Abstract
Purpose of Review
The aim of the review was to evaluate which diets are associated with higher TMAO levels.
Recent Findings
Several studies have shown that plasma and urinary levels of trimethylamine N-oxide (TMAO) are a reliable indicator of cardiovascular disease risk. Diet certainly has a strong influence on TMAO levels, but there is still uncertainty about which diet is the most effective in reducing this risk factor.
Summary
PubMed, Web of Science and Scopus were searched for studies that were published up until July 1, 2021 using specific keywords. In total, 447 studies were evaluated, of which papers on individual foods or supplements, or conducted in children, in vitro or in animal model studies were excluded. Twenty-five studies were included in this review. Three studies showed that caloric restriction and (visceral) weight loss improve TMAO levels. Six out of eight studies revealed beneficial effects of plant-based diets on plasma or urinary TMAO concentrations. Most of the studies demonstrated that a diet high in protein, particularly of animal origin, such as diets rich in fish or red meat, have negative effects on TMAO levels. Most studies that have evaluated the relationship between diet and plasma or urinary concentrations of TMAO seem to indicate that plant-based diets (Mediterranean, vegetarian and vegan) are effective in improving TMAO levels, while animal-based diets appear to have the opposite effect. Further long-term studies are needed to assess whether vegetarian or vegan diets are more effective than the Mediterranean diet in reducing TMAO levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
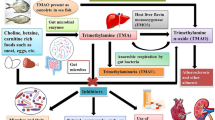
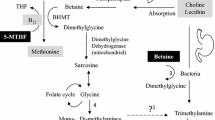
Trimethylamine N-oxide (TMAO) is a metabolite generated by gut microbial metabolism from nutrients such as choline, betaine, and l-carnitine. These nutrients are converted by the gut microbiota to trimethylamine (TMA) and processed into TMAO in the liver by the enzymes flavin monooxygenase activity 1 and 3 (FMO1 and FMO3). Diet, gut microbial flora, drugs, liver enzyme activity and other factors may determine the plasma level of TMAO. [1]
TMAO is associated with all-cause mortality, particularly in subjects with chronic kidney disease [2]. TMAO may be involved in the aetiology of metabolic and cardiovascular (CVD) diseases, as TMAO levels predict the risk of CVD independently of traditional cardiovascular risk factors.[3]. Despite the accumulating evidence, it is questioned whether TMAO is the mediator in the disease process or a prognostic marker of CVD [4].
In recent years, several methods have been proposed to reduce TMAO levels. Flavanol intervention [5], modulation of the gut microbiome with symbiotics or antibiotics [6], inulin supplementation [7] and faecal microbiota transplantation [8] have not shown effectiveness. Simpler approaches such as minimising foods rich in choline and L-carnitine in the diet [9•] or calorie restriction with exercise appear to be more effective in reducing TMAO [10]. Our previous review [11] revealed a positive association between intake of saltwater seafood, dark meat fish and shellfish with TMAO concentrations. We also discovered an increase in TMAO associated with the intake of animal-based foods such as meat and eggs. The mechanism underlying the relationship between diet and TMAO levels is still unclear. With the aim of evaluating the different effects of several types of diets on blood or urinary TMAO levels, we conducted a review of the current literature on the subject.
Methods
Research Strategy
The present review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We searched PubMed, Scopus and the Cochrane Central Register of Controlled Trials (CENTRAL) electronic databases using the following keywords: (“Trimethylamine N-oxide” OR “TMAO” OR “TMA”) AND “diet” AND (“Mediterranean” OR “plant” OR “vegetarian” OR “vegan” OR “paleo” OR “protein” OR “glycaemic load” OR “seafood”). We researched papers from January 1, 1990 to July 1, 2021.
Study Selection Criteria
Studies were excluded if they were abstracts, conference proceedings, reviews, letters, short surveys, books, book chapters or did not evaluate a dietary pattern (e.g. studies describing the effects of a single food). We also excluded studies on functional foods, supplements or probiotics, or that were performed in or animal models or children, and those that were duplicate studies (i.e. different papers by the same authors describing the same data). All possible documents were combined into one file, and redundant records were removed after being manually checked. Two separate reviewers (M.L. and G.R.) judged the adequacy of inclusion. Where there was disagreement, a third reviewer (G.A.) was included in the review procedure.
In total, 447 studies with these attributes were elicited. Thirty-five articles were ruled out as they were duplicate studies, another 284 were eliminated using the mentioned criteria because they were reviews, letters/posters or animal studies, and 128 papers were considered for full-text review. Overall, 103 articles were finally excluded because they covered individual foods, or nutraceutical or supplement use. Twenty-five studies were finally identified. The full texts of the selected papers were examined, and the outcomes are provided in this review. Figure 1 shows the steps for applying the inclusion and exclusion criteria as provided in the PRISMA guidelines to determine the final group of studies considered in this review.
Results
Twenty-five studies were included in this review [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. Their main characteristics, such as study design and dietary outcomes on TMAO, are presented in Tables 1 and 2. The methodology used by the various studies is shown in Table 1S in the supplementary material.
