Abstract
Purpose of Review
The purpose of this study is to review the evidence for the tumorigenic effects of food-stimulated bile acids on the colon and interaction with the gut microbiota.
Recent Findings
High-fat diets promote the hepatic synthesis of bile acids and increase their delivery to the colonic lumen. Here, they stimulate the growth and activity of 7α-dehydroxylating bacteria, which convert primary into secondary bile acids that show tumorigenic activity, especially deoxycholic acid (DCA). Fecal levels of secondary bile acids correlate with mucosal and metabolic markers of colorectal cancer (CRC) risk in high- and low-risk adult individuals and can be modified within a few weeks by dietary change. While gut bacteria regulate the bile acid pool via complex microbial biotransformation, bile acids alter the gut microbiota composition due to their antimicrobial properties. This mutual reaction induces altered bile acid pools and dysbiotic compositions of the gut microbiota that may show tumor-promoting activity of bile acids beyond their conversion to DCA.
Summary
Bile acids act as tumor promoters in the colon. Diet and the gut microbiota are most likely the key drivers that mediate and confer bile acid-associated tumorigenic activity. Bacterial conversion of bile acids in the colon has a significant impact on their tumorigenic activity, substantiating the hypothesis that diet affects CRC risk through its effects on colonic microbial metabolism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2012, colorectal cancer (CRC) accounted for approximately 10% of worldwide cancers and was the second common cause of cancer in men and third in women [1]. Although some cases of CRC are primarily caused by inherited genes, such as familial adenomatous polyposis, the vast majority progress from a genetic susceptibility to cancer because of adverse environmental factors [2]. Based on the analysis of epidemiological studies around the world, Doll and Peto estimated that over 90% of gastric and colonic cancers could be attributed to diet [3]. In a recent analysis of the evidence linking diet to CRC risk, the joint World Cancer Research Fund and the American Institute for Cancer Research concluded that the evidence for red and processed meat increasing and fiber reducing CRC risk was convincing [4]. This helps explain the high rate of CRC in westernized populations who typically consume high-meat, high-fat, and low-fiber diets. High-fat consumption increases the hepatic synthesis of bile acids and their delivery to the colon, where they are metabolized by the microbiota into products with tumorigenic activity. Here, we discuss recent studies that provided new insight into the complex interactions of dietary fat, bile acids, and the gut microbiota in the context of CRC.
Human primary bile acids (cholic acid (CA) and chenodeoxycholic acid (CDCA)) are synthesized from cholesterol in liver hepatocytes and secreted into the hepatobiliary system as conjugates with either glycine or taurine. Following a meal, bile acids are released into the duodenum, where they emulsify dietary fat, which promotes micelle formation and renders the triglycerides susceptible for the action of lipases. After secretion to the intestinal lumen, primary bile acids are deconjugated by microbial bile salt hydrolases and the majority of bile acids are reabsorbed by passive diffusion and active transport during the small intestinal transit [5, 6]. The remaining bile acids enter the colon, where they undergo extensive biotransformation mediated by colonic bacteria, such as 7α-dehydroxylation to secondary bile acids. The dominant secondary bile acids are deoxycholic acid (DCA from CA) and lithocholic acid (LCA from CDCA). The secondary bile acids are excreted in feces, but are partially reabsorbed during colonic transit and account for approximately 20% (DCA) and 5% (LCA) of the circulating bile acid pool (while CA and CDCA account for 30–40% each) [5, 6].
High-Fat Diet Shapes the Colonic Milieu by Triggering Bile Acid Secretion
Early epidemiological observations and experimental studies using rodent models reported a positive correlation between high-fat diets, fecal excretion of bile acids, and CRC incidence [7,8,9]. Increased amounts of bile acids in feces were demonstrated in CRC patients and populations at high risk for CRC [10,11,12].
A series of studies demonstrated that healthy rural Africans, who rarely get CRC (< 5:100,000), have significantly lower fecal concentrations of primary and secondary bile acids compared to healthy African Americans, who have the highest CRC risk in the USA (65:100,000) [13]. This reduced level of fecal bile acids in rural Africans was attributed to their 2–3-fold lower dietary fat consumption and was confirmed by a follow up study, which examined the effect of switching diets for 2 weeks in a facility where food was prepared and exact intakes recorded. The switch to a high-fat diet led to increased fecal bile acids and microbes containing the functional gene for 7α-dehydroxylation of bile acids in rural Africans [14••]. African Americans on a low-fat diet showed reciprocal changes with reduced fecal bile acids and 7α-dehydroxylating bacteria in the feces. Critically, these changes correlated with reciprocal shifts in mucosal markers associated with CRC risk (proliferation of intestinal epithelial cells detected by Ki67+ cells) and inflammation (infiltration of CD3+ lymphocytes and lamina propria CD68+ macrophages into the colonic mucosa) [14••].
