Abstract
Purpose of Review
Since obesity is a major risk factor for many different types of cancer, examining one of the most closely associated comorbidities, such as hypercholesterolemia, is crucial to understanding how obesity causes cancer. Hypercholesterolemia is usually associated with many cardiovascular complications such as hypertension, angina, and atherosclerosis. In addition, cholesterol may be a major factor in increasing cancer risk. Cancer patients who received statins, an anti-hypercholesteremic medicine, demonstrated improved prognosis possibly through its effect on tumor proliferation, apoptosis, and oxidative stress. Cholesterol could also aid in tumor progression through reprogramming tumor immunological architecture and mediators. This review focuses on the immunomodulatory role of cholesterol on cellular and molecular levels, which may explain its oncogenic driving activity. We look at how cholesterol modulates tumor immune cells like dendritic cells, T cells, Tregs, and neutrophils. Further, this study sheds light on the modification of the expression pattern of the common cancer-related immune mediators in the tumor immune microenvironment, such as programmed cell death 1 (PD-1), cytotoxic T lymphocyte antigen-4 (CTLA-4), transforming growth factor-beta (TGF-β), interleukin 12 (IL-12), IL-23, and forkhead box protein P3 (FOXP3).
Recent Findings
We highlight relevant literature demonstrating cholesterol's immunosuppressive role, leading to a worse cancer prognosis. This review invites further research regarding the pathobiological role of cholesterol in many obesity-related cancers such as uterine fibroids, post-menopausal breast, colorectal, endometrial, kidney, esophageal, pancreatic, liver, and gallbladder cancers.
Summary
This review suggests that targeting cholesterol synthesis may be a fruitful approach to cancer targeting, in addition to traditional chemotherapeutics.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Cholesterol
Cholesterol is a steroid ring molecule found in both animals and plants [1]. In humans, about 80% of total cholesterol is biosynthesized in the body, while the remaining 20% is received through diet. Cholesterol comes in two forms: either free or esterified with fatty acids [2••]. Cholesterol ester – the predominant form of dietary cholesterol – is carried by Apo proteins as part of an Apo-lipoprotein complex (cholesterol carrier/transporter) in the body to form lipoproteins. Plasma lipoproteins are classified as chylomicrons, chylomicron remnants, very-low-density lipoprotein (VLDL), intermediate low-density lipoprotein (ILDL), low-density lipoprotein (LDL), lipoprotein A (Lp (a)), and high-density lipoprotein (HDL) [3, 4].
Cholesterol plays a role in cancer incidence, prognosis, and treatment outcomes. Cancer cells require ample cholesterol for rapid growth. Increased serum cholesterol levels have been linked to an increased risk of developing malignancies such as colon, rectal, prostatic, and testicular cancers [5]. Overexpression of LDLR and ACAT is found in most tumor tissue from cancer patients, which supports rapid cancer cell proliferation [1]. Most cancer tissues have higher LDL receptor expression than normal [6]. Using a combination of drugs to target cholesterol production and uptake was found to decrease cancer cell survival.
As hypercholesterolemia is also one of the major risk factors for hypertension, Cho et al. [7] showed that there was a substantial correlation between the long-term usage of ARBs and a decreased incidence of cancer. When compared to users of other kinds of antihypertensive pharmaceuticals, there was no overall increased risk of cancer for those who took typical antihypertensive medications. On the other hand, other analyses showed that there is no significant association was found between cancer and other antihypertensives [8, 9].
As obesity is one of the main risk factors for several types of cancer [10, 11], investigating one of the most related comorbidities such as hypercholesterolemia is essential to knowing how obesity induces cancer. Several recent studies have emphasized altering tumor progression by targeting cholesterol metabolic pathways [12,13,14]. From there, several studies were made to find how cholesterol is essential for cancer growth, especially on proliferation, apoptosis, and oxidative stress. On the other hand, few studies randomly discussed the immunomodulatory effect of cholesterol. As the immune microenvironment is a recent promising axis for cancer-targeting, investigating the role of cholesterol on the cancer immune milieu will open a new era of better immune-metabolome interaction and thus novel targeting approaches. The immune mediators and immune cells discussed here are among the most studied immune-related biomarkers related to the tumor immune microenvironment.
Antihypercholesterolemia
Statins are FDA-approved drugs that prevent cholesterol synthesis by inhibiting the enzyme 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase [15, 16]. Statins appear to inhibit the growth and survival of several cancer cells in vitro and in vivo experiments. Furthermore, LDLR downregulation/inhibition increases the efficacy of chemotherapeutics [17].