Caloric Restriction/BMI
The effects of weight and caloric restriction on TMAO were assessed by three studies [19, 23, 26]. All three studies demonstrated that caloric restriction and weight loss improve TMAO levels. An 8-week observational study showed that people following a Western diet may have higher TMAO levels and that plasma TMA may be associated with BMI [19]. Another study confirmed that a decrease in TMAO levels may be associated with improvement in insulin sensitivity, particularly among subjects who achieved more weight loss at 6 months [23]. Thus, in a moderately sized cohort of older adults with obesity and insulin-resistance, caloric restriction in combination with exercise induced a reduction in TMAO levels, whereas a eucaloric diet plus exercise induced a small increment [26].
Mediterranean and Plant-Based Diets
Eight studies evaluated the possible role of a plant-based diet in lowering urinary TMAO levels [16, 18, 20, 22, 32,33,34,35]. Six of the eight studies showed the benefit of MD or VD diets on TMAO concentrations. A higher Mediterranean diet (MD) adherence score was predictive of a lower urinary TMAO value [22]. Another trial showed no significant difference between the MD and vegetarian diet (VD); however, plasma concentrations of TMAO and l-carnitine were lower after the VD compared with baseline [33]. A crossover randomised controlled trial (RCT) that compared plant vs. animal intake demonstrated that plant-based foods improved a number of cardiovascular disease risk factors, including TMAO levels [32]. Another observational study of 103 subjects confirmed that VD and vegan diets significantly improved urinary TMAO concentrations compared with omnivorous diets [18]. TMAO levels were reduced at weeks 1 and 8 after beginning a vegan diet, and levels increased again after resuming a normal diet [35]. Plasma TMAO was not associated with the strictness of the vegetarian diet, and intra-individual variations in TMAO were low in vegans [20]. Contrary to the above studies, two papers demonstrated lower effectiveness of the MD on urinary TMAO concentrations compared with control [16, 34].
Animal-Based Diets
Four studies [12, 25, 27, 30] have evaluated the impact of a diet containing high levels of animal-based foods on TMAO concentrations. Three of the studies showed that an animal-based diet has negative effects on plasma or urinary TMAO levels. A crossover trial in healthy males comparing “vegetarian” vs. “low-meat” vs. “high-meat” diets showed that TMAO was elevated in the period of high meat consumption [12]. Another crossover comparing high- vs low-saturated fat diets demonstrated that long-term consumption of red meat increased circulating TMAO levels and that a washout diet without red meat reduced TMAO concentrations after 4 weeks [25]. In a study of subjects who were prescribed a paleo diet (a model that includes mainly protein-rich foods such as meat, fish, eggs, seeds and nuts), TMAO levels were higher in the strict Palaeolithic group compared to the pseudo-Palaeolithic and control groups, and inversely associated with whole-grain intake [27]. On the contrary, a post hoc analysis of both low-calorie normal protein (0.8 g/kg BW/d) and higher-protein (1.2 g/kg BW/day; for the most part from lean red meat) diets showed no changes in TMAO levels [30].
Diets with Both Animal and Plant Protein Sources
Six studies evaluated the influence of the type of protein in the diet on TMAO levels. A 10-week RCT found that protein intake from a combination of animal and plant sources at twice the Recommended Daily Allowance (RDA) increased systemic TMAO levels in older men [28]. Another study demonstrated that TMAO levels are linked with urinary nitrogen excretion and therefore with protein consumed [13]. In a study on healthy subjects with high cardiometabolic risk, protein intake (but not intake of meat, processed meat or dairy products) was directly associated with plasma TMAO levels [36]. Another study evaluated possible relationships between variations in the type of amino acids in the diet and showed that there were no significant associations between ΔTMAO and variations in amino acids across subjects [23]. Two studies comparing diets with different protein ratios showed no significant difference in TMAO values [30, 31].
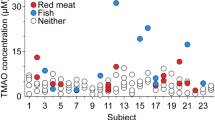
Seafood-Based Diets
Three studies evaluated the effects of diets based on seafood consumption [21, 24, 36]. The first study evaluating this aspect found significantly higher fasting and postprandial serum TMAO levels, which are assumed to originate from the TMAO content of fish [24]. Another study compared four diets, of which diet 1 was the most guideline-compliant, and diet 4 was the least guideline-compliant. Urinary concentrations of TMAO were significantly higher after consumption of diet 1 than after diet 4, probably due to increased consumption of fish (salmon), the food type most present in this diet along with fruits, cruciferous vegetables, and chicken [21]. A post hoc analysis of diets with differing proportions of long-chain n–3 fatty acids (LCn3) showed that diets rich in LCn3 significantly increase plasma levels of TMAO [36].