It is important to note that the changes in mucosal and metabolic markers associated with CRC risk could equally been caused by the changes in dietary fiber intake. Fiber and its fermentation by the gut microbiota to short-chain fatty acids (SCFA) are inversely correlated with CRC risk [15]. Experimental evidence supports the significant impact of SCFA, especially butyrate, produced by the gut microbiota on intestinal tumorigenesis [16]. The addition of butyrate to the diet of K-ras G12Dint mice, which are susceptible to intestinal tumor formation due to overexpression of oncogenic K-ras in the intestinal epithelium, attenuated the high-fat diet-induced tumor progression [17•]. An inverse relationship of SCFA and secondary bile acids in the intestinal lumen was demonstrated in healthy individuals and correlated with CRC risk [12, 14••]. This was confirmed when CA was added at different concentrations to the diet of wild-type rats resulting in proportionally increasing DCA and decreasing SCFA levels in the cecal lumen [18]. Featuring another experimental setup, the supplementation of agaro-oligosaccharides to a high-fat diet suppressed the induction of aberrant crypt foci in the colon of wild-type mice treated with azoxymethane (AOM) to induce colonic tumorigenesis [19]. Although not significant, murine serum DCA levels tended to increase after high-fat diet and were attenuated after the administration of agaro-oligosaccharides [19]. These studies suggest an inverse relationship between dietary fiber and corresponding levels of luminal SCFA on the one hand and dietary fat and induced luminal (secondary) bile acids on the other hand correlating with CRC risk. The underlying mechanisms remain unclear and may involve reduced abundance or impaired metabolic activity of bacteria that produce SCFA caused by high-fat diet or increased levels of luminal DCA, or an increased uptake of SCFA by the host due to altered energy metabolism induced by bile acids [18].
In summary, these studies show that high-fat diets trigger bile acid production and secretion, which correlates positively with mucosal markers of proliferation and inflammation in humans and susceptibility to intestinal tumor formation in rodent models. Importantly, the administration of fiber or butyrate antagonized the effect of dietary fat on CRC-associated markers. It is important to mention that these correlations do not prove a causal role for bile acids in human CRC risk and further studies need to be examined, if bile acids are major contributors to colonic tumorigenesis.
Bile Acids Act as Tumor Promoters
The secondary bile acids have the potential to induce oxidative stress and DNA damage [20, 21]. Fecal or serum levels of DCA are increased in individuals at high risk for CRC and in CRC patients compared to healthy controls [13, 22]. The administration of exogenous DCA via intrarectal instillation or addition to the diet potentiated the chemically induced tumor formation in the colon in different rodent models [23,24,25]. The increased enterohepatic circulation of DCA in mice was also shown to trigger inflammation- and tumor-promoting factors as well as neoplasia in liver [26].
However, in many studies that examined colonic tumor formation, exogenous DCA was orally administered to the mice, which does not represent physiologic conditions. To assess the tumor-promoting activity of increased fecal transit of endogenous bile acids, a recent study by Raufman et al. used mice that lack the ileal apical sodium-dependent bile acid transporter (ASBT, encoded by Slc10a2), which is responsible for the main uptake of bile acids in the distal ileum [27•]. Compared to wild-type mice, Slc10a2−/− mice showed an increased synthesis and attenuated absorption and secretion of bile acids due to impaired enterohepatic cycling [28]. Following exposure to AOM and dextran sodium sulfate (DSS), Slc10a2−/− mice were highly susceptible to neoplastic transformations in the colon displaying significantly more intestinal tumors and increased tumor sizes in comparison to wild-type controls [27•]. Treatment with AOM/DSS also induced an altered growth pattern of Ki67+ cells in colonic crypts of Slc10a2−/− mice [27•]. This suggests that bile acid malabsorption in the ileum promotes aberrant growth of intestinal epithelial cells, which may trigger neoplastic transformation in a susceptible host. However, it remains unclear how the bacterial metabolism of bile acids was affected in this rodent model, which may have significant impact on the tumorigenic activity of increased bile acid excretion in Slc10a2−/− mice. In addition, it would be interesting to examine changes in the gut microbiota composition induced by the increased levels of fecal bile acids, especially the fraction of bacteria that mediate the conversion to DCA.
