Abstract
Purpose of Review
Sarcoidosis is a systemic inflammatory disorder affecting multiple organ systems, with heterogenous clinical sequelae. Cardiac sarcoidosis (CS) is an underrecognized manifestation with significant clinical implications. Advances in cardiac imaging and new therapeutic options are rapidly changing the clinical approach to CS.
Recent Findings
Cardiac magnetic resonance imaging (CMR), 18Fluorodeoxyglucose positron emission tomography (FDG-PET), and hybrid CMR/FDG-PET imaging have emerged as powerful diagnostic and prognostic tools for CS and provide a means to monitor disease activity. Therapeutic options are similarly changing, with steroid-sparing agents and biologic therapy showing clinical promise, though ongoing clinical trials will potentially provide necessary evidence to definitively guide treatment.
Summary
In this review, we critically evaluate a variety of diagnostic and therapeutic options for CS. Additionally, we propose algorithms for the diagnostic evaluation and treatment of CS, which incorporate current guidelines as well as recent advances in CS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sarcoidosis is a systemic disease characterized by non-necrotizing granulomatous inflammation in a variety of organ systems. While a definitive etiology of sarcoidosis has not been identified, there is robust evidence supporting the combination of multiple genetic and environmental triggers leading to a dysregulated immune response [1,2,3]. Worldwide prevalence of sarcoidosis varies by region, ethnicity, and gender. In the USA, prevalence is 5–60 per 100,000 individuals, with a nearly threefold higher annual incidence among African American women [4,5,6]. Pulmonary involvement is common, occurring in 90% of those affected by sarcoidosis; however, organ involvement and clinical presentation is heterogenous [7].
Cardiac sarcoidosis (CS) is of particular significance due to its high morbidity and mortality, commonly believed to account for 25–85% of sarcoidosis-related deaths [8]. When present, symptoms include palpitations, syncope, symptoms of heart failure (HF), and sudden cardiac death [9]. Importantly, ventricular tachyarrhythmias or sudden cardiac death (SCD) may be the initial manifestation of CS [10]. The clinical manifestations of CS are best categorized by three primary disease sequalae: conduction abnormalities, arrhythmias, and HF [11••].
Cardiac symptoms are present in an estimated 5% of individuals with sarcoidosis; however, autopsy studies identified CS in 25–45% of individuals, suggesting cardiac involvement is significantly under-recognized [12, 13]. Additionally, there may be regional or racial variability, as Japanese studies report a much higher incidence of CS at 58–68% [14, 15]. Finally, isolated cardiac sarcoidosis (ICS) may occur in the absence of extracardiac disease and accounts for an estimated 27–54% of CS cases, though true prevalence is unknown owing to specific challenges related to diagnosis of ICS [16].
The first diagnostic criteria for CS were published in 1993 by the Japanese Ministry of Health and Welfare (JMHW), with updates in 2007 and 2016 by the Japanese Circulation Society (JCS). In JCS guidelines, CS may be diagnosed histologically based on presence of non-necrotizing granulomatous inflammation on endomyocardial biopsy (EMB) or by meeting a variety of major and minor criteria for clinical diagnosis in the absence of EMB [17••]. Additionally, the World Association of Sarcoidosis and Other Granulomatous Diseases (WASOG) provided an organ assessment tool to determine the likelihood of CS [18]. Finally, the Heart Rhythm Society (HRS) published diagnostic criteria for CS in 2014 aligned with the WASOG organ assessment tool, which provides diagnostic pathways for CS similar to JCS based on (1) EMB or (2) clinical criteria in the setting of known extracardiac disease [11••].
This review will be organized into two parts: we will first review diagnostic modalities used to evaluate CS, followed by a review of therapeutic options. In both sections, we will focus on recent literature and advancements in the field, though it should be highlighted there is limited evidence-based literature on this topic. Finally, we will discuss our approach to diagnosis and management of CS in addition to proposing algorithms based on current evidence as well as clinical experience from managing individuals with sarcoidosis at our quaternary care academic medical center.