Carbohydrates: Quantity and Type
The possible effects of dietary carbohydrates on TMAO levels were evaluated by six studies. A cross-sectional, multicentre study evaluated the effects of a low-glycaemic load (LGL) diet on TMAO levels, showing that TMAO levels were 37% higher after the LGL versus the high-glycaemic load diet [14]. Thus, people with reduced dietary carbohydrate intake had higher levels of TMAO [23]. In contrast, in a crossover RCT, an increased carbohydrate intake or high intake of resistant starch was independently sufficient to stimulate TMAO production by the microbiota [17]. In a cross-sectional study on the effects of the Palaeolithic diet, TMAO concentrations were inversely related to total and whole grain consumption [27]. A post hoc analysis to evaluate the effects of diets based on foods rich in polyphenols and/or LCn3 or whole-grain cereals on plasma TMAO showed that TMAO levels directly correlated with the intake of fish, vegetables and whole-grain products [36]. An MD intervention that increased intake of fibre demonstrated that TMAO levels were negatively correlated with fibre intake [34].
Quality and Quantity of Fat in the Diet
The effects of dietary fat quantity and quality on TMAO levels have been evaluated by nine studies. In healthy, non-obese, young males, a short-term high-fat diet raises postprandial TMAO plasma levels, but does not significantly elevate fasting TMAO concentrations [15]. Thus, a higher intake of dietary fat at baseline was associated with higher levels of TMAO [23], and a low-fat diet was associated with metabolites indicating higher excretion of TMAO [16]. A short-term diet containing high levels of saturated fat from animal sources was associated with changes in TMAO levels, and 4 weeks of a normal diet were sufficient to restore normal TMAO blood concentrations [29]. Regarding the quality of fat in the diet, two studies reported that consumption of foods high in saturated fat correlated with increased TMAO levels [12, 25]. In another paper, TMAO levels were predicted by intake of LCn3, eicosapentaenoic acid (EPA; 20:5n-3) and protein, but not by intake of saturated fatty acids, fibre or monounsaturated fatty acids [36]. One trial [34] aimed to increase fibre intake and the relative amount of monounsaturated fat in the diet in a population of subjects at high risk of colon cancer, which led to no change in serum concentrations of TMA-precursors or TMAO in the whole sample. In another study, diets with different amounts of fat did not influence TMAO levels after 6 months [31].
Discussion
In recent years, studies on humans and animal models [37, 38] have demonstrated a contribution of TMAO to CVD-related risk factors. Identifying which type of diet might have the best effects on TMAO reduction might be helpful in reducing patients’ disease risk factors. Many studies included in our review have shown that TMAO levels are influenced by excess calories and that weight reduction improves TMAO levels (Table 1). In humans, TMAO may increase CVD risk by triggering immune and inflammatory reactions, impairing cholesterol metabolism and enhancing platelet hyperactivity, which increases the risk of atherosclerotic thrombosis [1, 39]. A positive correlation between TMAO levels and visceral adiposity has been also demonstrated. Furthermore, an animal study showed that FMO3, an enzyme that regulates TMAO production in the liver, plays a key role in obesity risk and white adipose tissue transdifferentiation [23]. The Western-style diet (WD) carries a number of widely documented health risks. Of these, the excessive energy intake probably has the most deleterious effects. Thus, the WD, which is high in saturated fat, animal protein and sugar, has been shown to contribute to dysbiosis of the gut microbiota, increased plasma levels of TMAO and an increased risk of CVD [40]. Moreover, WD-fed mice have higher plasma TMAO concentrations and develop anatomical and functional alterations of the heart. They also exhibit enhanced levels of pro-inflammatory cytokines, such as interleukin-1β (IL-1β) and tumour necrosis factor-α (TNF-α), and reduced levels of anti-inflammatory cytokines such as IL-10 [41].
Interestingly, the diets that are most effective in reducing TMAO levels seem to be those that guidelines promote as the healthiest for humans and more beneficial for the environment in the long term [42••]. Most studies (Table 2) have indeed shown a possible benefit of plant-based diets (vegetarian, vegan or Mediterranean) for improving TMAO. Only one study assessed the importance of the strictness of adherence to a plant-based diet, finding very little difference associated with this variable. An exception is diets with high fish consumption, whose beneficial polyunsaturated fats may outweigh the negative effects of TMAO [11]. Results of a recent trial have shown that a diet rich in soluble dietary fibre, a nutrient found mainly in certain vegetables, fruit and legumes, can reduce the metabolism of TMA and TMAO by intestinal microflora by 40.6 and 62.6%, respectively [43]. Thus, the consumption of a high-fibre, low-glycaemic load diet results in changes in the structure of the gut microbiome community that increase TMAO production [14].
