Abstract
Psoriasis is a non-contagious, chronic, relapsing inflammatory skin disease with cutaneous manifestations such as red, raised scaly plaques. Current treatment approaches for psoriasis comprise topical therapy, systemic therapy, phototherapy, psoralen with UVA(PUVA) and biologics. Regardless of the progression in therapeutic approaches (novel therapies like biologics) in psoriasis, phototherapy is also an economical, compelling and safe treatment option that lacks the immunosuppressive properties as well as the toxicities of traditional modalities. It can be combined safely with other therapeutic options such as topical therapies and novel biologics and provide effective therapy. The aim of the current review is to analyze the literature on the safety as well as the efficacy of phototherapy with various treatment modalities in the management of psoriasis. This review summarizes randomized controlled clinical trials addressing combinations of phototherapy with other treatment modalities for the management of psoriasis. The findings of these clinical studies are elaborated.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psoriasis is a non-contagious, chronic, relapsing inflammatory skin disease with cutaneous manifestations such as red, raised scaly plaques caused by aberrant terminal differentiation and proliferation of keratinocytes that may be limited or widespread in extent (Dainichi et al. 2018). It is a complex illness with an unknown cause. Its prevalence is 11.8%. Psoriasis affects > 125 million individuals worldwide. Men and women are equally prone to this disease (Gudjonsson and Elder 2007). It has a bimodal age frequency distribution with two distinct onset spikes, one at 16 years and the other at 60 years in females and one at 22 years and the other at 57 years in males. It has a complicated etiology that involves an interaction between environmental factors and genetic factors (Neimann et al. 2006a). The disease can be classified into plaque, guttate, pustular, erythrodermic, palmoplantar, flexural, nail, scalp, genital and inverse psoriasis (Dubois Declercq and Pouliot 2013). However, the common form of psoriasis prevalent amongst most patients is plaque psoriasis (Kim et al. 2017). Psoriasis is said to have a notable influence on the patient’s well-being which is also linked with depression, diminished productivity, anxiety as well as frequent encounter with social stigmatization and denial (Jankowiak Beata Kowalewska El and Krajewska-Kułak Dzmitry Khvorik 2020; Kwan et al. 2018; Salman et al. 2018). Psoriasis is associated with psoriatic arthritis, a debilitating illness and also has a prevalence of comorbidities like cardiovascular disease and metabolic syndrome (Neimann et al. 2006b; Naldi and Gambini 2007; Gottlieb et al. 2008).
In healthy skin, the proportion of keratinocytes i.e., proliferating to non-proliferating is 60% whereas it is 100% in psoriatic skin. The average mitotic cycle time is lowered from 311 to 36 h in psoriatic lesions. It is a T-cell-arbitrated disease. In 1983, the first postulation that T-cells have a prominent part in the etiology of psoriasis emerged (Valdimarsson et al. 2004). Skin is made up epidermis, dermis as well as a subcutaneous layer (Woo 2019). The epidermis is majorly composed of keratinocytes which undergo maturation every 30–40 days and are shed off in non-psoriatic patients. But in psoriatic skin, despite shedding off after its multiplication which occurs within a week it is present as lesions on the surface of the skin (Jyothi et al. 2021). The distinctive feature of psoriasis is unrelenting inflammation which leads to unrestrained proliferation of keratinocytes which is mainly due to dysregulation of the adaptive as well as innate immune systems in addition to keratinocytes associated with vascular changes (Rendon and Schäkel 2019). The hallmarks of psoriasis vulgaris are acanthosis (epidermal thickening), parakeratosis (retention of the nucleus in stratum corneum) and cutaneous inflammatory infiltrates (Lowes et al. 2007).
The disease process starts in the epidermis. Due to some unknown trigger, stressed keratinocytes release their DNA which binds with antimicrobial peptides (LL37/β-defensins/S100 proteins) (ORTONNE 1999). These antimicrobial peptide-DNA complexes activate plasmacytoid dendritic cells(pDCs) which are very nasty and release Interferon-α (INF- α) which acts on dermal dendritic cells (DDCs) which further releases interleukin-23 (IL-23) and IL-12 (Lowes et al. 2014). DDCs are very plastic and change into inflammatory dendritic cells in psoriatic skin which further release IL-23 TNF-α, NO. These DDCs migrate to lymphoid and thus cause naive T-cell differentiation into Th1 as well as Th17 in the presence of IL-23 and IL-12, respectively. These interleukins act via Tyk2-Jak2 and STAT3 receptors and release important inflammatory mediators (Ogawa et al. 2018). Th17 and Th1 are major culprits in psoriasis (Schön 2019; Fletcher et al. 2020). These cells migrate from the lymphoid to the bloodstream through lymphatics and get back to the skin. Th1 cells release INFγ, IL-1, tumor necrosis factor- α (TNF-α). These cytokines act on keratinocytes as well as dendritic cells to produce more inflammatory cytokines so that an inflammatory cascade goes on. Th17 releases IL-17A, IL-17F, INFγ as well as IL-22 which act on keratinocytes and increase keratinocyte proliferation. These interleukins released by Th1 and Th17 are key underpinnings (Baliwag et al. 2015). These interleukins also recruit leukocytes to the epidermis. Psoriatic skin resides a substantial amount of CD8 + and CD4 + T cells which release cytokines responsible for inflammation (Ho and Kupper 2019; Chen and Shen 2020; Vu et al. 2021). Keratinocytes also release angiopoietin, vascular endothelial growth factor (VEGF) as well as basic fibroblast growth factor (BFGF) which causes angiogenesis of keratinocytes thereby causing increased vascularization and inflammation. NO causes vasodilation leading to erythema (Lee et al. 2021).
Current treatment and unmet clinical needs of psoriasis
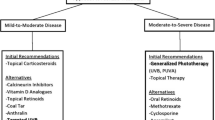
Psoriasis is incurable and has only suppressive therapy. Current treatment approaches for psoriasis include systemic therapy, topical therapy and phototherapy in addition to psoralen with ultraviolet-A radiation (PUVA). The selection of appropriate treatment is determined by the subject’s overall wellness, comorbidities, age, severity and form of the disease along with the affected body parts (Smith and Barker 2006). Psoriatic patients are often categorized into two groups based on the percentage of body surface area (BSA) involved as well as the severity of lesions: mild (psoriasis involving less than 10% of BSA (or PASI ≤ 10), moderate to severe (skin involvement greater than 10% of the BSA) (Nast et al. 2012). Mild psoriasis is managed with topical therapy whereas systemic or phototherapy is chosen to cure moderate to severe disease (Jain et al. 2021).
Topical therapy
Topical treatments approved currently for psoriasis therapy include corticosteroids, dithranol, calcineurin inhibitors (like tacrolimus, pimecrolimus), vitamin D analogs (calcipotriol), retinoids (tazarotene), coal tar (Reich and Bewley 2011; Verallo-Rowell et al. 2018). They also act as adjuvants for moderate to severe diseases that are contemporarily alleviated either using systemic medications or UV light (Reich and Bewley 2011; Buechler et al. 2021). Despite being effective and convenient, they are associated with several drawbacks (Arndt et al. 2016). The major issue with topical treatment includes adherence which is very poor in the majority of patients (Torsekar and Gautam 2017). Topical corticosteroids have been linked to a variety of adverse effects comprising hypothalamic–pituitary–adrenal (HPA) axis suppression, cutaneous atrophy, telangiectasia formation, striae formation, steroid rosacea, tachyphylaxis, perioral dermatitis, skin infections (Afifi et al. 2005; Laws and Young 2010; Horn et al. 2010).
Systemic therapy
Systemic therapy is preferred to alleviate moderate to severe forms of psoriasis (Sandoval et al. 2014). This comprises methotrexate, acitretin, mycophenolic acid, cyclosporine, esters of fumaric acid, hydroxyurea, etc (van Winden et al. 2020). To synergize the efficacy of these drugs, combination therapy is frequently used. These drugs often impose many adverse effects and cumulative toxicity risks upon long-term use which limit their applicability (Kaushik and Lebwohl 2019). For instance, skeletal toxicity, fetal abnormalities or fetal deaths, hyperlipidemia and hepatotoxicity are the repercussions of long-term administration of acitretin and so as gastrointestinal adverse effects, flushing, lymphocytopenia, proteinuria, elevated liver enzymes are the repercussions with esters of fumaric acid (Sascha et al. 2020). Cyclosporine causes nephrotoxicity upon chronic use (Lebwohl and Ali 2001). Methotrexate is the primary treatment for the systemic therapy of psoriasis (Peter Kozub 2011). It is used to treat osteosarcoma, Hodgkin’s disease, breast cancer, lung cancer, choriocarcinoma, breast cancer, non-Hodgkin’s disease, acute lymphocytic leukemia and head and neck cancers. It has an immunosuppressive, anti-inflammatory effect which enables it to be used in the management of psoriasis (Mrowietz 2001). It has long-term safety concerns such as myelosuppression, pulmonary fibrosis, liver toxicity, gastrointestinal irritation, and several skin irritabilities. Phototherapy is an exceptional substitute if systemic treatment fails.