Importantly, in most experimental studies, the increased fecal bile acid levels alone were not sufficient to induce intestinal tumor formation, but it required increased susceptibility of the rodent model mediated by chemical treatment or genetic disposition [23, 24, 27•, 29]. One study found an inherent tumor-inducing effect for DCA in wild-type mice when this secondary bile acid was added to the diet [25], but tumor formation was only observed after a comparatively long time frame (8–10 months). Taken together, this suggests that bile acids, especially DCA, act as tumor promoters rather than inducers. In addition, these studies highlight the microbial conversion of primary to secondary bile acids as an essential step not only directing the composition of the bile acid pool, but also its tumor promoting activity. Hence, the substantial contribution of gut bacteria to the tumorigenic activity of bile acids needs to be considered when evaluating the influence of bile acids on CRC risk.
Gut Bacteria Mediate the Conversion to Secondary Bile Acids and Regulate the Bile Acid Pool
The idea “that the intestinal microflora directly or indirectly played a modifying role in promoting or accelerating colon tumor production” by bile acids was proposed decades ago [9]. Since then, several studies have demonstrated that interactions between microbiota and host in bile acid metabolism are complex and have substantial impact on the host beyond colonic tumorigenesis [30, 31]. In the context of CRC, it is hypothesized that excess levels of secreted bile acids (due to high-fat diet) are able to enter the colon, which facilitates enhanced conversion of primary to secondary bile acids by colonic bacteria via 7α-dehydroxylation leading to higher levels of tumor-promoting DCA. Consistently, the luminal levels of secondary bile acids are abolished in the intestine in germ-free mice [32]. So far, only a few species of the genus Clostridium were identified to have the enzymatic capacities (encoded by the bile acid-inducible (bai) operon) to perform 7α-dehydroxylation, with Clostridium scindens being a well-characterized member [6]. The mono-association of a germ-free CRC mouse model with wild-type C. scindens or a mutant lacking functional genes of the 7α-dehydroxylation pathway and administration of a high-fat diet would be helpful to demonstrate whether the bacterial conversion to DCA is essential for the tumorigenic activity of bile acids—but since 7α-dehydroxylating bacteria of the human gut microbiota are only able to convert unconjugated bile acids, an association with additional bacteria that express bile salt hydrolases would be required. In addition, a knockout of genes in the 7α-dehydroxylation pathway was not reported so far and extensive DCA-mediated interactions with other bacteria of the gut microbiota need to be considered as well [30].
It was demonstrated that intestinal bacteria regulate the bile acid pool by different mechanisms. Comparing conventionally and germ-free raised mice, Sayin et al. showed that in the presence of gut bacteria, luminal levels of tauro-β-muricholic acid were reduced due to biotransformation of bile acids by gut bacteria. The altered bile acid pool resulted in increased activation of the farnesoid X receptor (FXR), which inhibited the expression of genes involved in bile acid synthesis [32]. Additional evidence regarding the complex interactions between bile acids and gut microbiota composition was demonstrated in patients with advanced cirrhosis, who have reduced levels of fecal secondary bile acids. Compared to early-stage patients or healthy controls, these patients showed impaired fecal conversion of primary to secondary bile acids, which correlated with increased abundance of Enterobacteriaceae and lower numbers of 7α-dehydroxylating bacteria in the feces [33]. Although showing reduced levels of fecal secondary bile acids, individuals with cirrhosis do not have a lower risk for CRC [34]. Assuming that bile acids are involved in cirrhosis-associated CRC, this suggests that not only secondary bile acids have tumor-promoting effects or that the remaining levels of luminal DCA are sufficient to promote tumorigenesis. But it also remains unclear if the tumorigenic activity of bile acids depends only on their microbial conversion and dose-dependent effects. Other factors, such as an altered microbiota composition (increase in Enterobacteriaceae, for example [33]) that is mediated by shifts in the bile acid pool, may also contribute to CRC pathogenesis.