The majority of studies evaluated show that diets high in animal-based products lead to increases in TMAO (Table 3). Since TMAO was first identified, correlations have been established between dietary choline [44] and carnitine [45] concentrations in animal foods, and blood or urinary TMAO levels. Diets based on the consumption of red meat provide about four times as much carnitine as those based on white meat. Increased microbial production of TMA/TMAO from carnitine, but not from choline; increased presence of TMA precursors in these types of diets, and reduced renal excretion of TMAO could be caused by which a diet high in red meat (such as the paleo or high-protein diet) may cause an increase in TMAO. Confirming this association, after 4 weeks of ceasing red meat consumption or a shift from a diet high in red meat to a white or non-meat protein source could potentially lower plasma TMAO concentrations [25]. Thus, consumption of any animal meat, particularly when kidney function is impaired, increases plasma levels of other metabolites of the gut microbiome, such as p-cresylsulfate, hippuric acid, indoxyl sulfate, p-cresylglucuronide, phenylacetylglutamine and phenylsulfate [46]. Inverse relationships have been demonstrated between consumption of diets rich in plant proteins and nuts with TMAO concentrations [47]. Interestingly, legumes and nuts, key constituents of vegetarian diets, contain large amounts of choline. A recent review hypothesised that the gut microbiota could convert choline to TMA at different rates with typical diets [48]. These data are confirmed by RCT studies, which showed that TMAO levels were lower in prediabetic subjects who were prescribed walnuts in combination with the MD than in patients following an MD diet alone [49]. Another study has shown that the effects of animal-based foods on TMAO concentrations may vary between populations [50]. For example, consumption of red meat/eggs is most associated with risk of raised TMAO levels and thus CVD in US populations, but this is less likely in European or Asian populations, which have a higher level of fish consumption [51•]. A recent review has suggested that salt consumption may be an important factor in explaining the correlation between processed red meat and TMAO levels. Excessive salt consumption affects the gut in both rodents and humans to such an extent that a triangular relationship is established between salt, hypertension and the gut microbiota. Excessive salt consumption is said to increase TMAO levels and decrease short-chain fatty acid levels in the gut. These alterations in the microbiota may affect the integrity of the intestine and interfere with the efficacy of antihypertensive drugs [52].
A diet high in red meat may increase TMAO levels through increased microbial production of TMA/TMAO from carnitine (but not choline), increased nutrient density of TMA precursors and decreased ability of the kidneys to regulate excretion of TMAO and its metabolites. The transformation of carnitine into TMA and TMAO is mediated by the intestinal microbiome through the formation of a proatherogenic intermediate, γBB. In mouse models, carnitine supplementation has been demonstrated to accelerate the development of atherosclerosis [53]. Higher circulating concentrations of l-carnitine have been seen in omnivores compared with vegans or vegetarians [54]. One study [25] demonstrated a reduced renal clearance of TMAO after 1 month on a high-red meat diet and suggested that the cause may be that the kidney becomes less efficient at clearing TMAO when consuming this type of diet. Thus, high plasma carnitine concentrations seem to be associated with CVD risk independently of traditional CVD risk factors, but only in the presence of elevated TMAO [55].
Our narrative review has some limitations. The search was limited to a few databases and to the English language only; however, despite this, we were able to include a large number of papers. We decided not to consider studies assessing the correlation between TMAO and consumption of individual foods, which excluded a great deal of potentially interesting studies. There is however a paucity of evidence from RCTs comparing different dietary approaches, and this limitation necessarily had an influence on the effectiveness of the review results.
Conclusions
Most of the studies evaluated showed a greater efficacy of plant-based diets (Mediterranean, vegetarian and vegan) in improving blood or urinary concentrations of TMAO. These data add to the many other studies suggesting that these diets are the most suitable for reducing cardiovascular disease risk factors. However, further studies are needed to assess whether the strictness of plant-based diets impacts the reduction in TMAO levels.
Abbreviations
- TMAO:
-
Trimethylamine N-oxide
- TMA:
-
Trimethylamine
- MD:
-
Mediterranean diet
- VD:
-
Vegetarian diet
- RCT:
-
Randomised controlled trial
- CVD:
-
Cardiovascular disease
- LCn3:
-
Long chain omega-3 polyunsaturated fatty acids
- WD:
-
Western-style diet
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Yang S, Li X, Yang F, Zhao R, Pan X, Liang J, Tian L, Li X, Liu L, Xing Y, Wu M. Gut microbiota-dependent marker TMAO in promoting cardiovascular disease: inflammation mechanism, clinical prognostic, and potential as a therapeutic target. Front Pharmacol. 2019;10:1360. https://doi.org/10.3389/fphar.2019.01360. PMID: 31803054; PMCID: PMC6877687.
Gruppen EG, Garcia E, Connelly MA, et al. TMAO is associated with mortality: impact of modestly impaired renal function. Sci Rep. 2017;7:13781. https://doi.org/10.1038/s41598-017-13739-9.
Tang WHW, Li XS, Wu Y, Wang Z, Khaw KT, Wareham NJ, Nieuwdorp M, Boekholdt SM, Hazen SL. Plasma trimethylamine N-oxide (TMAO) levels predict future risk of coronary artery disease in apparently healthy individuals in the EPIC-Norfolk prospective population study. Am Heart J. 2021;236:80–6. https://doi.org/10.1016/j.ahj.2021.01.020. Epub 2021 Feb 21. PMID: 33626384; PMCID: PMC8085024.
Janeiro MH, Ramírez MJ, Milagro FI, Martínez JA, Solas M. Implication of trimethylamine N-oxide (TMAO) in disease: potential biomarker or new therapeutic target. Nutrients. 2018;10(10):1398. https://doi.org/10.3390/nu10101398. Published 2018 Oct 1.
Angiletta CJ, Griffin LE, Steele CN, Baer DJ, Novotny JA, Davy KP, Neilson AP. Impact of short-term flavanol supplementation on fasting plasma trimethylamine N-oxide concentrations in obese adults. Food Funct. 2018;9(10):5350–61. https://doi.org/10.1039/c8fo00962g. PMID: 30264073.