Regardless of the progression in therapeutic approaches (novel therapies like biologics) in psoriasis, phototherapy yet has a role as an economical, safe as well as well-established treatment option that lacks the immunosuppressive properties and toxicities of traditional modalities. It is a very effective, safe and available treatment without sustaining any adverse effects of systemic therapy. It can be combined safely with other therapeutic options such as topical therapies and novel biologics and provide effective treatment. Phototherapy confers therapeutic benefits in psoriasis via a variety of pathways. Lately, new progressions have been made in this field in developing effective as well as safe targeted therapy to treat regions that are challenging to treat such as scalp psoriasis. Targeted phototherapy involves the use of handheld ultraviolet-B(UVB) devices which enables the targeting of light to psoriatic lesions that provide high patient satisfaction (Zhang and Wu 2018).
Phototherapy as an alternative therapeutic option in psoriasis
Phototherapy involves nonionizing electromagnetic radiation for therapeutic use. Phototherapy of dermatological diseases is primarily limited to the use of ultraviolet (UV) radiation. It entails exposing the patient to ultraviolet radiation, which is typically delivered via a special type of fluorescent light bulb (Matz 2010).
For centuries, Heliotherapy which involves the application of sunlight for the management of several skin ailments. Primordial Chinese, Hindu, and Egyptian medicine all used sun therapies. The use of artificial sources of ultraviolet radiation has evolved throughout these centuries. The transformation of heliotherapy to artificial light phototherapy intervened latterly in the nineteenth century. Boiled plant extract (derived from Ammi majus L., a weed growing in the Nile delta) in combination with sun exposure was employed in the treatment of vitiligo in ancient Egypt 3500 years ago. The founder of modern phototherapy is Nils Ryberg Finsen (1860–1904) who devised a carbon-arc lamp for the management of skin disorders such as lupus vulgaris (Campbell 2020). In 1923, William Henry Goeckerman prescribed the administration of artificial broadband UVB (BB-UVB) plus topical coal tar for the management of psoriasis which became very popular predominantly in the USA and was used for decades for the management of psoriasis (Hönigsmann 2013). With the advent of UVA radiation-emitting high-intensity lamps, effective psoriasis treatment is made possible. Photochemotherapy combines UVA radiation with psoralen (8-MOP) oral intake (Grzybowski et al. 2016). BB-UVB was discovered in the 1970s and when given in doses is effective in clearing mild forms of psoriasis subsequently a narrowband UVB (NB-UVB), a more defined wavelength was discovered which was effective in treating psoriasis (Wong et al. 2013a).
Action spectra of UV phototherapy
UV radiation is classified into:
-
UVA: 315–400 nm
-
UVB: 280–315 nm (narrowband UVB: 311-313 nm)
-
UVC: 100–280 nm.
Psoriatic lesions are unaffected by shorter wavelengths (254 nm, 280 nm, 290 nm). Longer wavelengths (296 nm, 300 nm, 304 nm) are required to clear psoriatic lesions. Complete clearance of psoriatic lesions was observed in all subjects after suberythemogenic exposure to 313 nm. NB-UVB phototherapy is a commonly used type of UV phototherapy. The use of broadband UV-B is very limited these days after the advent of NB- UVB (Torres et al. 2021). NB-UVB is safer than psoralen-plus-UVA phototherapy and more effective than BB-UVB phototherapy (Ontario Health 2020).
Principles and mechanisms of phototherapy
UV radiation is absorbed by chromophores (molecules that can absorb specific wavelengths), which include DNA, lipids, amino acids, nucleotides, melanin and trans-urocanic acid. UV radiation alters the structure as well as the function of chromophores. Photoproducts are molecules that have been modified in this way and are involved in apoptosis, photocarcinogenesis, immunosuppression and inflammation (Hönigsmann 2001).
There are four mechanisms proposed to understand the repercussions of phototherapy on psoriasis: (1) cytokine profile modification, (2) apoptosis induction, (3) immunosuppression and (4) other potential mechanisms (Wong et al. 2013b).
Cytokine profile modification
Host immune response is mediated by T-helper cells by converting into discrete effector cells namely Th1, Th2 and Th17, respectively, with Th1 and Th17 being the major ones in humans. Psoriasis is predominantly mediated by Th1/Th17 cytokines. A characteristic upregulation of Th1, as well as Th2 cytokines, occurs in psoriatic skin leading to keratinocyte hyperproliferation as well as inflammation. Psoriatic lesions show a rise in Th1, Th17 cytokine levels as well as a relative decline in Th2 cytokine level in contrast to healthy skin. Phototherapy has been shown to reverse the psoriasis cytokine profile by altering the immune response from the inflammatory Th1/Th17 axis towards the counterregulatory Th2 axis. Several studies have reported upregulation of Th2 cytokines with increased expression of IL-10 levels and other cytokines like IL-4 and IL-5 in both dermis and epidermis of psoriatic patients. In psoriatic lesions, phototherapy reduces the expression of Th1/Th17 cytokine levels like IL-23, IL-22, IL-17, IL-12 and IL-20 (Batycka-Baran et al. 2016; Bajaj et al. 2017). A decreased IFN-γ activators such as IL-23, IL-18 and IL-12 were observed upon exposure to NB-UVB radiation. An investigation performed with 34 psoriasis patients found lower plasma concentrations of TNF-α as well as IL-23 plus lower levels of IL-17, IL-22 after treatment with PUVA as well as NB-UVB phototherapies (Wong et al. 2013b).
Apoptosis induction
Apoptosis, also known as programmed cell death is induced by multiple external stimuli like infection, hypoxia, radiation and heat exposure (D’Arcy 2019). It has been implicated in several studies that phototherapy acts effectively via apoptosis as a major mechanism. Several studies describe mechanisms that cause apoptosis. The best-known target of UVB is DNA. Photoproducts involved in two key photochemical reactions caused by UVB in DNA include (6–4) pyrimidine-pyrimidone as well as cyclobutane pyrimidine dimers (CPDs). UV radiation causes damage to DNA which is followed by activation of p53 as well as cytochrome c leakage from mitochondria. Several plasma membrane death receptors (extrinsic pathway) are stimulated upon exposure to UV which results in caspase cascade activation in addition to apoptosis. Furthermore, UV radiation induces activation of death receptors followed by translocation of Bax to mitochondria that results in the release of cytochrome c. It brings about an elevation in reactive oxygen species (ROS) production which damages several important structural proteins, functional proteins, DNA and causes cytochrome c release from damaged mitochondria (Lee et al. 2013). A study with 30 psoriasis patients who received PUVA therapy showed increased keratinocyte apoptosis after 6 weeks of treatment. Likewise, the skin of five recovered psoriasis patients who received NB-UVB irradiation has shown enhanced apoptosis of keratinocytes and considerable expression of the tumor suppressor protein epidermal tp53 was observed (Wong et al. 2013) (Fig. 1).
Schematic representation of the mechanism of UV-induced apoptosis in psoriatic skin. (i) UV-induced DNA damage: UV induces DNA damage followed by cytochrome c release from mitochondria. (ii) UV-induced death receptor activation: death receptors are activated upon UV exposure followed by caspase-3 activation and apoptosis. (iii) UV-induced reactive oxygen species (ROS): UV induces an increase in the production of ROS which damages DNA as well as several important structural and functional proteins and also causes cytochrome c release from damaged mitochondria. (iv) UV-induced Bax translocation: translocation of Bax which causes activation of caspase thus leading to apoptosis. The Figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license
Immunosuppression
Since UV rays penetrate poorly into the skin, “photoreceptors”, the chromophores present at the body surface initiate the complex pathway leading to immunosuppression. Primary events are membrane changes, DNA impairment and isomerization to cis-urocanic acid from trans-urocanic acid (UCA). The major chromophore for UVR is DNA. As previously stated, the most common products generated as a result of phototherapy found in keratinocytes, Langerhans cells in the epidermis, as well as dendritic cells in lymph nodes draining irradiated sites, are cyclobutane pyrimidine dimers (CPDs) and (6–4)-photoproducts. Damage of DNA in the skin occurs followed by contact hypersensitivity response suppression (CHS). Immunosuppression occurs as a result of the liberation of IL-10, a cytokine that promotes Th2 proliferation. In the superior layers of the epidermis, histidase converts histidine to trans-isomer of UCA which accumulates in this site. It is a major absorber of UV radiation in the skin which isomerizes to cis-UCA. Some studies hypothesize that immunosuppression upon exposure to UVR is initiated by cis-UCA. UVR has been shown to target the cell membrane because the following multi-faceted sequence of actions can occur in cells that are devoid of nuclei. When exposed to UV radiation, alteration in cellular redox equilibrium occurs leading to peroxidation of membrane lipids as a result of the formation of free radicals. As a result, various enzymes were activated at the cell membrane which led to the production of numerous mediators followed by the activation as well as phosphorylation of several transcription factors. Upon exposure to UV radiation, phosphatidylcholine in the membrane gets oxidized which stimulates keratinocytes to release a molecule called platelet-activating factor (PAF). When PAF binds to its receptors present on mast cells, monocytes, and keratinocytes, it stimulates the liberation of cytokines (IL-10, IL-4) and prostaglandins that cause immunosuppression (Norval 2006). Regulatory T-cells (Treg) like CD4+ CD25+ Foxp3+ T cells are activated as well as responsible for immunosuppression upon irradiation with UV. These cells possess immune regulatory functions. These Treg cells release IL-10 upon activation thus causing immunosuppression (Morita 2018) (Fig. 2).