Cholesterol and Tumor Pathobiology
Cholesterol’s Effect on Cancer Proliferation
Enhanced proliferation is one of the main hallmarks of tumors. Cholesterol induces cancer proliferation by affecting multiple pathways. Afrin et al. [18•] found that simvastatin, one of the statins, inhibits uterine leiomyoma stem cell proliferation and induces apoptosis. In a patient-derived xenograft mouse model, El Sabeh et al. [19] showed that simvastatin significantly reduced tumor volume and inhibited the proliferation marker Ki67 expression when compared to the control group. Borahay et al. [20] revealed that simvastatin decreased tumor development and Ki67 expression in xenograft tumor tissue. Afrin et al. [21] found that simvastatin dramatically decreased proliferating cell nuclear antigen (PCNA) expression and E2-induced proliferation in leiomyoma cells. El Sabeh et al. [22] showed that both primary and immortalized human leiomyoma cells showed decreased levels of β-catenin following simvastatin treatment.
Cholesterol’s Effect on Cancer Apoptosis
Cholesterol inhibits cancer apoptosis by affecting multiple pathways. Caspase-3 is one of the apoptosis-inducing enzymes. Malik et al. [23] found that in simvastatin-treated cells, caspase-3 level decreased in a concentration-dependent manner. Borahay et al. [24] showed that simvastatin strongly induced leiomyoma cell death.
Cholesterol’s Effect on Cancer Oxidative Stress
Cholesterol induces oxidative stress and thus cancer progression by affecting multiple pathways. Homma et al. [25] found that long-term feeding of cholesterol induced atypical prostatic hyperplasia and increased tissue oxidative stress. Rauchbach et al. [26] found that in a model of non-alcoholic steatohepatitis, cholesterol may trigger hepatic stellate cells lipid peroxidation and death in the liver.
Cholesterol and Tumor Immune Microenvironment
Cholesterol and Immune Mediators
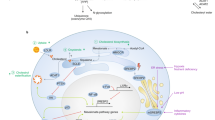
Cholesterol is found to suppress the immune microenvironment by suppressing immunostimulant cytokines and stimulating immunosuppressive cytokines. Immune mediators and cells discussed here are chosen based on their significance for cancer progression (Fig. 1).
Transforming Growth Factor-beta (TGF-β)
Transforming growth factor-beta (TGF-β) is an immunosuppressive cytokine that encourages cell growth and promotes cancer growth [27]. In HCC, TGF-β is linked to immune cell exhaustion, whereas inactivated TGF-β is linked to inadequate DNA repair [28, 29]. By controlling immune cells in the liver, TGF-β maintains a balance between immunological tolerance and activation. TGF-β is also a growth factor that regulates immune cells [30, 31] (Fig. 2).
Effect of cholesterol on tumor immune mediators. Cholesterol alters the tumor microenvironment in favor of immunosuppressive activity via a variety of mechanisms, including (1) Stimulation of both PDL1, IL-23, and TGF-β1, which stimulates both Treg cells and tolerogenic DCs while inhibiting cytotoxic T cells and mature DCs, (2) Stimulation of FOXP3, and CTLA-4, which stimulates Treg cells and inhibits cytotoxic T cells, and (3) Inhibition of IL-12 which stimulates Treg cells and M2 macrophages and inhibits cytotoxic T cells and M1 macrophages. CTLA-4: Cytotoxic T lymphocyte antigen-4; Il-12: interleukin 12; PD-L1: Programmed death-ligand 1; TGF-β: Transforming growth factor-β; DCS: Dendritic cells; FOXP3: Forkhead box protein P3; Treg: Regulatory T cells
Cholesterol induces TGF-β expression. Zhou et al. [32••] found that the levels of plasma TGF-β1 and cholesterol were positively correlated. Feeding high cholesterol elevated glomerular TGF-β1 and fibronectin mRNA levels in a nephrosis rat model. Furthermore, Statins decreased TGF-β activity as well as expression of TGF-β targets ZYX and SERPINE1. These effects were observed in GBM and GBM-initiating cell (GIC) lines [33]. In UL stem cells, simvastatin has been observed to suppress the production of TGF-β1 (Fig. 2) [18•].
Programmed Cell Death 1 (PD-1)
Programmed cell death 1 (PD-1) and its ligand, PD-L1, deplete T cells and prevent the action of proinflammatory mediators. PD-L1 induces tumor growth, activated T-cell immune suppression [34, 35]. Cholesterol induces PD-L1 expression. Anti–p PD–1 immunotherapies may be more effective if cholesterol is reduced [36]. Simvastatin was found to inhibit PD-L1 expression promoting anti-tumor in colorectal cancers (CRCs) [37]. In melanoma and lung cancer cells, simvastatin, atorvastatin, lovastatin, and fluvastatin reduced PD-L1 expression [38••]. Cholesterol increased PD-1, decreased interferon-gamma, and granzyme B production, and increased apoptosis in T cells [39] (Fig. 2).