Bile Acids Alter Gut Microbiota Composition
In addition to their tumor-promoting activities, bile acids have direct and indirect antimicrobial effects on the gut microbiota. Due to their hydrophobic properties, bile acids exert a potent antimicrobial effect in vitro by inducing membrane damage in bacteria [35, 36]. DCA was shown to have the highest antimicrobial effect (about 10 times higher than CA) [36]. Since most of the CA that enters the colon is converted to DCA, this suggests a strong selective pressure on bacteria in the colonic lumen, especially after high-fat diets or high luminal CA levels, respectively [18]. In addition to the direct antimicrobial effects, bile acids were shown to modulate gut microbiota composition by stimulating FXR, a nuclear receptor for bile acids also expressed in the intestine. The activation of FXR induced several genes in the small intestine that are involved in epithelial barrier integrity and mucosal immune homeostasis and prevented bacterial translocation in the murine ileum [37]. Since different gut bacteria also have different bile salt resistance levels, luminal bile acids are thought to alter the composition of the gut microbiota, which may also affect their tumorigenic activity.
The addition of two different concentrations of CA to the diet of wild-type rats shifted the cecal microbiota at phylum level towards increased abundance of Firmicutes (mainly class Clostridia) and reduced numbers of Bacteroidetes [18]. This coincided with an overall impaired diversity. As suggested by the authors, these shifts may be driven by the increased resistance of members of the Firmicutes phylum to the antimicrobial activity of bile acids [18]. Further substantiating this hypothesis, the class of Gammaproteobacteria, which were more abundant when more CA was added to the diet, was shown to be highly resistant to antimicrobial effects of bile acids [38]. Importantly, the levels of DCA increased proportionally when more CA was added to the diet, suggesting that altered luminal DCA concentrations contributed to the changes in microbiota composition [18]. Another study in mice confirmed higher numbers of 7α-dehydroxylating bacteria when 1% CA was added to the diet [39]. Similarly, lower fecal levels of CA observed in cirrhosis patients coincided with reduced abundance of 7α-dehydroxylating bacteria in the feces [33]. In contrast, a recent study detected different compositional shifts of the gut microbiota after adding DCA to the diet of Apc min/+ mice [29]; at phylum level, the administration of DCA triggered an increased abundance of Bacteroidetes and decreased numbers of Firmicutes compared to Apc min/+ mice that received a diet without additional DCA. However, this may be not surprising, since DCA was shown to have more potent antimicrobial activity than CA [36] and Apc min/+ mice are likely to have an altered gut microbiota compared to wild-type mice. Interestingly, the shifts in gut microbiota following the addition of CA to the diet are mainly similar to gut microbial patterns detected in mice after administration of a high-fat diet [17•, 40, 41], suggesting that the bile acid associated shifts in the microbiota are induced by high-fat diet.
Do Bile Acids Induce a Dysbiotic Microbiota with Tumorigenic Activity?
It is tempting to speculate that specific bile acid patterns modulate the gut microbiota to a dysbiotic composition, which mediates or triggers tumor-promoting activities in the gut beyond the conversion to secondary bile acids. It was demonstrated that CRC patients have an altered fecal microbiota with lower diversity including significant shifts in composition, altered metabolic functions and overrepresentation of pathobionts [42, 43]. However, a distinct microbial pattern of bile acid-induced dysbiosis that is causally linked with human CRC has not been detected so far and may be more related to presence of particular metabolic key features rather than abundance of specific species.
Two recent studies investigated how a high-fat diet or oral DCA supplementation affected the gut microbiota and its tumorigenic capacity in the small intestine of mice using microbiota transfer experiments. In a first study by Schulz et al., the administration of a high-fat diet to K-ras G12Dint mice, which are susceptible to tumor formation in the small intestine, accelerated tumor progression compared to a control diet [17•]. The triggered tumor formation correlated with shifts in the fecal microbiota from the small intestine demonstrating an increase in Clostridiaceae and Enterobacteriaceae. Interestingly, similar shifts in microbiota composition were described for the colonic/fecal microbiota when CA was added to the diet of wild-type rats [18]. Despite showing no tumor formation in the colon, K-ras G12Dint mice also had increased levels of Enterobacteriaceae and Desulfovibrionaceae and reduced abundance of Bifidobacteriaceae and of members belonging to the genus Roseburia and Butyrococcus in the colonic microbiota [17•]. Remarkably, a transfer of fecal samples from K-ras G12Dint mice on a high-fat diet to susceptible K-ras G12Dint mice on a control diet induced small intestinal tumor formation. In contrast, the fecal transfer of the high-fat-shaped microbiota to LSL-K-ras G12D/+ control mice on control diet did not accelerate intestinal tumorigenesis [17•], suggesting that the tumorigenic activity of a high-fat diet-shaped microbiota depends on a susceptible host.