Other potential mechanisms
Many other hypotheses have been raised in the literature. One such hypothesis is cell cycle arrest which attributes to the effectiveness of phototherapy. Cell-cycle suppressor protein, tp53 levels and cell-cycle promoter protein, cyclin D1 levels were normalized after exposure to NB-UVB or PUVA therapy. Insulin-like growth factor binding protein 7 (IGFBP7) gene is an anti-tumor gene present in the epidermis whose expression is presumed to be altered in the psoriatic epidermis. Some investigations have proclaimed that this gene is under-expressed in the psoriatic epidermis, but its levels were restored by NB-UVB phototherapy (Morita 2018).
PUVA (photochemotherapy) (psoralen + UVA radiation)
Photochemotherapy which is also called PUVA is a treatment method that employs psoralen with ultraviolet A radiation (320–400 nm) (Morita 2018). Psoralens are tricyclic compounds that contain a furan ring connected to a coumarin moiety. The absorption bands of most psoralens are strong in the 250–300 nm range and gradually fade into the visible region of the spectrum. PUVA is a commonly used systemic antipsoriatic therapy and its efficacy has been extensively studied. When exposed to UVA, psoralens are flanked between DNA base pairs and form DNA cross-linkages which effectively prevent the replication of DNA. It promotes the formation of ROS which causes damage to the plasma membrane thus leading to cell death as well as the diminution of lymphoid cells in the skin (Doppalapudi et al. 2017; Elmets et al. 2019). It primarily suppresses keratinocyte proliferation, induces apoptosis in them, regulates lymphocyte as well as antigen-presenting cell function suppresses cytokines production such as TNF-α (Shaker et al. 2013). Psoralens can be employed systemically, as bath therapy, or as a topical preparation. 8-methoxy psoralen (8-MOP) (brand name-methoxsalen) is the most commonly prescribed medication at present, though 5-methoxy psoralen (5-MOP) under brand name bergapten is also used in cases where it is available. Oral preparations containing crystals or micronized crystals, and solubilized psoralens in a gel matrix are existing for 8-MOP and 5-MOP. The liquid preparation produces peak plasma levels earlier, higher and more reproducibly than the crystalline preparations. Bath PUVA entails dissolving a psoralen in bathwater followed by soaking the affected areas prior to UVA light treatment (Elmets et al. 2019). PUVA has only a few advantages, such as faster clearance with fewer exposures, resulting in fewer hospital visits. Various studies found that PUVA treatment increased the jeopardy of long-term carcinogenic side effects (Gnaneshwar Rao and Jagadevapuram 2021).
Combination treatments with phototherapy
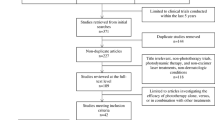
There are myriad psoriasis therapies available such as phototherapy, oral systemic agents, steroid and non-steroid topical agents as well as biologics with the ability to merge different therapies. It is difficult to attain and maintain remission in subjects with moderate to severe psoriasis particularly with monotherapy. Combination therapy is a safer and more effective option in contrast to monotherapy. It is desirable to merge agents with complementary adverse effects. Combination therapy provides certain advantages over monotherapies such as a decline in dose, a decline in dosing frequency, synergistic effects, improved safety and efficacy profiles, targeting multiple signaling pathways involved in the disease pathogenesis along with improved clinical outcomes. When phototherapy is combined with other conventional treatments, such as retinoids, Vitamin-D analogues, corticosteroids, and biologics, the following benefits are achieved such as prolonged disease-free intervals, greater rates of clearance, and reduced risk of carcinogenesis. Here we have stipulated ongoing preclinical as well as clinical studies involving combination therapies with phototherapy. Figure 3 provides the classification of conventional therapies used in combination with phototherapy (Fig. 4).
Outcomes of NBUVB radiation and drug treatment on pathophysiological alterations in mice induced with psoriasis. Outcomes of NBUVB radiation and drug treatment on pathophysiological alterations in mice induced with psoriasis. A Photographs representing skin lesions of mice post-indicated treatments. B Histopathological modifications in skin tissues from mice as treated in A. HE staining images are shown (×200). C Histochemical analysis of MMP13 expression in skin tissues from mice with indicated therapies. Pictures of MMP13 staining are presented (×200). Reprinted from Xi et al. (2021) under the license CC BY 4.0 (https://creativecommons.org/licenses/by-nc/4.0/).
Retinoids and phototherapy
Retinoids, namely vitamin A analogs have been used for the management of psoriasis since the early 1980s. Retinoids, in general bind to retinoid X receptors or nuclear retinoic acid receptors. This is followed by further binding to their respective response elements to regulate gene transcription as shown in Fig. 5. Topical as well as systemic retinoids are employed in the management of psoriasis. Nonetheless, they have many adverse effects despite being effective. Their combination with phototherapy has shown a marked decrease in unwanted effects and improved effectiveness in treating psoriasis (Koo et al. 2000).
Schematic representation of the mechanism of action of phototherapy in combination with conventional therapies (corticosteroids, Retinoids, vitamin D analogues, biologics). The Figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license
Xi et.al have investigated the expression of matrix metalloproteinase 13 (MMP13) in psoriatic patients and further scrutinized the therapeutic potential of acitretin/tazarotene and NB-UVB combination in psoriasis management. They have concluded that MMP13 is highly expressed in patients with psoriasis. Their findings in HaCaT keratinocytes (cultured Human keratinocytes) and psoriasis mouse models suggest that the combination of NB-UVB and acitretin inhibits MMP13 expression. MMP13 is released by keratinocytes and fibroblasts which is said to be involved in psoriasis pathogenesis. Greater levels of serum MMP13 were observed in imiquimod (IMQ)-induced mice than in control mice (p < 0.001). Likewise, NB-UVB radiation and tazarotene hindered the expression of MMP13 synergistically in this disease-induced mouse model. Serum levels of MMP13 in mice that were treated with combination therapy have shown a 1.17-fold decrease when compared to IMQ-induced mice. After acitretin or NB-UVB radiation therapy, HaCaT cell proliferation was reduced. The epidermal dynamics and microenvironment of keratinocytes change with augmented MMP13 expression in psoriatic skin subsequently influencing the proliferation of keratinocytes. When compared to IMQ-induced mice, mice treated with NB-UVB and acitretin have shown a 3.7-fold decrease in epidermal thickness (Xi et al. 2021).
Gahalaut and team have appraised the efficacy of PUVA in combination with oral isotretinoin by comparing it with PUVA (alone) in subjects with psoriasis. In this study, 40 human volunteers with psoriasis were allocated into 2 groups namely: Group A and B. Group A received only PUVA. Group B is given both PUVA as well as oral isotretinoin (0.5 mg/kg per day). In contrast to subjects in group A, the average percent decrease in Psoriasis Area and Severity Index (PASI) scores were reliably more for subjects in group B at 4,8,12 weeks. Group B subjects attained PASI 75 in an average of just 8 weeks, which is significantly faster than subjects in group A, who attained PASI 75 in an average of 10 weeks. This demonstrates the effectiveness as well as a quicker action of combination therapy of isotretinoin and PUVA. The Dermatology Life Quality Index (DLQI) scores of both study groups were improved (Gahalaut et al. 2014). Koo et.al have assessed the effectiveness of topical tazarotene with NB-UVB phototherapy in psoriasis. They randomly assigned each patient's bilateral target plaques to one of three treatments such as vehicle gel, 0.1% tazarotene gel or no treatment for 14 days. Following that, similar treatments were resumed three times a week for an additional 67 days. The findings demonstrated that the addition of 0.1% tazarotene gel improved the overall efficacy and rate of UVB phototherapy. With the incorporation of tazarotene together with UVB phototherapy, 50–75% improvement in psoriatic lesions was achieved and the median UVB exposure required was significantly declined. There were no reports of unusual photosensitivity amid target plaques treated with tazarotene and UVB phototherapy. As a result, combining tazarotene with UVB phototherapy was considered to be one of the least risky and most convenient options for treating recalcitrant psoriatic plaques while maintaining acceptable safety and tolerability (Koo et al. 2000). A similar study was performed by Dayal and his co-workers in an unblinded trial in 30 patients for 12 weeks to assess the safety as well as the effectiveness of NB-UVB plus tazarotene 0.05% gel. Two target plaques of psoriasis with identical dimensions were chosen from the same areas on the left as well as right sections of the body. Groups A & B represent the target plaques on either section of the body administered with tazarotene gel along with NB-UVB and NB-UVB alone, respectively. Group A has shown a significant decline in target plaque scaling and target plaque thickness by 98.7% and 99.9%, respectively, when compared to group B which has shown a 78.54% and 79% reduction, respectively. 83.33% and 3.33% of plaques in group A and Group B accomplished ≥ 50% reduction within 4 weeks whereas 100% and 86.67% of target plaques of the respective groups attained ≥ 50% reduction in 8 weeks. Thus, these findings have proven that a combination of tazarotene gel and NB-UVB has demonstrated a greater effect than either treatment alone (Dayal et al. 2018).