Diagnostic Evaluation of Cardiac Sarcoidosis
Diagnostic modalities for CS include electrocardiography (EKG), transthoracic echocardiography (TTE), serum biomarkers, ambulatory rhythm monitoring (ARM), cardiac MRI (CMR), 18Fluorodeoxyglucose Positron Emission Tomography (FDG-PET), hybrid CMR/PET, and endomyocardial biopsy (EMB). In this section, we will review the clinical utility, diagnostic accuracy, and characteristic findings of CS with a focus on CMR, FDG-PET, and hybrid imaging.
Electrocardiography
EKG findings in CS are non-specific and include abnormal Q waves, ST changes, atrioventricular (AV) blocks (type I, II or III), bundle branch blocks, axis deviation, or ventricular arrhythmias [17••]. Prospective studies have suggested new EKG abnormalities occur prior to onset of symptomatic CS [19]. With this in mind, American Thoracic Society (ATS) and HRS guidelines recommend obtaining an initial screening EKG in all individuals with extra-cardiac sarcoidosis [11••, 20]. Notably, no clear recommendations are made for or against serial EKG screening if initial EKG is normal, a question worthy of further research. Importantly, the absence of pathologic EKG findings does not exclude the presence of CS [21].
Transthoracic Echocardiography
TTE findings can include reduction in left ventricular ejection fraction (LVEF), LV dilation, diastolic dysfunction, right ventricular (RV) dysfunction, pericardial effusion, or valvular disorders. Wall motion abnormalities may be present in a non-coronary distribution [22]. TTE lacks sensitivity, limiting its diagnostic role in CS, though is considered a useful adjunct to screen for CS in all individuals with extra-cardiac disease per HRS guidelines, and in select individuals with EKG abnormalities or idiopathic arrhythmias per JCS [17••, 23]. LV function is often described in terms of EF which is a volumetric assessment based on changes in longitudinal, circumferential, and radial length measurements. Strain imaging assesses each component separately and can therefore detect subtle deformities indicative of early LV dysfunction. Echocardiography with strain imaging may further improve diagnostic sensitivity and better predict adverse cardiac outcomes [24, 25]. Similar to an EKG, a TTE alone remains insufficient to detect CS and has potential to underdiagnose cardiac involvement [21].
Serum Biomarkers
No single biomarker is sensitive for diagnosis of CS. High-sensitivity cardiac troponin (hs-CT), N-terminal pro brain natriuretic peptide (NT-pro BNP), erythrocyte sedimentation rate (ESR), and C-reactive peptide (CRP) may be elevated in active disease, though are nonspecific [23, 26, 27]. Angiotensin converting enzyme (ACE) and soluble interleukin-2 receptor (sIL-2R) levels are variable in CS [26, 28]. Few studies show ACE levels correlate with the presence of late gadolinium enhancement (LGE) on CMR, and elevated sIL-2R levels predict adverse cardiac outcomes [29, 30]. A definitive role of serum biomarkers for screening and/or diagnosis of CS remains uncertain.
Ambulatory Rhythm Monitoring
ARM is a readily available diagnostic tool to monitor for arrhythmias.
Abnormalities on ARM may include supraventricular tachycardia, premature ventricular contractions, atrioventricular block, and ventricular tachycardia [31]. In a study of 126 patients with extracardiac sarcoidosis, abnormal ARM was more predictive for positive imaging findings on CMR or FDG-PET compared to EKG or TTE [32, 33]. ARM may also have utility in assessing response to therapy; however, the precise role of ARM is not well defined in guidelines [34].
Cardiac Magnetic Resonance Imaging
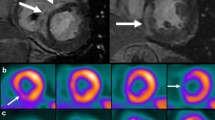
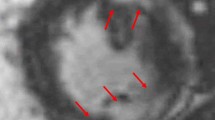
CMR with gadolinium can detect subtle myocardial abnormalities and provide information on ventricular function and morphology [35]. Gadolinium has delayed clearance from areas of active inflammation or fibrosis. The presence of LGE, especially patchy LGE in a non-coronary distribution, is suggestive of CS in the appropriate clinical setting [36].