Interleukin 12 (IL-12)
IL-12, a heterodimeric cytokine consisting of p40 and p35 subunits, is mostly thought to be pro-inflammatory. It is produced by antigen-presenting cells (APCs) – including macrophages and dendritic cells (DCs) – and is essential for CD8+ T and NK cell recruitment and effector functions. Thus, IL-12 plays a significant role in promoting anti-tumor immune responses [40, 41]. Cholesterol was found to inhibit IL-12 expression. In a central nervous system (CNS) autoimmune illness model, atorvastatin decreased STAT4 phosphorylation and suppressed the release of IL-12 [42]. Coward et al. [43] found that the primary mechanisms by which statins induce a proinflammatory response in activated peripheral blood monocytes (PBMCs) are the activation of caspase-1 and IL-18 production in the monocytes, with IL-12 playing a secondary role (Fig. 2).
Forkhead Box Protein P3 (FOXP3)
Forkhead box protein P3 (FOXP3) is a transcription factor and member of the forkhead box (FOX) protein family. FOXP3 acted as a master regulator in the maturation and operation of the immunosuppressive regulatory T cells (Tregs) [44]. Tregs can suppress several immunostimulant cells as NK cells, macrophages, DCs, and B cells by generating immunosuppressive substances and thus encourage tumor growth [45,46,47]. A large number of tumor-infiltrating Tregs are associated with HCC [48]. Li et al. [49] found that in stage B HCC patients, Treg levels independently predicted a poor prognosis.
Cholesterol induces FOXP3 expression. High-cholesterol diets increase the CD4+ FOXP3+ Treg cell population in the liver [50]. Mailer et al. [51] found that a high-cholesterol diet increased FOXP3 expression. Wen et al. [52] noted that cholesterol replenishment could prevent nicotine-induced p-STAT5/ FOXP3 pathway suppression, and Treg frequency (Fig. 2).
Cytotoxic T-lymphocyte Associated Antigen 4 (CTLA-4)
As a T cell suppressor, cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) was the first molecule effectively targeted for immune checkpoint therapy [53,54,55]. Cholesterol induces CTLA-4 expression. By targeting Ras-activated mTOR signaling, atorvastatin has been shown to suppress the expression of CTLA-4, implying an indirect link between cholesterol and immune checkpoint expression [56]. According to Zeng et al. [57], the mevalonate pathway, which is responsible for cholesterol synthesis, is necessary to produce CTLA-4 (Fig. 2).
IL-23
Interleukin-23 is an immunosuppressive cytokine that is overexpressed in many human malignancies, consistent with its involvement in increasing tumor growth in mice [58]. Infiltration of the immunosuppressive M2 macrophages, neutrophils, TGF-β, IL-10, and VEGF into tumor tissues is promoted by IL-23. IL-23 also raises the expression of the endothelial and proliferative markers CD31 and Ki67 in malignancies. Furthermore, IL23 suppresses the immune system by lowering the invasion of CD4+ and CD8+ T lymphocytes into tumor tissues [59]. Mice lacking IL-23p19 were resistant to DMBA/TPA-induced skin papillomas [58]. Cholesterol induces IL-23 expression. In obese patients, adiposity is a potential biological source of IL-17 and IL-23, as well as a source of pro-inflammatory mediators and invading immune cells [60]. In the synovitis, acne, pustulosis, hyperostosis, and osteitis group, serum IL-23 was associated positively with total cholesterol and HDL cholesterol [61].
Ma et al. [62] found that one week after starting statin medication, there was a decrease in IL-23 levels in peripheral blood [62]. Furthermore, statin inhibits the phosphorylation of the transcription factors STAT3 and STAT1, which are implicated in the control of IL-6 and IL-23 [63] (Fig. 2).
All data mentioned in the immune mediators’ part and their correlations to cholesterol are summarized in Table 1.
Cholesterol and Immune Cells
Cholesterol is found to suppress the immune microenvironment by suppressing immunostimulant cells and stimulating immunosuppressive cells.
