodermatol Photoimmunol Photomed. 2014;30(6):294–301. https://doi.org/10.1111/phpp.12125.
Yilmaz A, Ozkiraz S, Akcan AB, Canpolat M. Low-cost home-use light-emitting-diode phototherapy as an alternative to conventional methods. J Trop Pediatr. 2015;61(2):113–8. https://doi.org/10.1093/tropej/fmu076.
Pfaff S, Liebmann J, Born M, Merk HF, von Felbert V. Prospective randomized long-term study on the efficacy and safety of UV-free blue light for treating mild psoriasis vulgaris. Dermatology. 2015;231(1):24–34. https://doi.org/10.1159/000430495.
Weinstabl A, Hoff-Lesch S, Merk HF, von Felbert V. Prospective randomized study on the efficacy of blue light in the treatment of psoriasis vulgaris. Dermatology. 2011;223(3):251–9. https://doi.org/10.1159/000333364.
Kleinpenning MM, Otero ME, van Erp PEJ, Gerritsen MJP, van de Kerkhof PCM. Efficacy of blue light vs. red light in the treatment of psoriasis: a double-blind, randomized comparative study. J Eur Acad Dermatol Venereol. 2012;26(2):219–25. https://doi.org/10.1111/j.1468-3083.2011.04039.x.
Kemeny L, Csoma Z, Bagdi E, Banham AH, Krenacs L, Koreck A. Targeted phototherapy of plaque-type psoriasis using ultraviolet B-light-emitting diodes. Br J Dermatol. 2010;163(1):167–73. https://doi.org/10.1111/j.1365-2133.2010.09763.x.
•• Anderson KL, Feldman SR. A guide to prescribing home phototherapy for patients with psoriasis: the appropriate patient, the type of unit, the treatment regimen, and the potential obstacles. J Am Acad Dermatol. 2015;72(5):868–78. https://doi.org/10.1016/j.jaad.2015.02.003. Provides explanation pertaining to home phototherapy prescription for patients with psoriasis.
•• Armstrong AW, Robertson AD, Wu J, Schupp C, Lebwohl MG. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the National Psoriasis Foundation surveys, 2003-2011. JAMA Dermatol. 2013;149(10):1180–5. https://doi.org/10.1001/jamadermatol.2013.5264. Highlights important psoriasis treatment topics such as patient dissatisfaction, undertreatment and treatment trends.
Funding
There was no funding received for this review article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Feldman has received research, speaking, and/or consulting support from a variety of companies including Galderma, GSK/Stiefel, Almirall, Leo Pharma, Baxter, Boeringer Ingelheim, Mylan, Celgene, Pfizer, Valeant, Taro, Abbvie, Cosmederm, Anacor, Astellas, Janssen, Lilly, Merck, Merz, Novartis, Regeneron, Sanofi, Novan, Parion, Qurient, National Biological Corporation, Caremark, Advance Medical, Sun Pharma, Suncare Research, Informa, UpToDate, and National Psoriasis Foundation. He is the founder and majority owner of www.DrScore.com and the founder and part owner of Causa Research, a company dedicated to enhancing patients’ adherence to treatment. Seth Howell and Leah Cardwell have no conflicts to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Psoriasis
Rights and permissions
About this article
Cite this article
Howell, S.T., Cardwell, L.A. & Feldman, S.R. A Review and Update of Phototherapy Treatment Options for Psoriasis. Curr Derm Rep 7, 43–51 (2018). https://doi.org/10.1007/s13671-018-0211-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13671-018-0211-3