The sensitivity of CMR in detecting CS has been reported between 91 and 95%, superior to other modalities, including FDG-PET [37••, 38,39,40]. In a cohort of 300 individuals with biopsy-proven extracardiac sarcoidosis, CMR detected cardiac involvement in 9% of individuals who had no cardiac symptoms and a normal baseline EKG [41]. CMR has an excellent negative predictive value, making it useful in early evaluation of patients with suspected CS [42].The prognostic value of CMR has been validated by multiple studies which have identified an association between the presence of LGE and adverse cardiovascular events, including all-cause mortality and arrhythmias [39, 43,44,45].
CMR does have limitations. Most importantly, while the presence of LGE is specific for identifying affected myocardium in CS, it does not discern active from inactive or fibrotic disease [36]. The addition of T1 and T2 weighted images can mitigate this limitation and improve detection of active disease. T2 mapping detects myocardial edema which may be more representative of active inflammation [46, 47]. Finally, there are absolute and relative contraindications to CMR including renal dysfunction, allergy to gadolinium contrast, presence of non-MRI compatible devices, and claustrophobia [48].
18Fluorodeoxyglucose Positron Emission Tomography
FDG-PET to evaluate for CS utilizes radionuclide-labeled glucose in combination with myocardial perfusion imaging. Inflammatory cells, including macrophages and CD4 + T-lymphocytes present in granulomas, readily take up the glucose analog and appear bright on the PET images [49]. The combination of FDG and perfusion imaging is useful in differentiating between metabolically active myocardium versus inactive CS or fibrotic scar from prior infarct. A pattern of focal FDG uptake with or without perfusion defects suggests active inflammation, while a perfusion defect without associated FDG uptake is indicative of fibrotic scar [50, 51].
The sensitivity of FDG-PET for diagnosis of CS is reported between 84 and 89%, while specificity is 78 and 83%, comparable to CMR [38, 52, 53]. One key advantage of FDG-PET is the detection of active inflammation, which is paramount in treatment decisions; for instance, abnormalities seen on CMR may be further delineated as active myocardial inflammation (FDG-PET positive) or chronic fibrosis or scar (FDG-PET negative) [50]. Hence, CMR and FDG-PET are frequently used in tandem with one another. FDG-PET also has a role in risk stratification; specific uptake patterns, including the right ventricle and basal anterolateral left ventricle, may be predictive of adverse outcomes [54]. Additionally, several observational studies note utility in serial FDG-PET for monitoring disease activity and titration of immunosuppression, though data surrounding this practice is limited [55,56,57].
Individuals must adhere to a strict, high-fat and carbohydrate-restricted diet prior to FDG-PET in order to suppress native myocardial FDG uptake [51]. This limitation has been overcome to some extent with highly structured protocols [58], though FDG-PET remains non-diagnostic in up to 15% of individuals [59]. Initial and serial FDG-PET exams increase exposure to ionizing radiation [60]. Finally, a post-transplant study found a high false-positive rate of FDG-PET where only 33% of probable CS cases identified by FDG-PET were confirmed on histologic analysis [61].
Hybrid CMR and FDG-PET
Hybrid CMR/PET has recently emerged as an imaging strategy to diagnose and stratify CS, with the potential advantage of recognizing myocardial abnormalities and differentiating active from chronic disease simultaneously [62]. This advanced imaging modality appears to be highly sensitive for diagnosis of CS, has a strong prognostic role, and is superior to either CMR or FDG-PET alone in predicting adverse cardiac events [63].
In a prospective study of 43 individuals with biopsy-proven extracardiac sarcoidosis, the use of hybrid CMR/PET detected active CS in 36% of individuals and fibrotic CS in 14% [37••]. Additionally, hybrid imaging improves interpretation of potential false-positive FDG-PET results; the finding of negative CMR with positive FDG-PET, especially when FDG uptake is diffuse rather than focal, may be more indicative of inadequate suppression of myocardial glucose uptake [62]. Additionally, the inclusion of T2 images may improve sensitivity of FDG-PET in identifying earlier stages of disease [64].