Neutrophils
Neutrophils comprise 50–70% of human leukocytes in the bloodstream. They serve as the host's first line of defense, protecting against pathogen attacks by phagocytosis, granule release, and cytokine synthesis. Tumor-associated neutrophils (TANs) that infiltrate the body exhibit either protumorigenic (N2) or anttumorigenic (N1) properties [65]. Xiong et al. [66] found that cholesterol metabolite causes an increase in neutrophil counts. Akinyemi et al. [67] and Grzywa et al. [68] found that arginase activity, one of the common inducers of N2 immunosuppressive neutrophils, was significantly increased in rats fed a high-cholesterol diet. Guasti et al. [69] demonstrated that patients on long-term statin therapy consistently showed a reduction in AT1-R expression in primed PMNs and a reversion of the pro-inflammatory oxidative functional response.
Cytotoxic CD8+ T Cells
Cytotoxic T lymphocytes (Cytotoxic CD8+ T Cells; CTLs) induce cancer cell death through temporary cell-cell interaction and paracrine distribution of cytotoxic effector chemicals [45, 54, 70]. Interleukin-2 and IFN-γ promote T cell priming, activation, and cytotoxicity, which culminates in anti-tumor action [71]. Upregulated T cell expression of PD-1, 2B4, TIM-3, and LAG-3 was positively and gradually linked with T cell exhaustion in tumor tissues enriched with cholesterol and cholesterol content in tumor-infiltrating CD8+ T cells [39]. Picarda et al. [72] found that T cell exhaustion was induced by tumor-derived or exogenous cholesterol via upregulating immunological checkpoints on CD8 T cells and inducing death (Fig. 2).
Dendritic Cells
Dendritic cells are APCs that can recognize diseases and alert the immune system, mainly T cells, of their presence. Major histocompatibility molecules (MHCs) are required for naive T cells to locate, identify, capture, and process pathogens in peripheral tissues before conveying antigenic peptides from pathogens to naive T cells in lymphoid organs. These methods result in a critical role for DCs in the development of antigen-specific immune responses [73]. Cholesterol shifts the equilibrium in DCs from the mature type (immunostimulant) to the tolerogenic (immunosuppressive) type (Fig. 3). Ramakrishnan et al. [74] found that cross-presentation was inhibited by oxidized lipids. DCs with a high lipid content could not efficiently excite allogeneic T lymphocytes or present tumor-associated antigens, and their ability to digest antigens was diminished [75]. Please see Table 2 for a summary of the data of Cholestertol’s correlation to immune cells.
Effect of cholesterol on tumor immune archetype. Cholesterol alters the tumor microenvironment by various mechanisms, including (1) Shifting T cell equilibrium away from the cytotoxic type and towards the Treg cell type; (2) Shifting DCs equilibrium away from the mature type and towards the tolerogenic cell type; (3) Shifting Neutrophils equilibrium away from the N1 type and towards N2 cell type. DCs: Dendritic cells; Tregs: regulatory T cells
Conclusion
Despite decades of considerable research on cancer immunophenotyping that has yielded intriguing results, there is still a need to investigate the pathobiological role of different metabolites in tumor immune microenvironment.
Several studies proved the carcinogenic role of cholesterol through its pro-proliferative, pro-oxidant, and anti-apoptotic properties. Cholesterol was found to suppress tumor immune fitness. Cholesterol decreases immunostimulant mediators like IL-12 while increasing immunosuppressive mediators, including TGF-β, FOXP3, IL-23, PD-L1, and CTLA-4. Furthermore, immune-stimulatory cells such as cytotoxic T cells, DCs, and neutrophils were blocked by cholesterol, whereas immunosuppressive cells like Treg cells were activated. Based on our review, we suggest more research be done on different types of cancer, studying the effect of cholesterol and cholesterol-lowering medication on cancer immune milieu.
Data Availability
No datasets were generated or analysed during the current study.
References
Mayengbam SS, Singh A, Pillai AD, Bhat MK. Influence of cholesterol on cancer progression and therapy. Transl Oncol. 2021;14(6):101043.
•• Cardoso D, Perucha E. Cholesterol metabolism: a new molecular switch to control inflammation. 2021;135(11):1389–408. This article focuses on how cholesterol metabolism affects different immune cells' function.
Yoon H, Shaw JL, Haigis MC, Greka A. Lipid metabolism in sickness and in health: emerging regulators of lipotoxicity. Mol Cell. 2021;81(18):3708–30.
Pinzon Grimaldos A, Bini S, Pacella I, Rossi A, Di Costanzo A, Minicocci I, D'Erasmo L, Arca M, Piconese S. The role of lipid metabolism in shaping the expansion and the function of regulatory T cells. Clin Exp Immunol. 2022;208:181–92.
Ding X, Zhang W, Li S, Yang H. The role of cholesterol metabolism in cancer. Am J Cancer Res. 2019;9(2):219–27.