Tripolt NJ, Leber B, Triebl A, Köfeler H, Stadlbauer V, Sourij H. Effect of Lactobacillus casei Shirota supplementation on trimethylamine-N-oxide levels in patients with metabolic syndrome: an open-label, randomized study. Atherosclerosis. 2015;242(1):141–4. https://doi.org/10.1016/j.atherosclerosis.2015.05.005. Epub 2015 Jul 8 PMID: 26188537.
Baugh ME, Steele CN, Angiletta CJ, et al. Inulin supplementation does not reduce plasma trimethylamine N-oxide concentrations in individuals at risk for type 2 diabetes. Nutrients. 2018;10(6):793. https://doi.org/10.3390/nu10060793. Published 2018 Jun 20.
Smits LP, Kootte RS, Levin E, Prodan A, Fuentes S, Zoetendal EG, Wang Z, Levison BS, Cleophas MCP, Kemper EM, Dallinga-Thie GM, Groen AK, Joosten LAB, Netea MG, Stroes ESG, de Vos WM, Hazen SL, Nieuwdorp M. Effect of vegan fecal microbiota transplantation on carnitine- and choline-derived trimethylamine-N-oxide production and vascular inflammation in patients with metabolic syndrome. J Am Heart Assoc. 2018;7(7):e008342. https://doi.org/10.1161/JAHA.117.008342. PMID: 29581220; PMCID: PMC5907601.
• Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–85. https://doi.org/10.1038/nm.3145. Epub 2013 Apr 7. PMID: 23563705; PMCID: PMC3650111. This study demonstrated that consumption of foods rich in L-carnitine and pro-atherosclerotic mechanism observed for TMAO may be the causes of the relationship between dietary red meat ingestion and atherosclerosis.
Erickson ML, Malin SK, Wang Z, Brown JM, Hazen SL, Kirwan JP. Effects of lifestyle intervention on plasma trimethylamine N-oxide in obese adults. Nutrients. 2019;11(1):179. https://doi.org/10.3390/nu11010179. Published 2019 Jan 16.
Lombardo M, Aulisa G, Marcon D, Rizzo G, Tarsisano MG, Di Renzo L, Federici M, Caprio M, De Lorenzo A. Association of urinary and plasma levels of trimethylamine N-oxide (TMAO) with foods. Nutrients. 2021;13(5):1426. https://doi.org/10.3390/nu13051426. PMID: 33922680; PMCID: PMC8145508.
Stella C, Beckwith-Hall B, Cloarec O, Holmes E, Lindon JC, Powell J, van der Ouderaa F, Bingham S, Cross AJ, Nicholson JK. Susceptibility of human metabolic phenotypes to dietary modulation. J Proteome Res. 2006;5(10):2780–8. https://doi.org/10.1021/pr060265y. PMID: 17022649.
Rasmussen LG, Winning H, Savorani F, Toft H, Larsen TM, Dragsted LO, Astrup A, Engelsen SB. Assessment of the effect of high or low protein diet on the human urine metabolome as measured by NMR. Nutrients. 2012;4(2):112–31. https://doi.org/10.3390/nu4020112. Epub 2012 Feb 20. PMID: 22413065; PMCID: PMC3296994.
Barton S, Navarro SL, Buas MF, Schwarz Y, Gu H, Djukovic D, Raftery D, Kratz M, Neuhouser ML, Lampe JW. Targeted plasma metabolome response to variations in dietary glycemic load in a randomized, controlled, crossover feeding trial in healthy adults. Food Funct. 2015;6(9):2949–56. https://doi.org/10.1039/c5fo00287g. PMID: 26165375; PMCID: PMC4558254.
Boutagy NE, Neilson AP, Osterberg KL, Smithson AT, Englund TR, Davy BM, Hulver MW, Davy KP. Short-term high-fat diet increases postprandial trimethylamine-N-oxide in humans. Nutr Res. 2015;35(10):858–64. https://doi.org/10.1016/j.nutres.2015.07.002. Epub 2015 Jul 11 PMID: 26265295.
Vázquez-Fresno R, Llorach R, Urpi-Sarda M, Lupianez-Barbero A, Estruch R, Corella D, Fitó M, Arós F, Ruiz-Canela M, Salas-Salvadó J, Andres-Lacueva C. Metabolomic pattern analysis after mediterranean diet intervention in a nondiabetic population: a 1- and 3-year follow-up in the PREDIMED study. J Proteome Res. 2015;14(1):531–40. https://doi.org/10.1021/pr5007894. Epub 2014 Nov 13 PMID: 25353684.
Bergeron N, Williams PT, Lamendella R, Faghihnia N, Grube A, Li X, Wang Z, Knight R, Jansson JK, Hazen SL, Krauss RM. Diets high in resistant starch increase plasma levels of trimethylamine-N-oxide, a gut microbiome metabolite associated with CVD risk. Br J Nutr. 2016;116(12):2020–9. https://doi.org/10.1017/S0007114516004165. Epub 2016 Dec 20. PMID: 27993177; PMCID: PMC5885763.