Vitamin D analogues and phototherapy
Vitamin d analogues have been proposed to be used in psoriasis since the early 1990s after oral vitamin D analogues have shown effectiveness in psoriasis treatment (Reichrath and Holick 2018). They bind to vitamin D receptors present in keratinocytes and T-lymphocytes followed by transporting this complex into the nucleus and binding to the genes which regulate the proliferation of keratinocytes and synthesis of pro-inflammatory cytokines as represented in Fig. 5 and reduce inflammation (Reich and Bewley 2011). Vitamin D analogues may be beneficial as phototherapy adjuncts in psoriasis patients. This combination not only improves the effectiveness of phototherapy but also reduces the irritation caused by vitamin D analogs.
Elsherif and his colleagues performed a study in psoriatic patients to assess the efficacy of NBUVB alone as well as NBUVB with calcipotriol ointment (combination therapy). 55 subjects were randomized into two treatment groups. Group I received NBUVB phototherapy a couple of times a week as monotherapy. Subjects in Group II received calcipotriol ointment twice a day together with NBUVB twice per week. After 8 weeks of therapy, a significant decline in PASI scores was witnessed in Group II in contrast to Group I. In Group II, PASI 75 was accomplished in 50% of patients after 6 weeks of treatment whereas in group I, it was achieved in 10 weeks. The average total NBUVB dose for attaining PASI 75 was found to be 42 ± 21 J/cm2 in group I in contrast to 19 ± 6.4 J/cm2 in group II (P < 0.05). The average NBUVB sittings required for the subjects was 17.5 ± 4.0 in group II (P < 0.05) and 34.7 ± 11.2 in group I. Both the treatment strategies decreased PASI score, nevertheless the combination of NBUVB and calcipotriol have shown synergistic effects and enhanced response of psoriasis to phototherapy (Nadia Elsherif 2015). A similar study was performed by Ali and his co-workers in 100 subjects with psoriasis who were allocated randomly to either group B group A. Subjects in group A received only NB-UVB. Subjects in group B received NB-UVB radiation together with Calcipotriol ointment. PASI scores were reduced in the calcipotriol combination group in contrast to NBUVB (Table 1).
The mean total dose of calcipotriol ointment in patients of group A was 34.3 J/cm2 whereas it was 21.5 J/cm2 in group B patients. This implies the achievement of faster clearance rates in the calcipotriol combination group with a lower cumulative dose. These findings explain the therapeutic effectiveness of calcipotriol when administered together with NB-UVB phototherapy in contrast to NB-UVB monotherapy (Hassan et al. 2020). Nejad et al. have investigated the efficacy of Daivonex cream (calcipotriol 5 mg) administered together with NBUVB phototherapy in comparison to Daivonex cream (5 mg) alone in psoriasis treatment. They have established that the group treated with Daivonex cream alone has shown 30.2% of complete recovery whereas the group treated with Daivonex and NB-UVB phototherapy combination has shown 73.8% complete recovery implying that combining calcipotriol ointment with NB-UVB phototherapy twice weekly improves thickness, erythema, scaling, and investment of psoriatic lesions (Nejad et al. 2015). Ozkan et al. randomly allocated 30 individuals with plaque psoriasis to one of the following groups group 1—received only targeted NB-UVB phototherapy, group 2—received NB-UVB with psoralen gel (0.1% gel), and group 3- received NB-UVB with calcipotriol ointment thrice a week. There were improved PASI and Psoriasis Severity Index (PSI) scores in group 3 with 67.2% and 59%, respectively. This implies the enhancement of the therapeutic outcomes of targeted phototherapy in combination with calcipotriol (Özkan et al. 2012). Tacalcitol, vitamin D analogue is less irritating than calcipotriol (Aggarwal et al. 2016). Aggarwal et al. defended this finding by establishing the effectiveness and safety of tacalcitol ointment with NBUVB radiation in plaque psoriasis. They have demonstrated that combination therapy of topical tacalcitol and NBUVB has shown positive clinical outcomes in an intra-individual, open-label study in 30 subjects with plaque psoriasis. Two psoriasis target plaques of roughly equal size and from similar sites were selected from the right and left portions of the body of each patient. Groups were divided into two namely, group 1—which represents target plaques on the right portion were treated with topical tacalcitol once a day and group 2 which represent target plaques on the left portion without any topical treatment. Subsequently, NBUVB phototherapy was given three times a week. Group 1 has lower mean plaque scores than group 2, with a significant difference from 2 to 8 weeks (p < 0.05). Target plaque scores, erythema scores, scaling scores and thickness scores were markedly reduced with combination treatment in contrast to NBUVB therapy alone. Total NBUVB exposures were significantly lesser in the tacalcitol-treated group which decreases the jeopardy of photocarcinogenesis and long-term treatment costs (87).
Corticosteroids and phototherapy
Topical corticosteroids stand as the main treatment modality for psoriasis in the United States due to their short-term efficacy, high patient acceptability and low cost. They bind to glucocorticoid receptor and forms a complex. This complex transports into the nucleus and further binds to glucocorticoid response elements and blocks the transcription of genes responsible for inflammation and synthesis of inflammatory cytokines (Fig. 5) (Uva et al. 2012). However, they are associated with unwanted effects comprising cutaneous atrophy, telangiectasia formation, striae formation, Acne rosacea, perioral folliculitis as well as HPA axis suppression, etc. Thus, it has been combined with phototherapy which provides a synergistic effect with significantly lower cumulative doses of steroids and UV phototherapy which decrease the extent of adverse reactions (Gagan Jot Kaur 2013).
Kaur and his co-workers carried out a randomized, placebo-controlled study with 20 subjects allocated into two groups. Subjects in one group received bath PUVA as well as betamethasone propionate cream (30/70) dilution cream and the other received PUVA and placebo cream. In the placebo group, eight patients achieved complete remission, while all patients in the steroid group achieved complete remission. The mean baseline PASITUL (Psoriasis Area Severity Index- Trunk, upper and lower limb score) for the steroid group declined to 0.4 from 19.7 whereas it declined to 1.5 from 23.9 in the placebo group. In the steroid group, the median number of UVA sittings required for > 90% clearance was 11 whereas 14 UVA sittings were required for the placebo group (p = 0.35). The average cumulative UVA doses utilized were 40.60 J/cm2 and 32.85 J/cm2 for placebo and steroid groups respectively. Seven in ten volunteers in the group subjected to steroids, whereas one in eight volunteers in the placebo group relapsed in 6 months (p = 0.23). A statistically significant difference was not observed in overall clearance as well as the mean cumulative UVA dose used. Early relapses were observed in the group treated with steroids which were statistically significant (Gagan Jot Kaur 2013). Levin et al. have performed an open-label pilot study to investigate the efficacy of clobetasol propionate with excimer laser and topical calcitriol in the management of plaque psoriasis. Patients were given excimer laser treatments two times a week. On a monthly basis, topical therapy was rotated. For the first month, 0.05% clobetasol spray was used two times a day and during the second month, they received calcitriol ointment (3 µg/g) twice a day. Patients used clobetasol spray as well as calcitriol ointment during the third month. By week 12, 76% of patients had a PASI-75 (75% decline in PASI) and 52% had a physician global assessment (PGA) score of ≤ 1 demonstrating the synergistic effect of the excimer laser in conjunction with calcitriol as well as clobetasol propionate ointment (Levin et al. 2015).
Biologics
The advancement of biological therapies has changed the paradigm of psoriasis over the past couple of decades (Kamata and Tada 2018). These treatment options, which primarily target IL-17, IL-12, IL-23 and TNF-α is found to be efficacious and safe in the psoriasis treatment. The main side effect of biologics is systemic immunosuppression. Many clinicians are concerned about this immunosuppressive effect of biologics which might increase patient’s risk of cancer or infection. Phototherapy shows up to have no systemic immunosuppressive effects and thus can be utilized in combination with biologics to treat recalcitrant lesions. Clinical trials involving the combination of biologics with phototherapy are discussed in Table 2.
Other potential drug molecules
Methotrexate
Methotrexate is a folic acid antagonist used in the treatment of psoriasis (Coates et al. 2020). It inhibits dihydrofolate reductase (DHFR) and inhibits the synthesis of DNA. Inhibition of DNA synthesis reduces epidermal hyperproliferation, recruitment of neutrophils and causes programmed cell death of activated T cells. It also inhibits the production of several pro-inflammatory cytokines such as IL-1, TNF-α (Czarnecka-Operacz and Sadowska-Przytocka 2014) (Fig. 7). It remains a cost-effective option in developing countries among other treatment modalities like biologics, cyclosporine, and retinoids (Yélamos and Puig 2015). Despite being effective, it produces adverse effects. Methotrexate monotherapy often requires quite a lot of weeks to achieve adequate clearance of lesions. Thus, it can be combined with NB-UVB phototherapy to achieve greater clearance of lesions in a lesser period of time with significantly minimal cumulative doses of both methotrexate and NB-UVB (Soliman et al. 2015).