Matsushita Y, Nakagawa H, Koike K. Lipid metabolism in oncology: why it matters, how to research, and how to treat. Cancers (Basel). 2021;13.
Cho IJ, Shin JH, Jung MH, Kang CY, Hwang J, Kwon CH, Kim W, Kim DH, Lee CJ, Kang SH, Lee JH, Kim HL, Kim HM, Cho I, Lee HY, Chung WJ, Ihm SH, Kim KI, Cho EJ, Sohn IS, Park S, Shin J, Ryu SK, Kim JY, Kang SM, Cho MC, Pyun WB, Sung KC. Antihypertensive drugs and the risk of cancer: a nationwide cohort study. J Clin Med. 2021;10.
Wang S, Xie L, Zhuang J, Qian Y, Zhang G, Quan X, et al. Association between use of antihypertensive drugs and the risk of cancer: a population-based cohort study in Shanghai. BMC Cancer. 2023;23(1):425.
Copland E, Canoy D, Nazarzadeh M, Bidel Z, Ramakrishnan R, Woodward M, et al. Antihypertensive treatment and risk of cancer: an individual participant data meta-analysis. Lancet Oncol. 2021;22(4):558–70.
Pati S, Irfan W, Jameel A, Ahmed S, Shahid RK. Obesity and cancer: a current overview of epidemiology, pathogenesis, outcomes, and management. Cancers (Basel). 2023;15.
Krupa-Kotara K, Dakowska D. Impact of obesity on risk of cancer. Cent Eur J Public Health. 2021;29(1):38–44.
Peltomaa AI, Talala K, Taari K, Tammela TLJ, Auvinen A, Murtola TJ. Inverse association between statin use and cancer mortality relates to cholesterol level. Cancers (Basel). 2022;14.
Narii N, Zha L, Komatsu M, Kitamura T, Sobue T, Ogawa T. Cholesterol and breast cancer risk: a cohort study using health insurance claims and health checkup databases. Breast Cancer Res Treat. 2023;199(2):315–22.
Hartmann P, Trufa DI, Hohenberger K, Tausche P, Trump S, Mittler S, et al. Contribution of serum lipids and cholesterol cellular metabolism in lung cancer development and progression. Sci Rep. 2023;13(1):5662.
da Costa RF, Freire VN, Bezerra EM, Cavada BS, Caetano EW, de Lima Filho JL, et al. Explaining statin inhibition effectiveness of HMG-CoA reductase by quantum biochemistry computations. Phys Chem Chem Phys. 2012;14(4):1389–98.
Abd El-Fattah EE. IDO/kynurenine pathway in cancer: possible therapeutic approaches. J Transl Med. 2022;20(1):347.
Broadfield LA, Pane AA, Talebi A, Swinnen JV, Fendt SM. Lipid metabolism in cancer: new perspectives and emerging mechanisms. Dev Cell. 2021;56(10):1363–93.
• Afrin S, Ali M, El Sabeh M, Yang Q, Al-Hendy A, Borahay MA. Simvastatin inhibits stem cell proliferation in human leiomyoma via TGF-β3 and Wnt/β-Catenin pathways. 2022;26:1684–98. This article shows how statins (antihypercholesteremic) inhibit leiomyoma cell proliferation.
El Sabeh M, Vincent KL, Afrin S, Motamedi M, Saada J, Yang J, et al. Simvastatin-loaded liposome nanoparticles treatment for uterine leiomyoma in a patient-derived xenograft mouse model: a pilot study. J Obstet Gynaecol. 2022;42(6):2139–43.
Borahay MA, Vincent K, Motamedi M, Sbrana E, Kilic GS, Al-Hendy A, et al. Novel effects of simvastatin on uterine fibroid tumors: in vitro and patient-derived xenograft mouse model study. Am J Obstet Gynecol. 2015;213(2):196.e1–.e8.
Afrin S, El Sabeh M, Islam MS, Miyashita-Ishiwata M, Malik M, Catherino WH, et al. Simvastatin modulates estrogen signaling in uterine leiomyoma via regulating receptor palmitoylation, trafficking and degradation. Pharmacol Res. 2021;172:105856.
El Sabeh M, Saha SK, Afrin S, Borahay MA. Simvastatin inhibits Wnt/β-Catenin pathway in uterine leiomyoma. Endocrinology. 2021;162.
Malik M, Catherino WH, Laknaur A, Ali M, Al-Hendy A, Segars J, et al. Synergistic effects of simvastatin and ulipristal acetate on uterine leiomyoma. Fertility and sterility. 2017;108(3, Supplement):e65.