De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, Serrazanetti DI, Di Cagno R, Ferrocino I, Lazzi C, Turroni S, Cocolin L, Brigidi P, Neviani E, Gobbetti M, O’Toole PW, Ercolini D. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65(11):1812–21. https://doi.org/10.1136/gutjnl-2015-309957. Epub 2015 Sep 28 PMID: 26416813.
Malinowska AM, Szwengiel A, Chmurzynska A. Dietary, anthropometric, and biochemical factors influencing plasma choline, carnitine, trimethylamine, and trimethylamine-N-oxide concentrations. Int J Food Sci Nutr. 2017;68(4):488–95. https://doi.org/10.1080/09637486.2016.1256379. Epub 2016 Nov 17 PMID: 27855528.
Obeid R, Awwad HM, Keller M, Geisel J. Trimethylamine-N-oxide and its biological variations in vegetarians. Eur J Nutr. 2017;56(8):2599–609. https://doi.org/10.1007/s00394-016-1295-9. Epub 2016 Aug 25 PMID: 27562778.
Garcia-Perez I, Posma JM, Gibson R, et al. Objective assessment of dietary patterns by use of metabolic phenotyping: a randomised, controlled, crossover trial. Lancet Diabetes Endocrinol. 2017;5(3):184–95. https://doi.org/10.1016/S2213-8587(16)30419-3.
Barrea L, Annunziata G, Muscogiuri G, Laudisio D, Di Somma C, Maisto M, Tenore GC, Colao A, Savastano S. Trimethylamine N-oxide, Mediterranean diet, and nutrition in healthy, normal-weight adults: also a matter of sex? Nutrition. 2019;62:7–17. https://doi.org/10.1016/j.nut.2018.11.015. Epub 2018 Nov 24 PMID: 30822745.
Heianza Y, Sun D, Li X, Di Donato JA, Bray GA, Sacks FM, Qi L. Gut microbiota metabolites, amino acid metabolites and improvements in insulin sensitivity and glucose metabolism: the POUNDS Lost trial. Gut. 2019;68(2):263–70. https://doi.org/10.1136/gutjnl-2018-316155. Epub 2018 Jun 2. PMID: 29860242; PMCID: PMC6275143.
Schmedes M, Balderas C, Aadland EK, Jacques H, Lavigne C, Graff IE, Eng Ø, Holthe A, Mellgren G, Young JF, Sundekilde UK, Liaset B, Bertram HC. The effect of lean-seafood and non-seafood diets on fasting and postprandial serum metabolites and lipid species: results from a randomized crossover intervention study in healthy adults. Nutrients. 2018;10(5):598. https://doi.org/10.3390/nu10050598. PMID: 29751643; PMCID: PMC5986478.
Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, Koeth RA, Li L, Wu Y, Tang WHW, Krauss RM, Hazen SL. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J. 2019;40(7):583–94. https://doi.org/10.1093/eurheartj/ehy799. PMID: 30535398; PMCID: PMC6374688.
Erickson ML, Malin SK, Wang Z, Brown JM, Hazen SL, Kirwan JP. Effects of lifestyle intervention on plasma trimethylamine N-oxide in obese adults. Nutrients. 2019;11(1):179. https://doi.org/10.3390/nu11010179. PMID: 30654453; PMCID: PMC6356515.
Genoni A, Christophersen CT, Lo J, Coghlan M, Boyce MC, Bird AR, Lyons-Wall P, Devine A. Long-term Paleolithic diet is associated with lower resistant starch intake, different gut microbiota composition and increased serum TMAO concentrations. Eur J Nutr. 2020;59(5):1845–58. https://doi.org/10.1007/s00394-019-02036-y. Epub 2019 Jul 5. PMID: 31273523; PMCID: PMC7351840.
Mitchell SM, Milan AM, Mitchell CJ, Gillies NA, D’Souza RF, Zeng N, Ramzan F, Sharma P, Knowles SO, Roy NC, Sjödin A, Wagner KH, Zeisel SH, Cameron-Smith D. Protein intake at twice the RDA in older men increases circulatory concentrations of the microbiome metabolite trimethylamine-N-oxide (TMAO). Nutrients. 2019;11(9):2207. https://doi.org/10.3390/nu11092207. PMID: 31547446; PMCID: PMC6770800.
Park JE, Miller M, Rhyne J, Wang Z, Hazen SL. Differential effect of short-term popular diets on TMAO and other cardio-metabolic risk markers. Nutr Metab Cardiovasc Dis. 2019;29(5):513–7. https://doi.org/10.1016/j.numecd.2019.02.003. Epub 2019 Feb 22 PMID: 30940489.
Porter-Starr KN, Connelly MA, Orenduff M, McDonald SR, Sloane R, Huffman KM, Kraus WE, Bales CW. Impact on Cardiometabolic risk of a weight loss intervention with higher protein from lean red meat: combined results of two randomized controlled trials in obese middle-aged and older adults. J Clin Lipidol. 2019;13:920–31. https://doi.org/10.1016/j.jacl.2019.09.012.