Potential cost and availability of advanced imaging limits routine use of hybrid CMR/PET. A retrospective, observational study demonstrates potential cost-effectiveness of hybrid imaging with lower lifetime cost. Notably, the reference standard for cost analysis was CMR followed by FDG-PET, which is currently not consistent with most major guidelines [65].
Endomyocardial Biopsy
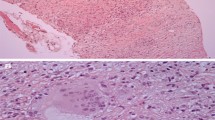
EMB demonstrating non-necrotizing granulomas is the gold standard for diagnosis of CS; however, diagnostic yield is estimated at 25% due to the patchy distribution of granulomas in CS [63]. Using electrogram-guided EMB or preprocedural CMR/PET to direct biopsy increases diagnostic yield to 41% [66]. EMB has an associated procedural risk ranging from 0.6 to 0.8% [67]. The limitations of EMB are reflected in changes in major society guidelines which have more recently emphasized the diagnostic utility of non-invasive advanced cardiac imaging [11••, 63].
Our Diagnostic Approach to CS
Our approach to evaluating for the presence of CS incorporates recommendations from ATS, HRS, and JCS guidelines and is summarized in Fig. 1. Individuals undergoing evaluation for CS are categorized into one of three groups: (1) presence of extracardiac sarcoidosis without high clinical suspicion, (2) presence of extracardiac sarcoidosis with high clinical suspicion, and (3) absence of extracardiac sarcoidosis but high clinical suspicion for CS. High clinical suspicion for CS in individuals with extracardiac sarcoidosis includes the presence of palpitations lasting > 2 weeks, unexplained syncope or pre-syncope, unexplained life-threatening cardiac arrhythmia, and/or abnormal screening EKG or TTE. Individuals with extra-cardiac sarcoidosis without high clinical suspicion for CS undergo screening EKG and TTE. If these studies are normal, then routine clinical follow-up is suggested. The development of high-risk symptoms and/or new abnormalities on EKG should prompt further evaluation with CMR. Admittedly, this approach risks missing subclinical CS [21]; however, there is not sufficient evidence to recommend advanced imaging modalities for all individuals with extra-cardiac sarcoidosis, and it remains uncertain if subclinical disease warrants therapy [11••]. Individuals with extracardiac sarcoidosis with high clinical suspicion for CS are evaluated with CMR and FDG-PET. Twenty-four-hour ARM for 5 to 7 days is also obtained if there is clinical suspicion for arrhythmia. The utility of obtaining both CMR and FDG-PET enables identification and characterization of CS (active inflammatory CS versus inactive fibrotic CS) and provides baseline imaging to guide therapy. If both CMR and FDG-PET are negative, an alternative diagnosis should be considered. EMB may be pursued in select patients, though there is limited data regarding the utility of EMB in patients with negative findings on advanced imaging modalities. Individuals without extra-cardiac sarcoidosis are considered high risk for ICS if they have cardiac symptoms of unclear etiology and are aged < 60 with unexplained 2nd or 3rd degree AV block or ventricular arrhythmias. In these individuals, screening with EKG and TTE should be obtained, and if abnormalities are noted on both studies, advanced imaging including both CMR and FDG-PET should be pursued, as well as 24-h ARM for 5 to 7 days if there is suspicion for arrhythmia. We commonly obtain whole-body PET to evaluate for potential extracardiac disease and other sites amenable to biopsy, though admittedly, this practice is not specifically addressed by most guidelines. Positive results on CMR and FDG-PET would fulfill JCS criteria for diagnosis of ICS [17••].