Similar evidence was demonstrated in a second study by Cao et al., who used Apc min/+ mice, a model for tumor formation in the small intestine. The addition of exogenous DCA to the diet of Apc min/+ mice resulted in accelerated tumorigenesis in the small intestine compared to Apc min/+ mice that received no additional DCA [29]. The transfer of the fecal microbiota from DCA-treated Apc min/+ mice to Apc min/+ mice, which were treated before with antibiotics, transmitted the increased tumor-promoting activity. This suggests that DCA mediates a dysbiotic composition of the gut microbiota that triggers tumor formation in a genetically susceptible host. Importantly, the fecal transfer from the DCA-treated Apc min/+ to wild-type mice as well as the DCA-supplemented diet in wild-type mice did not induce intestinal tumor formation [29]. Again, this highlights the importance of genetic susceptibility of the host as a major factor in bile acid-mediated tumorigenic activity.
Taken together, both studies suggest that an altered bile acid pool induces shifts in the composition of the gut microbiota, which demonstrates dysbiotic characteristics and has tumor-promoting activity in susceptible hosts. However, in both mentioned studies, the fecal concentrations of all major bile acids or the capacity of the transferred microbiota to generate DCA as possible tumorigenic or antimicrobial agent were not determined after the fecal microbiota transfer. Thus, it remains unclear, which mechanisms were involved in the tumor-promoting activity of the high-fat- or DCA-shaped gut microbiota in recipient mice. Since conversion of primary bile acids takes place in the colon; other bile acids or an increased enterohepatic circulation of DCA may be responsible for the observed effects in the small intestine. Importantly, both mouse models showed tumor formation in the small intestine, which is not representative for human CRC, especially considering the different luminal milieu affecting the gut bacteria. K-ras G12Dint mice feature defects in Paneth cell function resulting in decreased antimicrobial defense in the small intestine [17•], which may trigger a non-physiologic condition such as small intestinal bacterial overgrowth and affect the assembly of the gut microbiota independent of dietary factors. Similarly, the genetic susceptibility of Apc min/+ mice may result in an altered microbiota independent of diet or bile acid-mediated shifts. In summary, these two studies provide experimental evidence that a high-fat- or DCA-shaped gut microbiota has increased tumorigenic activity, but the relevance to human CRC remains unclear due to model limitations.
With regard to the transmission of tumor-promoting activity by the high-fat- or bile acid-shaped gut microbiota to a genetically susceptible host, the identification of over/underrepresented species or bacterial metabolites has revealed interesting information. It was demonstrated that a diet rich in saturated fat (derived from milk) promoted taurine conjugation of CA and resulted in a bloom of sulphite-reducing Bilophila wadsworthia due to increased luminal levels of sulfur substrate [44]. Interestingly, B. wadsworthia was also enriched in the fecal microbiota following a high-fat or animal-based diet in healthy individuals [14••, 45]. Increased abundance of B. wadsworthia promoted colonic inflammation in the IL-10−/− mouse model by stimulating a potent TH1 immune response in the mucosa [44]. Hydrogen sulfide, which is produced by B. wadsworthia, showed genotoxic activity in vitro [46, 47] and provides another possible mechanism how bile acid-mediated shifts of the gut microbiota may trigger colonic tumorigenesis.