A. Soliman et al. have investigated the effectiveness of methotrexate together with NBUVB phototherapy in comparison to methotrexate alone in chronic plaque psoriasis therapy. This study included 40 psoriasis subjects who had psoriasis that covered more than 20% of their BSA. They were assigned to two groups with 20 subjects each- Group A is treated with oral methotrexate (2.5 mg/tablet). The treatment regimen is initiated with 7.5 mg once a week as a test dose in 3 divided doses. The dose was then gradually increased by 5 mg over the next few weeks until it reached the individual effective dose of 30 mg/week. Group B patients were administered methotrexate orally in a similar regimen as given to patients in group A together with NB-UVB phototherapy (2 times a week). Every two weeks, all subjects were assessed. PASI score reduction by 30% reflects the onset of improvement of which a significant difference has been reported between the two groups with more improvement in group B (p < 0.001). PASI score decline by ≥ 90% represents end point clearance. Subjects in group B have shown a higher clearance rate of 100% as compared to group A which has shown 83% clearance. The mean total methotrexate dose was higher in group A than in group B (p < 0.05) with 165.5 ± 61.3 mg in group A and 89.8 ± 65.6 in Group B. No significant difference was reported concerning side effects and relapse. Thus, this study illustrated the effect of combining methotrexate with NBUVB in psoriasis treatment (Soliman et al. 2015). Another study on 120 patients with plaque psoriasis was performed by Al-Hamamy and his team to investigate the efficacy of methotrexate in combination with NB-UVB radiation when compared to NBVB alone and methotrexate alone. Patients were randomly allocated to three groups. Group-MN was treated with the combination of NBUVB and methotrexate at a dose of 0.2 mg per kg once a week. The groups received a maximum of 20 mg of methotrexate per week. Group N was treated with only NBUVB phototherapy. Group M- was administered with similar doses of methotrexate as Group MN. NBUVB phototherapy was given three times a week on alternate days via UV therapy cabin (311; TL-01 100 W lamps; Philips). The authors concluded that the average dose of NBUVB required to achieve clearance is statistically significant among the studied groups. Group MN required an NB-UVB dose of 12.13 ± 4.02 J/cm2 in contrast to group N (p < 0.0001) which required 34.48 ± 13.13 J/cm2. Likewise, the average dose of methotrexate in group MN is 2.5-folds lesser than in group N. The average weeks needed to attain clearance in the group treated with both NBUVB and methotrexate is twofold lesser than that required for group N and group M. When compared to group MN, the average number of phototherapy sessions required has significantly increased for group N. These outcomes have exemplified the synergistic effects of methotrexate with NB-UVB with fewer cumulative doses of both treatment modalities (Al-Hamamy et al. 2014). A randomized patient-blinded clinical trial has been performed by Mahajan et al. with forty patients diagnosed with chronic psoriasis vulgaris split into two groups comprising 20 patients each- with group A-treated with methotrexate tablet at a dose of 0.5 mg/kg once a week (30 mg/week) and NB-UVB while group-B received placebo together with NB-UVB. On the succeeding day of oral medication, NB-UVB phototherapy was initiated and was given three times a week on alternate days. PASI scores among the groups are statistically significant. Group A patients attained PASI 75 to a greater extent than those in the B group (p < 0.04). Similarly, average weeks (p = 0.001), average NB-UVB dose (p = 0.001), as well as average sessions of phototherapy (p = 0.0001) needed to attain PASI 75 in group B, are more than in group A. Assessment for relapse was also made after a follow-up for 12 weeks and concluded that none of the patients relapsed during the course (Mahajan et al. 2010). In a similar randomized controlled trial performed by DK and his co-workers substantial progress in the psoriatic area was observed. Seventy-nine patients diagnosed with plaque psoriasis were randomized (double-blind study) into two groups. Patients in group A received methotrexate followed by NB-UVB irradiation. Patients in group B received methotrexate alone (0.4 mg/kg/week with a maximum of 25 mg/week for 12 weeks). 89% of subjects in group A and 85% of subjects in group B (p = 0.052) attained PASI 75 in the 3rd week and 8th week, respectively (p = 0.031). A significant decline in the average cumulative dose of NB-UVB as well as average phototherapy sittings needed to attain PASI 75 was observed in group A in contrast with group B. DLQI assessment was also done and a substantial reduction of DLQI was detected in group A at 12 weeks (p = 0.022). At the completion of the study, neither treatment group experienced a significant number of relapses, and no serious adverse events occurred. Thus, methotrexate and NB-UVB is a potential combination in the management of psoriasis (Khadka et al. 2016).
Fumaric acid esters
European guidelines recommended fumaric acid esters (FAEs) for prolonged systemic therapy of adults suffering from moderate to severe psoriasis when topical treatment is inadequate. This treatment modality is approved in Germany and is an initial course of treatment in the management of moderate to severe psoriasis (Hamm et al. 2021). The mechanism of FAEs in psoriasis is still unclear. It is found to induce cytoprotective and antioxidative agents (Balak 2014). It is also hypothesized to inhibit the production of proinflammatory mediators thus causing immunosuppression. Furthermore, it is said to increase IL-10, IL-4 production which causes immunosuppression (Fig. 6). FAEs manifest a major drawback- which is slow onset of action(Tzaneva et al. 2018). This limitation can be addressed by combining this short-acting drug with NB-UVB which bestows itself as an affordable approach to upsurge patient adherence (Tzaneva et al. 2018).
To scrutinize the synergistic efficacy of FAEs with NB-UVB radiation in moderate to severe psoriasis patients, Tzaneva et al. have performed a randomized controlled trial (assessor-blinded) in thirty psoriatic patients for 6-weeks and were analyzed for DLQI, PASI as well as Psoriasis Long Based Area and Severity Index (PLASI) scores. Patients were randomly allocated to two treatment groups- one that was administered with only FAE and the other that was administered with both FAE and NB-UVB phototherapy. 68.9% of patients in the combination category and 36.5% of patients in the FAE category achieved a significant reduction of PASI (p = 0.016) after 6 weeks. On the other hand, 71.4% of patients in the combination group achieved PLASI75 whereas none in the group treated with FAE alone attained PLASI75. A significant reduction of DLQI was observed with the combination group when compared to FAE alone after 8 weeks. The outcomes of this prospective randomized trial indicate that a 6-week treatment of NB-UVB and FAE combined therapy resulted in a significant improvement in clinical short-term outcomes (Tzaneva et al. 2018). A similar study was performed by Weisenseel et al. in a larger patient cohort with plaque psoriasis using phototherapy (UV) concurrently with Fumaderm®. Safety, dosage, as well as efficacy (in terms of PASI score, DLQI, PGA score, EQ-5D) were evaluated for the combination in due course of twelve months. These scores are successively assessed in comparison to the data acquired from a historic cohort study carried out using FAE alone. 363 subjects participated in the study. FAEs were used as the primary systemic treatment in 79.1% of patients, with the remaining receiving FAEs as a follow-up after cessation of their prior systemic therapy. Phototherapy was administered to 96.1% of subjects as monotherapy and 3.9% received a combination of two different phototherapies. A continuous improvement in the PGA score was observed with 55% of subjects achieving a PGA score of at least 1 after 3 months. 72% and 78% of subjects attained a PGA score of at least 1 after 6 and 12 months, respectively. DLQI scores improved by 5 points in 73.3% of patients. By 12 months, 87.5% of patients with FAE/UVA combination achieved PASI50 response whereas 54.7% and 23.4% of patients attained PASI 75 and PASI 90 responses. These data were then compared with retrospective FAE monotherapy. A significant improvement in PASI, DLQI and PGA scores was observed in FAE/UVA treatment arm when compared with FAE monotherapy. After a year, the overall EQ-5D score dropped from 6.7 (n = 362) to 5.6 (n = 236). Individual well-being was estimated by means of a visual analogue scale (VAS), and it got upgraded from 53.0 (n = 359) to 80.1 (n = 234) by 12 months. Only 7% of patients have experienced AEs. Thus, FAEs administered alongside phototherapy were proven to be safe and well-tolerated (Weisenseel et al. 2017).
Sitagliptin
Psoriasis is linked with debilitating comorbidities related to metabolic and cardiovascular health. Keratinocytes show higher expression of dipeptidyl peptidase-4 (DPP-4) (Lynch et al. 2014b). The prospective of DPP-4 inhibition therapy in the management of psoriasis comes from its ability to suppress DPP-4 expression in keratinocytes in vitro and partial restoration of differentiation of keratinocytes in vivo. Sitagliptin is a dipeptidyl peptidase-4 (DPP-4) blocker that has been shown to ameliorate psoriasis and is approved at present for type 2 diabetes (T2DM) treatment. T-cells show expression of DPP-4 whose inhibition improves skin lesions by suppressing T-cell activation. It is also hypothesized to inhibit the production of various pro-inflammatory cytokines (Fig. 6). Combined with NB-UVB phototherapy, it has been demonstrated to improve psoriasis therapy rather than NB-UVB monotherapy (Lynch et al. 2014a).