Zhou T, Heianza Y, Chen Y, Li X, Sun D, DiDonato JA, Pei X, LeBoff MS, Bray GA, Sacks FM, Qi L. Circulating gut microbiota metabolite trimethylamine N-oxide (TMAO) and changes in bone density in response to weight loss diets: the POUNDS Lost Trial. Diabetes Care. 2019 Aug;42(8):1365–71. https://doi.org/10.2337/dc19-0134. Epub 2019 May 21. PMID: 31332027; PMCID: PMC6647048.
Crimarco A, Springfield S, Petlura C, Streaty T, Cunanan K, Lee J, Fielding-Singh P, Carter MM, Topf MA, Wastyk HC, Sonnenburg ED, Sonnenburg JL, Gardner CD. A randomized crossover trial on the effect of plant-based compared with animal-based meat on trimethylamine-N-oxide and cardiovascular disease risk factors in generally healthy adults: Study With Appetizing Plantfood-Meat Eating Alternative Trial (SWAP-MEAT). Am J Clin Nutr. 2020;112(5):1188–99. https://doi.org/10.1093/ajcn/nqaa203. PMID: 32780794; PMCID: PMC7657338.
Djekic D, Shi L, Brolin H, Carlsson F, Särnqvist C, Savolainen O, Cao Y, Bäckhed F, Tremaroli V, Landberg R, Frøbert O. Effects of a vegetarian diet on cardiometabolic risk factors, gut microbiota, and plasma metabolome in subjects with ischemic heart disease: a randomized, crossover study. J Am Heart Assoc. 2020;9(18):e016518. https://doi.org/10.1161/JAHA.120.016518. Epub 2020 Sep 6. PMID: 32893710; PMCID: PMC7726986.
Griffin LE, Djuric Z, Angiletta CJ, et al. A Mediterranean diet does not alter plasma trimethylamine N-oxide concentrations in healthy adults at risk for colon cancer. Food Funct. 2019;10(4):2138–47. https://doi.org/10.1039/c9fo00333a.
Argyridou S, Davies MJ, Biddle GJH, Bernieh D, Suzuki T, Dawkins NP, Rowlands AV, Khunti K, Smith AC, Yates T. Evaluation of an 8-week vegan diet on plasma trimethylamine-N-oxide and postchallenge glucose in adults with dysglycemia or obesity. J Nutr. 2021;151(7):1844–53. https://doi.org/10.1093/jn/nxab046. PMID:33784394; PMCID:PMC8245890.
Costabile G, Vetrani C, Bozzetto L, Giacco R, Bresciani L, Del Rio D, Vitale M, Della Pepa G, Brighenti F, Riccardi G, Rivellese AA, Annuzzi G. Plasma TMAO increase after healthy diets: results from 2 randomized controlled trials with dietary fish, polyphenols, and whole-grain cereals. Am J Clin Nutr. 2021;114:1342–50. https://doi.org/10.1093/ajcn/nqab188. Epub ahead of print. PMID: 34091663.
Geng J, Yang C, Wang B, Zhang X, Hu T, Gu Y, Li J. Trimethylamine N-oxide promotes atherosclerosis via CD36-dependent MAPK/JNK pathway. Biomed Pharmacother. 2018;97:941–7. https://doi.org/10.1016/j.biopha.2017.11.016. Epub 2017 Nov 7 PMID: 29136772.
Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WHW, DiDonato JA, Brown JM, Lusis AJ, Hazen SL. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165(1):111–24. https://doi.org/10.1016/j.cell.2016.02.011. Epub 2016 Mar 10. PMID: 26972052; PMCID: PMC4862743.
Guasti L, Galliazzo S, Molaro M, Visconti E, Pennella B, Gaudio GV, Lupi A, Grandi AM, Squizzato A. TMAO as a biomarker of cardiovascular events: a systematic review and meta-analysis. Intern Emerg Med. 2021;16(1):201–7. https://doi.org/10.1007/s11739-020-02470-5. Epub 2020 Aug 10 PMID: 32779113.
Zhang Y, Wang Y, Ke B, Du J. TMAO: how gut microbiota contributes to heart failure. Transl Res. 2021;228:109–25. https://doi.org/10.1016/j.trsl.2020.08.007. Epub 2020 Aug 22 PMID: 32841736.
Chen K, Zheng X, Feng M, Li D, Zhang H. Gut microbiota-dependent metabolite trimethylamine N-oxide contributes to cardiac dysfunction in western diet-induced obese mice. Front Physiol. 2017;8:139. https://doi.org/10.3389/fphys.2017.00139.
•• Heianza Y, Ma W, DiDonato JA, Sun Q, Rimm EB, Hu FB, Rexrode KM, Manson JE, Qi L. Long-term changes in gut microbial metabolite trimethylamine N-oxide and coronary heart disease risk. J Am Coll Cardiol. 2020;75(7):763–72. https://doi.org/10.1016/j.jacc.2019.11.060. PMID: 32081286; PMCID: PMC8140616. In this prospective study, the authors demonstrated a significant relationship between long-term changes in TMAO and CVD risk. This association was strengthened by unhealthy dietary patterns and attenuated by healthy dietary patterns.