Borahay MA, Kilic GS, Yallampalli C, Snyder RR, Hankins GD, Al-Hendy A, et al. Simvastatin potently induces calcium-dependent apoptosis of human leiomyoma cells. J Biol Chem. 2014;289(51):35075–86.
Homma Y, Kondo Y, Kaneko M, Kitamura T, Nyou WT, Yanagisawa M, et al. Promotion of carcinogenesis and oxidative stress by dietary cholesterol in rat prostate. Carcinogenesis. 2004;25(6):1011–4.
Rauchbach E, Zeigerman H, Abu-Halaka D, Tirosh O. Cholesterol induces oxidative stress, mitochondrial damage and death in hepatic stellate cells to mitigate liver fibrosis in mice model of NASH. Antioxidants. 2022;11(3):536.
Saad EE, Michel R, Borahay MA. Immunosuppressive tumor microenvironment and uterine fibroids: role in collagen synthesis. Cytokine Growth Factor Rev. 2024;75:93–100.
Abd El-Fattah EE, Zakaria AY. Metformin modulate immune fitness in hepatocellular carcinoma: molecular and cellular approach. Int Immunopharmacol. 2022;109:108889.
Abdelhamid AM, Saber S, Youssef ME, Gaafar AGA, Eissa H, Abd-Eldayem MA, et al. Empagliflozin adjunct with metformin for the inhibition of hepatocellular carcinoma progression: emerging approach for new application. Biomed Pharmacother. 2022;145: 112455.
Jeon H-S, Jen J. TGF-beta signaling and the role of inhibitory Smads in non-small cell lung cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2010;5(4):417–9.
Youssef ME, Abd El-Fattah EE, Abdelhamid AM, Eissa H, El-Ahwany E, Amin NA, Hetta HF, Mahmoud MH, Batiha GE, Gobba N, Ahmed Gaafar AG, Saber S. Interference with the AMPKα/mTOR/NLRP3 signaling and the IL-23/IL-17 axis effectively protects against the dextran sulfate sodium intoxication in rats: a new paradigm in empagliflozin and metformin reprofiling for the management of ulcerative colitis. Front Pharmacol. 2021;12:719984.
•• Zhou X, Johnston TP, Johansson D, Parini P, Funa K, Svensson J, et al. Hypercholesterolemia leads to elevated TGF-beta1 activity and T helper 3-dependent autoimmune responses in atherosclerotic mice. Atherosclerosis. 2009;204(2):381–7. This article show how cholesterol induce TGF-B1 expression (one of the most potent immunosuppressants).
Xiao A, Brenneman B, Floyd D, Comeau L, Spurio K, Olmez I, et al. Statins affect human glioblastoma and other cancers through TGF-β inhibition. Oncotarget. 2019;10(18).
Abd El-Fattah EE, Selim HM. Reprograming immune microenvironment modulates CD47 cancer stem cells in hepatocellular carcinoma. Int Immunopharmacol. 2022;113:109475.
Macek Jilkova Z, Aspord C, Decaens T. Predictive factors for response to PD-1/PD-L1 checkpoint inhibition in the field of hepatocellular carcinoma: current Status and Challenges. Cancers. 2019;11(10):1554.
Cholesterol modulates immune checkpoint expression in TILs. Cancer Discov. 2019;9:OF15-OF15.
Ni W, Mo H, Liu Y, Xu Y, Qin C, Zhou Y, et al. Targeting cholesterol biosynthesis promotes anti-tumor immunity by inhibiting long noncoding RNA SNHG29-mediated YAP activation. Mol Ther. 2021;29(10):2995–3010.
•• Lim W-J, Lee M, Oh Y, Fang X-Q, Lee S, Lim C-H, et al. Statins decrease programmed death-ligand 1 (PD-L1) by inhibiting AKT and β-catenin signaling. Cells. 2021;10(9):2488. This article focuses on how statins (anti-hypercholesterolemic medication) decrease PD-L1 and thus reverse its immunosuppressive effect.
Ma X, Bi E, Lu Y, Su P, Huang C, Liu L, et al. Cholesterol induces CD8(+) T cell exhaustion in the tumor microenvironment. Cell Metab. 2019;30(1):143-56.e5.
Yue T, Zheng X, Dou Y, Zheng X, Sun R, Tian Z, et al. Interleukin 12 shows a better curative effect on lung cancer than paclitaxel and cisplatin doublet chemotherapy. BMC Cancer. 2016;16(1):665.