As another example, increased fecal levels of the secondary bile acid ursodeoxycholic acid (UDCA) were reported in healthy individuals compared to CRC patients [42]. This correlated positively with the abundance of Ruminococcus spp. in the fecal microbiota, which exhibit enzymes (7α- and 7β-hydroxysteroid dehydrogenase) that mediate the epimerization of CDCA to UDCA [48]. Oral application of UDCA to patients with ulcerative colitis or primary sclerosing cholangitis suppressed colonic tumorigenesis [49], but high oral doses of UDCA were associated with a higher risk for CRC [50]. This may be explained by the presence of gut bacteria from the genus Clostridium that are able to convert UDCA to LCA, which shows cytotoxic properties in vitro [51]. However, under normal conditions LCA accounts only for a small amount of the pool of circulating bile acids. A recent study demonstrated that oral administration of UDCA to wild-type mice treated with DSS resulted in reduced intestinal inflammation and in LCA being the most common bile acid in the colonic lumen [52]. Interestingly, the inhibition of DSS-mediated colitis in mice was potentiated when LCA was administered instead of UDCA, suggesting that anti-inflammatory effects of UDCA rely on its microbial conversion to its primary metabolite LCA [52]. It also suggests different roles of LCA in the pathogenesis of intestinal inflammation and CRC and highlights the complex interplay between the gut microbiota and host in bile acid metabolism.
Conclusions
The regulation of the bile acid pool by diet, gut bacteria, and host factors exemplifies the complexity of diet-microbe-host interactions in maintaining intestinal homeostasis. Perturbations of this tightly balanced system caused by detrimental dietary patterns, for example, result in altered characteristics, which may be correlating with or causally linked to disease. Regarding the influence of bile acids on CRC risk, diet and the gut microbiota are most likely the key drivers that mediate and confer bile acid-associated tumorigenic activity (Fig. 1); high-fat diets mediate increased production and secretion of bile acids, which provides high luminal levels of bile acids in the colon, where they serve as substrate for bacterial metabolism. High-fat diet-associated bile acid levels impact on gut microbiota assembly favoring increased levels of 7α-dehydroxylating bacteria, which stimulates increased colonic levels of secondary bile acids with tumor-promoting activity, especially DCA. Additional complex microbial biotransformation of bile acids regulates the bile acid pool, which in turn affects the composition of the gut microbiota and may trigger tumor formation or progression by bile acid-induced dysbiosis. Alterations of the gut microbiota mediated by an altered bile acid composition and/or high-fat diet, feature high abundance of bacteria that convert primary to secondary bile acids and involve microbes that exert experimental inflammatory or genotoxic activity. The detailed contributions of the gut microbiota to tumorigenic activity of bile acids remain unclear and further studies are needed to dissect the role of microbial biotransformation of bile acids in the context of their tumor-promoting activity.
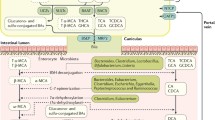
Impact of high-fat diet and selected biotransformation by the gut microbiota on tumor-promoting activity of bile acids in the intestine. The repeated intake of a high-fat diet stimulates the hepatic synthesis of bile acids (BA), their conjugation with taurine, and the secretion into the duodenum providing high levels of primary BA in the gut lumen. The deconjugation of BA by microbial bile salt hydrolases results in increased luminal levels of taurine, which promotes the growth of single members of the gut microbiota and high levels of unconjugated BA that enter the colon, where they are converted to secondary BA by 7α-dehydroxylating bacteria. This promotes high colonic concentrations of secondary BA, especially deoxycholic acid (DCA), which has tumorigenic activity and drives a dysbiotic composition of the colonic microbiota featuring increased abundance of 7α-dehydroxylating bacteria and microbes with potential genotoxic activity. The enhanced 7α-dehydroxylation capacity of the gut microbiota leads to an altered BA pool with higher levels of DCA entering enterohepatic circulation. Cholic acid (CA), chenodeoxycholic acid (CDCA), lithocholic acid (LCA)
In summary, secondary bile acids appear to function as tumor promoters in human CRC. Their interdependence with diet and the gut microbiota makes them an interesting target to modulate the risk for CRC associated with the consumption of western diets. Considering the major impact of diet on the gut microbiota and the bile acid pool of the host, long-term diet changes offer a promising strategy for CRC risk reduction [15]. In addition, the characterization of microbe-host interactions implicated in bile acid metabolism will help to further unravel the complex influence of bile acids on CRC risk.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86.
Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044–58.
Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66:1191–308.
World Cancer Research Fund/American Institute for Cancer Research (2011) Continuous update project report. Food, Nutrition, Physical Activity, and the Prevention of Colorectal Cancer.
Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med. 1999;159:2647–58.
Ridlon JM, Kang D-J, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–59.
Hill MJ, Drasar BS, Hawksworth G, Aries V, Crowther JS, Williams RE. Bacteria and aetiology of cancer of large bowel. Lancet Lond Engl. 1971;1:95–100.