Lynch and his co-workers designed a prospective, open-label clinical trial of oral sitagliptin plus NB-UVB radiation in psoriasis patients. Around 120 patients are randomly allocated to either sitagliptin 100 mg per day in combination with NB-UVB phototherapy or only NB-UVB phototherapy. The study was carried out for 24 weeks. A significant improvement in PASI scores was observed in the group administered with sitagliptin and NB-UVB phototherapy in contrast to the group administered with sitagliptin alone (p = 0.044). A significant difference in the EuroQol 5-item questionnaire (0.1 [95% CI 0.0–0.1]; p = 0.036) and Hospital Anxiety and Depression Scale (−2.5 [95% CI −4.0 to −1.0]; p = 0.002) values has been observed between group administered with sitagliptin and NB-UVB in contrast to group administered with sitagliptin alone (Lynch et al. 2016).
Apremilast
Bagel and his team have evaluated the efficacy of the combination of apremilast—a PDE-4 inhibitor and NB-UVB phototherapy in psoriasis. An open-label investigation has been performed with 29 Subjects for 12 weeks. Subjects were given 30 mg Apremilast two times a day and a total dose of NB-UVB thrice a week for a duration of 12 weeks. By week 12, 73% of subjects attained PASI75. At week 12, DLQI, VAS pain, PGA, VAS itch and PASI scores were improved by 70%,77%,67%, 69% and 77%, respectively (Bagel et al. 2017).
Pioglitazone
Pioglitazone is an antidiabetic drug that has been shown to be effective in psoriasis treatment. It has been proposed that a relationship exists between peroxisome proliferator-activated receptor γ (PPARγ) and psoriasis. There is a decline of PPARγ in psoriasis. PPARγ may influence numerous facets of psoriasis pathogenesis, such as keratinocytes, altered lipid metabolism, oxidative stress, angiogenesis, insulin sensitivity, lymphocytes and pro-inflammatory cytokines (Fig. 7). Pioglitazone is a PPARγ agonist shown to be effective in psoriasis management (Lin et al. 2022).
Schematic representation of mechanisms of action of some potential drugs in the management of psoriasis (Methotrexate, pioglitazone, methotrexate, fumaric acid esters). The Figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license
Ghiasi and his co-workers performed an investigation to evaluate the efficacy of pioglitazone with phototherapy in psoriasis treatment. Sixty subjects with psoriasis were randomly allocated to two batches: patients in one batch were administered pioglitazone and the other with placebo with subsequent administration of NB-UVB phototherapy sessions for both groups for 10 weeks. A significant decrease in PASI scores was reported among the two batches with an 83.5% reduction in the pioglitazone group and a 56.7% reduction in the placebo group (p < 0.05), respectively. Thus, pioglitazone in combination with phototherapy provides a synergistic effect in psoriatic patients when compared to NB-UVB phototherapy alone (Ghiasi et al. 2019).
Conclusion and future perspectives
Psoriasis is a chronic, non-contagious inflammatory skin disease that necessitates excruciating flare-ups as well as long-term disease management. There are myriad psoriasis therapies available, such as phototherapy, oral systemic agents, biologics as well as steroid and non-steroid topical agents with the ability to combine different therapies. Combination therapy entails combining two or more treatment modalities acting via numerous pathways as well as safety profiles to attain and sustain adequate disease control while limiting treatment toxicity. When a single therapy is inadequate to treat recalcitrant disease, combination therapy can be employed. Selecting a combination regimen that provides maximum effectiveness as well as safety while also considering patient usefulness and adherence can be difficult. The use of visible light with regard to inflammatory skin disease treatment has gained massive interest in recent times. Blue light, in particular (wavelength range 400 to 480 nm) has lesser detrimental outcomes on mammalian cells than ultraviolet light. The major advantage of phototherapy which enables it to be a backbone in psoriasis treatment is the lack of systemic immunosuppressive effects. Thus, it can be combined with various other treatment strategies and provide synergistic effects and thus reduce adverse effects associated with them by lowering their cumulative doses.
Phototherapy is safe as well as effective in clearing psoriatic lesions entirely. Still, they are often linked with side effects like pruritus, stinging, xerosis, burning, erythema and blistering in the short term. Long-term UV therapy side effects include photoaging as well as carcinogenesis. Combining nanoformulation with phototherapy could be an effective and safer option compared to the conventional formulation when combined with phototherapy. Nanoparticles act as vehicles to deliver phototherapeutic agents and can also act as adjuvants to augment the immune response. Nanoparticles have an important role in photodynamic therapy. It is a type of phototherapy involving a photosensitizer, oxygen as well as 3 components of visible light. This photodynamic therapy (PDT) system has been corroborated to be advantageous in psoriasis therapy and is rapidly evolving.
For instance, a study has been carried out to demonstrate the effectiveness of chitosan/alginate nanoparticles with curcumin incorporation (Cur-CS/Alg NPs) with blue light radiation in the suppression of hyperproliferation of TNF-α induced HaCaT cells (Gomez et al. 2019). They prepared Cur-CS/Alg nanoparticles by emulsifying curcumin in the solution of sodium alginate followed by crosslinking with chitosan and calcium chloride. MTT assay has been carried out which has shown that these CS/Alg nanoparticles at 0.5%(v/v) were not toxic to HaCat cells. The substantial overproliferation of keratinocytes in HaCat cells is induced by TNF-α which increased viable cells to 135.5%. Viable cell count in the free curcumin-treated group declined by 8% and 24.4% at 0.05 μg/ml and 0.1 μg/ml concentrations, respectively. Cells treated with curcumin followed by UV irradiation have shown a decline in viable cells by 30.8% and 42.7% at 0.05 μg/ml and 0.1 μg/ml concentrations, respectively. Upon exposure to blue light, the cells treated with Cur-CS/Alg nanoparticles showed a decline in viable cell numbers by 42.6% as well as 57.7%, respectively, at concentrations of curcumin of 0.05 μg/ml and 0.1 μg/ml, respectively. Cur-CS/Alg NPs alone have shown a decline of 26% and 41% in viable cell numbers at concentrations of curcumin of 0.05 μg/ml and 0.1 μg/ml, respectively. These results have proven the safety as well as the effectiveness of curcumin-based nanoparticles together with phototherapy. Thus, there is a need to explore nano-carrier-based systems in conjugation with phototherapy for greater patient compliance, and safety as well as an effective treatment strategy for psoriasis.