Lo CH, Chang CM, Tang SW, Pan WY, Fang CC, Chen Y, et al. Differential antitumor effect of interleukin-12 family cytokines on orthotopic hepatocellular carcinoma. J Gene Med. 2010;12(5):423–34.
Youssef S, Stüve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420(6911):78–84.
Coward WR, Marei A, Yang A, Vasa-Nicotera MM, Chow SC. Statin-induced proinflammatory response in mitogen-activated peripheral blood mononuclear cells through the activation of Caspase-1 and IL-18 secretion in Monocytes1. J Immunol. 2006;176(9):5284–92.
Jia H, Qi H, Gong Z, Yang S, Ren J, Liu Y, et al. The expression of FOXP3 and its role in human cancers. Biochim Biophys Acta. 2019;1871(1):170–8.
El-Ashmawy NE, Salem ML, Abd El-Fattah EE, Khedr EG. Targeting CD166+ lung cancer stem cells: molecular study using murine dendritic cell vaccine. Toxicol Appl Pharmacol. 2021;429:115699.
Abd El-Fattah EE, Abdelhamid AM. Benzo[a]pyrene immunogenetics and immune archetype reprogramming of lung. Toxicology. 2021;463:152994.
Zhang H, Chen Y, Liao W, Wang L, Xie X, Fei R, et al. FOXP3 expression in FOXP3+ tumor cells promotes hepatocellular cells metastasis. Transl Cancer Res. 2020;9(10):5868–81.
Tu J-F, Ding Y-H, Ying X-H, Wu F-Z, Zhou X-M, Zhang D-K, et al. Regulatory T cells, especially ICOS+ FOXP3+ regulatory T cells, are increased in the hepatocellular carcinoma microenvironment and predict reduced survival. Sci Rep. 2016;6(1):35056.
Li F, Guo Z, Lizée G, Yu H, Wang H, Si T. Clinical prognostic value of CD4+CD25+FOXP3+regulatory T cells in peripheral blood of Barcelona Clinic Liver Cancer (BCLC) stage B hepatocellular carcinoma patients. Clin Chem Lab Med. 2014;52(9):1357–65.
Mailer RKW, Gisterå A, Polyzos KA, Ketelhuth DFJ, Hansson GK. Hypercholesterolemia induces differentiation of regulatory T cells in the liver. Circ Res. 2017;120(11):1740–53.
Mailer RKW, Gisterå A, Polyzos KA, Ketelhuth DFJ, Hansson GK. Hypercholesterolemia enhances T cell receptor signaling and increases the regulatory T cell population. Sci Rep. 2017;7(1):15655.
Wen X, Zhao W-H, Chen L-Z, Qu W, Liu H-X, Yan H-Y, et al. Attenuated cholesterol metabolism pathway suppresses regulatory T cell development in prenatal nicotine exposed female mice. Toxicology. 2019;428:152309.
Perez-Ruiz E, Minute L, Otano I, Alvarez M, Ochoa MC, Belsue V, et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature. 2019;569(7756):428–32.
Salem ML, El-Ashmawy NE, Abd El-Fattah EE, Khedr EG. Immunosuppressive role of Benzo[a]pyrene in induction of lung cancer in mice. Chem Biol Interact. 2021;333:109330.
Sobhani N, Tardiel-Cyril DR, Davtyan A, Generali D, Roudi R, Li Y. CTLA-4 in regulatory T cells for cancer immunotherapy. Cancers. 2021;13(6):1440.
Okoye I, Namdar A, Xu L, Crux N, Elahi S. Atorvastatin downregulates co-inhibitory receptor expression by targeting Ras-activated mTOR signalling. Oncotarget. 2017;8:98215–32.
Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H. mTORC1 couples immune signals and metabolic programming to establish Treg-cell function. Nature. 2013;499(7459):485–90.
Yan J, Smyth MJ, Teng MWL. Interleukin (IL)-12 and IL-23 and their conflicting roles in cancer. Cold Spring Harb Perspect Biol. 2018;10(7):a028530.
Nie W, Yu T, Sang Y, Gao X. Tumor-promoting effect of IL-23 in mammary cancer mediated by infiltration of M2 macrophages and neutrophils in tumor microenvironment. Biochem Biophys Res Commun. 2017;482(4):1400–6.
Leite BF, Morimoto MA, Gomes C, Klemz BNdC, Genaro PDS, Damasceno NRT, et al. Higher bodily adiposity, fat intake, and cholesterol serum levels are associated with higher disease activity in psoriatic arthritis patients: is there a link among fat and skin and joint involvement? Lipids Health Dis. 2020;19(1):21.