Reddy BS, Wynder EL. Large-bowel carcinogenesis: fecal constituents of populations with diverse incidence rates of colon cancer. J Natl Cancer Inst. 1973;50:1437–42.
Reddy BS. Role of bile metabolites in colon carcinogenesis. animal models. Cancer. 1975;36:2401–6.
Reddy BS, Wynder EL. Metabolic epidemiology of colon cancer. fecal bile acids and neutral sterols in colon cancer patients and patients with adenomatous polyps. Cancer. 1977;39:2533–9.
Imray CH, Radley S, Davis A, Barker G, Hendrickse CW, Donovan IA, et al. Faecal unconjugated bile acids in patients with colorectal cancer or polyps. Gut. 1992;33:1239–45.
Ou J, DeLany JP, Zhang M, Sharma S, O’Keefe SJD. Association between low colonic short-chain fatty acids and high bile acids in high colon cancer risk populations. Nutr Cancer. 2012;64:34–40.
Ou J, Carbonero F, Zoetendal EG, DeLany JP, Wang M, Newton K, et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr. 2013;98:111–20.
•• O’Keefe SJD, Li JV, Lahti L, et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun. 2015;6:6342. This publication shows how high-fat or high-fiber diets correlate with mucosal, microbial, and metabolic markers associated with colorectal cancer risk in humans. It provides evidence that directed dietary changes (diet switch from high-fat/low-fiber to low-fat/high-fiber diet and vice versa) have significant impact on colorectal cancer risk in humans.
O’Keefe SJD. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13:691–706.
Donohoe DR, Holley D, Collins LB, et al. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner. Cancer Discov. 2014;4:1387–97.
• Schulz MD, Atay C, Heringer J, et al. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature. 2014;514:508–12. This publication evaluates how high-fat diet-mediated alterations of the gut microbiota impact on intestinal tumorigenesis in a mouse model genetically susceptible to tumor formation in the small intestine.
Islam KBMS, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, et al. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology. 2011;141:1773–81.
Higashimura Y, Naito Y, Takagi T, et al. Protective effect of agaro-oligosaccharides on gut dysbiosis and colon tumorigenesis in high-fat diet-fed mice. Am J Physiol Gastrointest Liver Physiol. 2016;310:G367–75.
Lechner S, Müller-Ladner U, Schlottmann K, Jung B, McClelland M, Rüschoff J, et al. Bile acids mimic oxidative stress induced upregulation of thioredoxin reductase in colon cancer cell lines. Carcinogenesis. 2002;23:1281–8.
Dvorak K, Payne CM, Chavarria M, et al. Bile acids in combination with low pH induce oxidative stress and oxidative DNA damage: relevance to the pathogenesis of Barrett’s oesophagus. Gut. 2007;56:763–71.
Bayerdörffer E, Mannes GA, Richter WO, Ochsenkühn T, Wiebecke B, Köpcke W, et al. Increased serum deoxycholic acid levels in men with colorectal adenomas. Gastroenterology. 1993;104:145–51.
Narisawa T, Magadia NE, Weisburger JH, Wynder EL. Promoting effect of bile acids on colon carcinogenesis after intrarectal instillation of N-methyl-N’-nitro-N-nitrosoguanidine in rats. J Natl Cancer Inst. 1974;53:1093–7.
Flynn C, Montrose DC, Swank DL, Nakanishi M, Ilsley JNM, Rosenberg DW. Deoxycholic acid promotes the growth of colonic aberrant crypt foci. Mol Carcinog. 2007;46:60–70.
Bernstein C, Holubec H, Bhattacharyya AK, Nguyen H, Payne CM, Zaitlin B, et al. Carcinogenicity of deoxycholate, a secondary bile acid. Arch Toxicol. 2011;85:863–71.
Yoshimoto S, Loo TM, Atarashi K, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101.
• Raufman J-P, Dawson PA, Rao A, Drachenberg CB, Heath J, Shang AC, et al. Slc10a2-null mice uncover colon cancer-promoting actions of endogenous fecal bile acids. Carcinogenesis. 2015;36:1193–200. This study evaluates the role of bile acids in colorectal cancer using a mouse model with increased fecal excretion of endogenous bile acids, highlighting their role as tumor-promoting agents.