References
Afifi T, de Gannes G, Huang C, Zhou Y (2005) Topical therapies for psoriasis: evidence-based review. Can Fam Phys 51:519–525
Aggarwal P, Aggarwal K, Jain VK (2016) Tacalcitol: a useful adjunct to narrow band ultraviolet B phototherapy in psoriasis. J Dermatol Treat 27:546–551. https://doi.org/10.3109/09546634.2016.1163318
Al-Hamamy HR, Al-Mashhadani SA, Mustafa IN (2014) Comparative study of the effect of narrowband ultraviolet B phototherapy plus methotrexate vs. narrowband ultraviolet B alone and methotrexate alone in the treatment of plaque-type psoriasis. Int J Dermatol 53:1531–1535. https://doi.org/10.1111/ijd.12444
Arndt KA, Leboit PE, Wintroub BU, Stein Gold LF (2016) Topical therapies for psoriasis: improving management strategies and patient adherence (guest editor)
Bagel J, Nelson E, Keegan BR (2017) Apremilast and narrowband ultraviolet-B combination therapy for treating moderate-to-severe plaque psoriasis. J Drugs Dermatol 16:957–962
Bajaj S, Gautam RK, Khurana A et al (2017) Effect of narrow band ultraviolet B phototherapy on T helper 17 cell specific cytokines (interleukins-17, 22 and 23) in psoriasis vulgaris. J Dermatol Treat 28:14–17. https://doi.org/10.1080/09546634.2016.1177162
Balak DMW (2014) Fumaric acid esters in the management of psoriasis. Psoriasis Targets Ther 5:9–23
Baliwag J, Barnes DH, Johnston A (2015) Cytokines in psoriasis. Cytokine 73:342–350
Batycka-Baran A, Besgen P, Wolf R et al (2016) The effect of phototherapy on systemic inflammatory process in patients with plaque psoriasis. J Photochem Photobiol B 161:396–401. https://doi.org/10.1016/j.jphotobiol.2016.05.023
Buechler CR, Veenstra J, Stein Gold L (2021) New topical therapies for psoriasis. Dermatol Rev 2:262–268. https://doi.org/10.1002/der2.84
Campbell J (2020) Safe and effective use of phototherapy and photochemotherapy in the treatment of psoriasis. Br J Nurs 29:547–552. https://doi.org/10.12968/bjon.2020.29.10.547
Chen L, Shen Z (2020) Tissue-resident memory T cells and their biological characteristics in the recurrence of inflammatory skin disorders. Cell Mol Immunol 17:64–75
Coates LC, Merola JF, Grieb SM et al (2020) Methotrexate in psoriasis and psoriatic arthritis. J Rheumatol 96:31–35. https://doi.org/10.3899/jrheum.200124
Czarnecka-Operacz M, Sadowska-Przytocka A (2014) The possibilities and principles of methotrexate treatment of psoriasis—the updated knowledge. Postepy Dermatol Alergol 31:392–400. https://doi.org/10.5114/pdia.2014.47121
D’Arcy MS (2019) Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int 43:582–592
Dainichi T, Kitoh A, Otsuka A et al (2018) The epithelial immune microenvironment (EIME) in atopic dermatitis and psoriasis. Nat Immunol 19:1286–1298
Dayal S, Kaura R, Sahu P, Jain VK (2018) Tazarotene gel with narrow-band UVB phototherapy: a synergistic combination in psoriasis. An Bras Dermatol 93:385–390. https://doi.org/10.1590/abd1806-4841.20186723
Doppalapudi S, Jain A, Chopra DK, Khan W (2017) Psoralen loaded liposomal nanocarriers for improved skin penetration and efficacy of topical PUVA in psoriasis. Eur J Pharm Sci 96:515–529. https://doi.org/10.1016/j.ejps.2016.10.025
Dubois Declercq S, Pouliot R (2013) Promising new treatments for psoriasis. Sci World J. https://doi.org/10.1155/2013/980419
Elmets CA, Lim HW, Stoff B et al (2019) Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol 81:775–804. https://doi.org/10.1016/j.jaad.2019.04.042
Fletcher JM, Moran B, Petrasca A, Smith CM (2020) IL-17 in inflammatory skin diseases psoriasis and hidradenitis suppurativa. Clin Exp Immunol 201:121–134
Gagan Jot Kaur SBPSUKN (2023) Randomized double blinded placebo controlled comparative study of topical steroid plus bath PUVA versus bath PUVA and placebo cream. J Pak Assoc Dermatol
Gahalaut P, Soodan PS, Mishra N et al (2014) Clinical efficacy of psoralen + sunlight vs. combination of isotretinoin and psoralen + sunlight for the treatment of chronic plaque-type psoriasis vulgaris: a randomized hospital-based study. Photodermatol Photoimmunol Photomed 30:294–301. https://doi.org/10.1111/phpp.12125
Ghiasi M, Ebrahimi S, Lajevardi V et al (2019) Efficacy and safety of pioglitazone plus phototherapy versus phototherapy in patients with plaque type psoriasis: a double blinded randomized controlled trial. J Dermatol Treat 30:664–667. https://doi.org/10.1080/09546634.2018.1544702
Gnaneshwar Rao A, Jagadevapuram K (2021) Comparative study of PUVA and NB-UVB in the management of chronic plaque psoriasis. Indian J Drugs Dermatol 7:79. https://doi.org/10.4103/ijdd.ijdd_26_20
Gomez C, Muangnoi C, Sorasitthiyanukarn FN et al (2019) Synergistic effects of photo-irradiation and curcumin-chitosan/alginate nanoparticles on tumor necrosis factor-alpha-induced psoriasis-like proliferation of keratinocytes. Molecules. https://doi.org/10.3390/molecules24071388
Gottlieb AB, Chao C, Dann F (2008) Psoriasis comorbidities. J Dermatol Treat 19:5–21
Grzybowski A, Sak J, Pawlikowski J (2016) A brief report on the history of phototherapy. Clin Dermatol 34:532–537. https://doi.org/10.1016/j.clindermatol.2016.05.002
Gudjonsson JE, Elder JT (2007) Psoriasis: epidemiology. Clin Dermatol 25:535–546. https://doi.org/10.1016/j.clindermatol.2007.08.007
Hamm H, Wilsmann-Theis D, Tsianakas A et al (2021) Efficacy and safety of fumaric acid esters in young patients aged 10–17 years with moderate-to-severe plaque psoriasis: a randomized, double-blinded, placebo-controlled trial. Br J Dermatol 185:62–73. https://doi.org/10.1111/bjd.19747
Hassan R, Ali S, Kallan F et al (2020) A study to compare the efficacy and safety of narrow band-UVB alone as well as in combination with topical calcipotriol in patients with psoriasis vulgaris. IP Indian J Clin Exp Dermatol 6:30–34. https://doi.org/10.18231/j.ijced.2020.008
Ho AW, Kupper TS (2019) T cells and the skin: from protective immunity to inflammatory skin disorders. Nat Rev Immunol 19:490–502
Hönigsmann H (2001) Phototherapy for psoriasis. Clin Exp Dermatol 26:343–350. https://doi.org/10.1046/j.1365-2230.2001.00828.x
Hönigsmann H (2013) History of phototherapy in dermatology. Photochem Photobiol Sci 12:16–21
Horn EJ, Domm S, Katz HI et al (2010) Topical corticosteroids in psoriasis: strategies for improving safety. J Eur Acad Dermatol Venereol 24:119–124
Jain H, Bhat AR, Dalvi H et al (2021) Repurposing approved therapeutics for new indication: addressing unmet needs in psoriasis treatment. Curr Res Pharmacol Drug Discov 2:100041
Jankowiak Beata Kowalewska El B, Krajewska-Kułak Dzmitry Khvorikzbieta F (2020) Stigmatization and quality of life in patients with psoriasis. Dermatol Ther. https://doi.org/10.6084/m9.figshare.11890032
Jyothi SL, Krishna KL, Ameena Shirin VK et al (2021) Drug delivery systems for the treatment of psoriasis: current status and prospects. J Drug Deliv Sci Technol 62:106324
Kamata M, Tada Y (2018) Safety of biologics in psoriasis. J Dermatol 45:279–286
Kaushik SB, Lebwohl MG (2019) Review of safety and efficacy of approved systemic psoriasis therapies. Int J Dermatol 58:649–658
Khadka DK, Agrawal S, Dhali TK (2016) Methotrexate plus narrow band ultraviolet B (NBUVB) versus methotrexate alone in the treatment of moderate to severe plaque psoriasis: a randomized clinical trial. Nepal J Dermatol Venereol Leprol 13:12–23. https://doi.org/10.3126/njdvl.v13i1.14298
Kim WB, Jerome D, Yeung J (2017) Diagnosis and management of psoriasis. Can Fam Phys 63:278–285
Koo JYM, Lowe NJ, Lew-Kaya DA et al (2000) Tazarotene plus UVB phototherapy in the treatment of psoriasis. J Am Acad Dermatol 43:821–828. https://doi.org/10.1067/mjd.2000.107940
Kwan Z, Bong YB, Tan LL et al (2018) Determinants of quality of life and psychological status in adults with psoriasis. Arch Dermatol Res 310:443–451. https://doi.org/10.1007/s00403-018-1832-x
Laws PM, Young HS (2010) Topical treatment of psoriasis. Expert Opin Pharmacother 11:1999–2009
Lebwohl M, Ali S (2001) Treatment of psoriasis. Part 2. Systemic therapies. J Am Acad Dermatol 45:649–664. https://doi.org/10.1067/mjd.2001.117047
Lee CH, Wu SB, Hong CH et al (2013) Molecular mechanisms of UV-induced apoptosis and its effects on skin residential cells: the implication in UV-based phototherapy. Int J Mol Sci 14:6414–6435. https://doi.org/10.3390/ijms14036414
Lee HJ, Hong YJ, Kim M (2021) Angiogenesis in chronic inflammatory skin disorders. Int J Mol Sci 22:169
Levin E, Debbaneh M, Malakouti M et al (2015) Supraerythemogenic excimer laser in combination with clobetasol spray and calcitriol ointment for the treatment of generalized plaque psoriasis: interim results of an open label pilot study. J Dermatol Treat 26:16–18. https://doi.org/10.3109/09546634.2013.860210