Przepiera-Będzak H, Fischer K, Brzosko M. Serum interleukin-23 protects, whereas methotrexate treatment stimulates selected components of the metabolic syndrome in patients with SAPHO syndrome. Arch Med Sci. 2021;17(1):120–6.
Ma X, Liu S, Li T, Yuan H. Intensive statin treatment ameliorate the Th17/Treg functional imbalance in patients with non-ST elevation acute coronary syndrome underwent percutaneous coronary intervention. Clin Cardiol. 2020;43(4):379–85.
Forero-Peña DA, Gutierrez FRS. Statins as modulators of regulatory T-cell biology. Mediators Inflamm. 2013;2013:167086.
Manti S, Leonardi S, Panasiti I, Arrigo T, Salpietro C, Cuppari C. Serum IL-10, IL-17 and IL-23 levels as “bioumoral bridges” between dyslipidemia and atopy. Cytokine. 2017;99:43–9.
Giese MA, Hind LE, Huttenlocher A. Neutrophil plasticity in the tumor microenvironment. Blood. 2019;133(20):2159–67.
Xiong S, Dong L, Cheng L. Neutrophils in cancer carcinogenesis and metastasis. J Hematol Oncol. 2021;14(1):173-.
Akinyemi AJ, Oboh G, Ademiluyi AO, Boligon AA, Athayde ML. Effect of two ginger varieties on arginase activity in hypercholesterolemic rats. J Acupunct Meridian Stud. 2016;9(2):80–7.
Grzywa TM, Sosnowska A, Matryba P, Rydzynska Z, Jasinski M, Nowis D, et al. Myeloid cell-derived arginase in cancer immune response. Front Immunol. 2020;11:938.
Guasti L, Marino F, Cosentino M, Maio RC, Rasini E, Ferrari M, et al. Prolonged statin-associated reduction in neutrophil reactive oxygen species and angiotensin II type 1 receptor expression: 1-year follow-up. Eur Heart J. 2008;29(9):1118–26.
Abdelhamid AM, Youssef ME, Abd El-Fattah EE, Gobba NA, Gaafar AGA, Girgis S, et al. Blunting p38 MAPKα and ERK1/2 activities by empagliflozin enhances the antifibrotic effect of metformin and augments its AMPK-induced NF-κB inactivation in mice intoxicated with carbon tetrachloride. Life Sci. 2021;286:120070.
Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25(18):2586–93.
Picarda E, Ren X, Zang X. Tumor cholesterol up T cells down. Cell Metabol. 2019;30(1):12–3.
Lu P, Yu B, Xu J. Cucurbitacin B regulates immature myeloid cell differentiation and enhances antitumor immunity in patients with lung cancer. Cancer Biother Radiopharm. 2012;27(8):495–503.
Ramakrishnan R, Tyurin VA, Veglia F, Condamine T, Amoscato A, Mohammadyani D, et al. (2014) Oxidized lipids block antigen cross-presentation by dendritic cells in cancer. J Immun (Baltimore, Md: 1950). 2014;192(6):2920–31.
Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. 2010;16(8):880–6.
Maher BM, Dhonnchu TN, Burke JP, Soo A, Wood AE, Watson RWG. Statins alter neutrophil migration by modulating cellular Rho activity—a potential mechanism for statins-mediated pleotropic effects? J Leukoc Biol. 2008;85(1):186–93.
Lenglet S, Quercioli A, Fabre M, Galan K, Pelli G, Nencioni A, et al. Statin treatment is associated with reduction in serum levels of receptor activator of NF-κB ligand and neutrophil activation in patients with severe carotid stenosis. Mediators Inflamm. 2014;2014:720987.
• Lim SA, Wei J, Nguyen TM, Shi H, Su W, Palacios G, Dhungana Y, Chapman NM, Long L, Saravia J, Vogel P, Chi H. Lipid signalling enforces functional specialization of T(reg) cells in tumours. Nature. 2021;591:306–11. This article indicates how lipids induce Tregs differentiation in tumors which aids in its progression.
Funding
This work was supported by NIH grant 1R01HD094380.
Author information
Authors and Affiliations
Contributions
Eslam E. Saad: Conceptualization, Methodology, Investigation, Writing- Original draft preparation. Rachel Michel: English editing. Borahay M: Supervision, Reviewing, and Editing. All authors reviewed the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The author declares that there is no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saad, E.E., Michel, R. & Borahay, M.A. Cholesterol and Immune Microenvironment: Path Towards Tumorigenesis. Curr Nutr Rep 13, 557–565 (2024). https://doi.org/10.1007/s13668-024-00542-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13668-024-00542-y