Dawson PA, Haywood J, Craddock AL, Wilson M, Tietjen M, Kluckman K, et al. Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J Biol Chem. 2003;278:33920–7.
Cao H, Xu M, Dong W, et al. Secondary bile acid-induced dysbiosis promotes intestinal carcinogenesis. Int J Cancer. 2017; https://doi.org/10.1002/ijc.30643.
Ridlon JM, Harris SC, Bhowmik S, Kang D-J, Hylemon PB. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes. 2016;7:22–39.
Staley C, Weingarden AR, Khoruts A, Sadowsky MJ. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl Microbiol Biotechnol. 2017;101:47–64.
Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall H-U, Bamberg K, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–35.
Kakiyama G, Pandak WM, Gillevet PM, et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol. 2013;58:949–55.
Komaki Y, Komaki F, Micic D, Ido A, Sakuraba A. Risk of colorectal cancer in chronic liver diseases: a systematic review and meta-analysis. Gastrointest Endosc. 2016; https://doi.org/10.1016/j.gie.2016.12.009.
Floch MH, Binder HJ, Filburn B, Gershengoren W. The effect of bile acids on intestinal microflora. Am J Clin Nutr. 1972;25:1418–26.
Kurdi P, Kawanishi K, Mizutani K, Yokota A. Mechanism of growth inhibition by free bile acids in lactobacilli and bifidobacteria. J Bacteriol. 2006;188:1979–86.
Inagaki T, Moschetta A, Lee Y-K, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A. 2006;103:3920–5.
Begley M, Gahan CGM, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev. 2005;29:625–51.
Ridlon JM, Alves JM, Hylemon PB, Bajaj JS. Cirrhosis, bile acids and gut microbiota. Gut Microbes. 2013;4:382–7.
Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–23.
Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen Y-Y, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:1716–24. e1–2
Weir TL, Manter DK, Sheflin AM, Barnett BA, Heuberger AL, Ryan EP. Stool microbiome and metabolome differences between colorectal cancer patients and healthy adults. PLoS One. 2013;8:e70803.
Feng Q, Liang S, Jia H, et al. Gut microbiome development along the colorectal adenoma–carcinoma sequence. Nat Commun. 2015; https://doi.org/10.1038/ncomms7528.
Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature. 2012;487:104–8.
David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–63.
Attene-Ramos MS, Wagner ED, Plewa MJ, Gaskins HR. Evidence that hydrogen sulfide is a genotoxic agent. Mol Cancer Res MCR. 2006;4:9–14.
Attene-Ramos MS, Nava GM, Muellner MG, Wagner ED, Plewa MJ, Gaskins HR. DNA damage and toxicogenomic analyses of hydrogen sulfide in human intestinal epithelial FHs 74 Int cells. Environ Mol Mutagen. 2010;51:304–14.
Lepercq P, Gérard P, Béguet F, Grill J, Relano P, Cayuela C, et al. Isolates from normal human intestinal flora but not lactic acid bacteria exhibit 7α- and 7β-hydroxysteroid dehydrogenase activities. Microb Ecol Health Dis. 2004;16:195–201.
Pardi DS, Loftus EV, Kremers WK, Keach J, Lindor KD. Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology. 2003;124:889–93.
Eaton JE, Silveira MG, Pardi DS, et al. High-dose ursodeoxycholic acid is associated with the development of colorectal neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Am J Gastroenterol. 2011;106:1638–45.
Kulkarni MS, Heidepriem PM, Yielding KL. Production by lithocholic acid of DNA strand breaks in L1210 cells. Cancer Res. 1980;40:2666–9.
Ward JBJ, Lajczak NK, Kelly OB, et al. Ursodeoxycholic acid and lithocholic acid exert anti-inflammatory actions in the colon. Am J Physiol Gastrointest Liver Physiol. 2017. ajpgi.00256.2016.
Acknowledgements
S. Ocvirk is supported by the German Cancer Aid.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Soeren Ocvirk and Stephen J.D. O’Keefe declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Cancer
Rights and permissions
About this article
Cite this article
Ocvirk, S., O’Keefe, S.J. Influence of Bile Acids on Colorectal Cancer Risk: Potential Mechanisms Mediated by Diet-Gut Microbiota Interactions. Curr Nutr Rep 6, 315–322 (2017). https://doi.org/10.1007/s13668-017-0219-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13668-017-0219-5