Li Q, Wu T, Liu R, Zhang M, Wang R. Soluble dietary fiber reduces trimethylamine metabolism via gut microbiota and co-regulates host AMPK pathways. Mol Nutr Food Res. 2017;61(12):1700473. https://doi.org/10.1002/mnfr.201700473. Epub 2017 Nov 16. PMID: 28884952.
Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. https://doi.org/10.1038/nature09922. PMID: 21475195; PMCID: PMC3086762.
Wu WK, Panyod S, Liu PY, et al. Characterization of TMAO productivity from carnitine challenge facilitates personalized nutrition and microbiome signatures discovery. Microbiome. 2020;8:162. https://doi.org/10.1186/s40168-020-00912-y.
Al-Shaar L, Satija A, Wang DD, Rimm EB, Smith-Warner SA, Stampfer MJ, Hu FB, Willett WC. Red meat intake and risk of coronary heart disease among US men: prospective cohort study. BMJ. 2020;371: m4141. https://doi.org/10.1136/bmj.m4141.
Cho CE, Taesuwan S, Malysheva OV, Bender E, Tulchinsky NF, Yan J, Sutter JL, Caudill MA. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: a randomized controlled trial. Mol Nutr Food Res. 2017;61(1):1600324. https://doi.org/10.1002/mnfr.201600324. Epub 2016 Aug 3. PMID: 27377678.
Arias N, Arboleya S, Allison J, et al. The relationship between choline bioavailability from diet, intestinal microbiota composition, and its modulation of human diseases. Nutrients. 2020;12(8):2340. https://doi.org/10.3390/nu12082340. Published 2020 Aug 5.
Hernández-Alonso P, Cañueto D, Giardina S, Salas-Salvadó J, Cañellas N, Correig X, Bulló M. Effect of pistachio consumption on the modulation of urinary gut microbiota-related metabolites in prediabetic subjects. J Nutr Biochem. 2017;45:48–53. https://doi.org/10.1016/j.jnutbio.2017.04.002. Epub 2017 Apr 12 PMID: 28432876.
Spence JD, Srichaikul KK, Jenkins DJA. Cardiovascular harm from egg yolk and meat: more than just cholesterol and saturated fat. J Am Heart Assoc. 2021 Apr 6;10(7):e017066. https://doi.org/10.1161/JAHA.120.017066. Epub 2021 Mar 15. PMID: 33719494; PMCID: PMC8174346.
• Yang JJ, Shu X-O, Herrington DM, Moore SC, Meyer KA, Ose J, Menni C, Palmer ND, Eliassen H, Harada S, Tzoulaki I, Zhu H, Albanes D, Wang TJ, Zheng W, Cai H, Ulrich CM, Guasch-Ferré M, Karaman I, Fornage M, Cai Q, Matthews CE, Wagenknecht LE, Elliott P, Gerszten RE, Yu D. Circulating trimethylamine N-oxide in association with diet and cardiometabolic biomarkers: an international pooled analysis. Am J Clin Nutr. 2021;113(5):1145–56, https://doi.org/10.1093/ajcn/nqaa430. This international study revealed that a higher consumption of animal protein may be associated with increased circulating TMAO, whereas TMAO associations with fish, shellfish, eggs, and red meat varied among populations.
Naqvi S, Asar TO, Kumar V, Al-Abbasi FA, Alhayyani S, Kamal MA, Anwar F. A cross-talk between gut microbiome, salt and hypertension. Biomed Pharmacother. 2021;134: 111156. https://doi.org/10.1016/j.biopha.2020.111156. Epub 2021 Jan 2 PMID: 33401080.
Koeth RA, Lam-Galvez BR, Kirsop J, Wang Z, Levison BS, Gu X, Copeland MF, Bartlett D, Cody DB, Dai HJ, Culley MK, Li XS, Fu X, Wu Y, Li L, DiDonato JA, Tang WHW, Garcia-Garcia JC, Hazen SL. l-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J Clin Invest. 2019;129(1):373–87. https://doi.org/10.1172/JCI94601. Epub 2018 Dec 10. PMID: 30530985; PMCID: PMC6307959.
Szabo Z, Koczka V, Marosvolgyi T, Szabo E, Frank E, Polyak E, Fekete K, Erdelyi A, Verzar Z, Figler M. Possible biochemical processes underlying the positive health effects of plant-based diets—a narrative review. Nutrients. 2021;13:2593. https://doi.org/10.3390/nu13082593.
Winther SA, Øllgaard JC, Hansen TW, von Scholten BJ, Reinhard H, Ahluwalia TS, Wang Z, Gæde P, Parving HH, Hazen S, Pedersen O, Rossing P. Plasma trimethylamine N-oxide and its metabolic precursors and risk of mortality, cardiovascular and renal disease in individuals with type 2-diabetes and albuminuria. PLoS ONE. 2021;16(3):e0244402. PMID: 33657115; PMCID: PMC7928450.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardiovascular Disease
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lombardo, M., Aulisa, G., Marcon, D. et al. The Influence of Animal- or Plant-Based Diets on Blood and Urine Trimethylamine-N-Oxide (TMAO) Levels in Humans. Curr Nutr Rep 11, 56–68 (2022). https://doi.org/10.1007/s13668-021-00387-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13668-021-00387-9