Lin X, Meng X, Song Z, Lin J (2022) Peroxisome proliferator-activator receptor γ and psoriasis, molecular and cellular biochemistry. Mol Cell Biochem 477:1905–1920. https://doi.org/10.1007/s11010-022-04417-0
Lowes MA, Bowcock AM, Krueger JG (2007) Pathogenesis and therapy of psoriasis. Nature 445:866–873
Lowes MA, Suárez-Fariñas M, Krueger JG (2014) Immunology of psoriasis. Annu Rev Immunol 32:227–255
Lynch M, Tobin AM, Ahern T et al (2014a) Sitagliptin for severe psoriasis. Clin Exp Dermatol 39:841–842
Lynch M, Ahern TB, Timoney I et al (2016) Dipeptidyl peptidase-4 inhibition and narrow-band ultraviolet-B light in psoriasis (DINUP): study protocol for a randomised controlled trial. Trials. https://doi.org/10.1186/s13063-016-1157-z
Mahajan R, Kaur I, Kanwar A (2010) Methotrexate/narrowband UVB phototherapy combination vs. narrowband UVB phototherapy in the treatment of chronic plaque-type psoriasis—a randomized single-blinded placebo-controlled study. J Eur Acad Dermatol Venereol 24:595–600. https://doi.org/10.1111/j.1468-3083.2009.03486.x
Matz H (2010) Phototherapy for psoriasis: what to choose and how to use: facts and controversies. Clin Dermatol 28:73–80. https://doi.org/10.1016/j.clindermatol.2009.04.003
Morita A (2018) Current developments in phototherapy for psoriasis. J Dermatol 45:287–292
Mrowietz U (2001) Advances in systemic therapy for psoriasis. Clin Exp Dermatol 26:362–367. https://doi.org/10.1046/j.1365-2230.2001.00835.x
Nadia A, Elsherif 1 IMESAE-D (2015) Narrow band UVB monotherapy versus topical calcipotriol ointment combined with narrow band UVB phototherapy for treatment of psoriasis vulgaris. Ibnosina J Med Biomed Sci
Naldi L, Gambini D (2007) The clinical spectrum of psoriasis. Clin Dermatol 25:510–518. https://doi.org/10.1016/j.clindermatol.2007.08.003
Nast A, Boehncke W-H, Mrowietz U et al (2012) S3-Guidelines on the treatment of psoriasis vulgaris (English version). Update guidelines on the treatment of psoriasis vulgaris S1 1 preface. JDDG. https://doi.org/10.1111/j.1610-0379.2012.07919.x
Neimann AL, Porter SB, Gelfand JM (2006a) The epidemiology of psoriasis. Expert Rev Dermatol 1:63–75. https://doi.org/10.1586/17469872.1.1.63
Neimann AL, Shin DB, Wang X et al (2006b) Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol 55:829–835. https://doi.org/10.1016/j.jaad.2006.08.040
Nejad SB, Ghadim HH, Ezzati A et al (2015) Comparison between the effects of Daivonex cream alone and its combination with narrowband ultraviolet B in treatment of psoriasis. Our Dermatol Online. https://doi.org/10.7241/ourd.20153.76
Norval M (2006) The mechanisms and consequences of ultraviolet-induced immunosuppression. Prog Biophys Mol Biol 92:108–118
Ogawa E, Sato Y, Minagawa A, Okuyama R (2018) Pathogenesis of psoriasis and development of treatment. J Dermatol 45:264–272
Ontario Health (Quality) (2020) Home narrowband ultraviolet B phototherapy for photoresponsive skin conditions: A health technology assessment
ORTONNE (1999) Recent developments in the understanding of the pathogenesis of psoriasis. Br J Dermatol 140:1–7. https://doi.org/10.1046/j.1365-2133.1999.140S54001.x
Özkan İ, Köse O, Özmen İ, Arca E (2012) Efficacy and safety of non-laser, targeted UVB phototherapy alone and in combination with psoralen gel or calcipotriol ointment in the treatment of localized, chronic, plaque-type psoriasis. Int J Dermatol 51:609–613. https://doi.org/10.1111/j.1365-4632.2011.05257.x
Peter Kozub M (2011) Systemic therapy of psoriasis: methotrexate
Reich K, Bewley A (2011) What is new in topical therapy for psoriasis? J Eur Acad Dermatol Venereol 25:15–20
Reichrath J, Holick MF (2018) Psoriasis and other skin diseases. In: Vitamin D. Elsevier, London, pp 1037–1051
Rendon A, Schäkel K (2019) Psoriasis pathogenesis and treatment. Int J Mol Sci 20:6
Salman A, Yucelten AD, Sarac E et al (2018) Impact of psoriasis in the quality of life of children, adolescents and their families: a cross-sectional study. An Bras Dermatol 93:819–823. https://doi.org/10.1590/abd1806-4841.20186981
Sandoval LF, Pierce A, Feldman SR (2014) Systemic therapies for psoriasis: an evidence-based update. Am J Clin Dermatol 15:165–180
Sascha DMWB, Aurora G, Laura Salgado-Boquete P (2020) Long-term safety of oral systemic therapies for psoriasis: a comprehensive review of the literature. Dermatol Ther. https://doi.org/10.6084/m9.figshare.12409523
Schön MP (2019) Adaptive and innate immunity in psoriasis and other inflammatory disorders. Front Immunol 10:1764
Shaker OG, Khairallah M, Rasheed HM et al (2013) Antiangiogenic effect of methotrexate and PUVA on psoriasis. Cell Biochem Biophys 67:735–742. https://doi.org/10.1007/s12013-013-9563-2
Smith CH, Barker JNWN (2006) Psoriasis and its management. BMJ 333:380–384. https://doi.org/10.1136/bmj.333.7564.380
Soliman A, Nofal EA, Nofal A et al (2015) Combination therapy of methotrexate plus NBUVB phototherapy is more effective than methotrexate monotherapy in the treatment of chronic plaque psoriasis. J Dermatol Treat 26:528–534. https://doi.org/10.3109/09546634.2015.1034069
Torres AE, Lyons AB, Hamzavi IH, Lim HW (2021) Role of phototherapy in the era of biologics. J Am Acad Dermatol 84:479–485
Torsekar R, Gautam M (2017) Topical therapies in psoriasis. Indian Dermatol Online J 8:235. https://doi.org/10.4103/2229-5178.209622
Tzaneva S, Geroldinger A, Trattner H, Tanew A (2018) Fumaric acid esters in combination with a 6-week course of narrowband ultraviolet B provides an accelerated response compared with fumaric acid esters monotherapy in patients with moderate-to-severe plaque psoriasis: a randomized prospective clinical study. Br J Dermatol 178:682–688. https://doi.org/10.1111/bjd.16106
Uva L, Miguel D, Pinheiro C et al (2012) Mechanisms of action of topical corticosteroids in psoriasis. Int J Endocrinol 2012:561018
Valdimarsson H, Gudjonsson JE, Johnston A et al (2004) Immunopathogenic mechanisms in psoriasis. Clin Exp Immunol 135:1–8. https://doi.org/10.1046/j.1365-2249.2004.02310.x
van Winden MEC, van der Schoot LS, van de L’Isle AM et al (2020) Effectiveness and safety of systemic therapy for psoriasis in older adults a systematic review. JAMA Dermatol 156:1229–1239
Verallo-Rowell VM, Katalbas SS, Evangelista MTP, Dayrit JF (2018) Review update on topical therapy for psoriasis. Curr Dermatol Rep 7:24–36
Vu TT, Koguchi-Yoshioka H, Watanabe R (2021) Skin-resident memory t cells: pathogenesis and implication for the treatment of psoriasis. J Clin Med 10:17
Weisenseel P, Reich K, Griemberg W et al (2017) Wirksamkeit und Sicherheit von Fumarsäureestern in Kombination mit Phototherapie bei Patienten mit moderater bis schwerer Plaque-Psoriasis (FAST). JDDG-J German Soc Dermatol 15:180–186. https://doi.org/10.1111/ddg.12837
Wong T, Hsu L, Liao W (2013a) Phototherapy in psoriasis: a review of mechanisms of action. J Cutan Med Surg 17:6–12. https://doi.org/10.2310/7750.2012.11124
Woo W-M (2019) Imaging technologies and transdermal delivery in skin disorders. Wiley, New York
Xi C, Xiong C, Wang H et al (2021) Combination of retinoids and narrow-band ultraviolet B inhibits matrix metalloproteinase 13 expression in HaCaT keratinocytes and a mouse model of psoriasis. Sci Rep 11:13328. https://doi.org/10.1038/s41598-021-92599-w
Yélamos O, Puig L (2015) Systemic methotrexate for the treatment of psoriasis. Expert Rev Clin Immunol 11:553–563. https://doi.org/10.1586/1744666X.2015.1026894
Zhang P, Wu MX (2018) A clinical review of phototherapy for psoriasis. Lasers Med Sci 33:173–180
Acknowledgements
The authors would like to acknowledge the research funding support by the Department of Pharmaceuticals (DoP), Ministry of Chemicals and Fertilizers, Government of India to “Pharmaceutical Innovation and Translational Research Lab (PITRL)” Department of Pharmaceutics, National Institute of Pharmaceutical Education and Research (NIPER) Hyderabad, India.
Funding
This study was funded by the Department of Pharmaceuticals (DoP), Ministry of Chemicals and Fertilizers, Government of India to National Institute of Pharmaceutical Education and Research (NIPER) Hyderabad, India.
Author information
Authors and Affiliations
Contributions
RS: Conceptualization, Methodology, Writing—original draft, Writing—review & editing. SN: Conceptualization, Methodology, Writing—review & editing. VP: writing—review and editing. SBS: Supervision and Project administration. SS: Supervision, Conceptualization, Project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Data availability
Enquiries about data availability should be directed to the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sreya, R., Nene, S., Pathade, V. et al. Emerging trends in combination strategies with phototherapy in advanced psoriasis management. Inflammopharmacol 31, 1761–1778 (2023). https://doi.org/10.1007/s10787-023-01257-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-023-01257-2