Management of Cardiac Sarcoidosis
Treatment of CS is focused on reducing myocardial inflammation and subsequent development of fibrotic scar and arrhythmia. Corticosteroids have long been the cornerstone of therapy; however, use of steroid-sparing agents and/or biologic therapy is increasingly recognized. Additionally, antiarrhythmics, device therapy, and optimization of HF are imperative to CS management [68]. In this section, we will review indications for therapy, treatment options, and follow-up evaluation. We will highlight various immunosuppressive therapies (ISTs) and discuss treatment approach in the context of the main clinical sequalae of CS: conduction abnormalities, arrhythmias, and HF. Finally, a proposed therapeutic algorithm will be presented.
Indications for IST in CS
The decision to initiate IST is individualized based on the clinical context. HRS guidelines suggest use of IST in individuals with evidence of active disease on FDG-PET or in individuals with high-risk features including high-degree AV block, frequent ventricular ectopy, and non-sustained or sustained ventricular arrhythmias [11••]. JCS guidelines suggest initiating IST in the presence of high-degree AV block, ventricular arrhythmias, or cardiac dysfunction [17••].
IST Use in CS
IST targets active inflammation in CS [17••]. A systematic meta-analysis of 34 studies demonstrated that IST, in most cases corticosteroids, is likely beneficial in treatment of conduction abnormalities or impaired left ventricular function; however, IST did not appear to reduce ventricular arrhythmias or overall mortality [69]. A separate meta-analysis corroborated the finding that IST improves conduction abnormalities and left ventricular function, though also found benefit in treatment of ventricular arrhythmias [70]. Obtaining an FDG-PET prior to treatment may help identify which individuals are more appropriate for IST [71].
Initial treatment regimens historically include high-dose prednisone and other corticosteroid equivalents up to 60 mg/day. More recently, a lower initial dose of prednisone (30 mg/day) showed similar outcomes with decreased adverse effects attributed to high-dose prednisone [72]. JCS guidelines suggest an initial induction phase of 4 weeks, followed by a slow taper of 5 mg every 2–4 weeks to a final maintenance dose of 5–10 mg prednisone/day [17••].
Duration of maintenance therapy is not well described, though commonly continued for a minimum of 12 months. Serial assessment with FDG-PET may be useful in guiding therapy [56]. In a study of 34 individuals with CS, serial FDG-PET led to treatment changes in 73% of individuals and was instrumental in weaning prednisone. At the end of an average 2.3-year follow-up, 48% of individuals were off steroids and 20% were weaned to a maintenance dose of 5–10 mg/day [55].
Steroid-sparing agents including methotrexate, mycophenolate, azathioprine, and cyclophosphamide are frequently incorporated in the management of CS in order to limit or eliminate corticosteroid use and associated adverse effects [73, 74]. Methotrexate is the recommended second-line agent in JCS guidelines and may be considered in addition to corticosteroids or as monotherapy [17••]. In a retrospective cohort study of 61 treatment-naïve individuals with CS, initial therapy with either prednisone, prednisone plus methotrexate, or methotrexate monotherapy all resulted in improvement in metabolic activity on FDG-PET and had no significant differences in adverse cardiovascular events or LV function [75••]. A smaller trial suggests superiority of combination corticosteroids plus methotrexate compared to corticosteroids alone for stabilization of LV function, though more robust comparative data is lacking [76]. The Cardiac Sarcoidosis Multi-Center Randomized Controlled Trial (CHASM CS-RCT) is an ongoing multicenter prospective randomized control trial comparing initial treatment regimen of prednisone with prednisone plus methotrexate and should provide valuable evidence for initial IST selection in individuals with CS [77••].
Biologic drugs, specifically the tumor necrosis factor-alpha (TNF-α) inhibitors infliximab and adalimumab, are frequently used for refractory CS [74]. In 2016, the American Heart Association recommended against use of TNF-α inhibitors in individuals with moderate to severe HF, a recommendation based on earlier data associating TNF-α inhibitor use with increased adverse outcomes, hospitalizations, worsening HF, and death in individuals who had preexisting HF; however, the evidence was inconsistent, and most individuals had ischemic cardiomyopathy [78]. More recent evidence supports the role of TNF-α and demonstrates good safety profile of these agents when used for treatment of individuals with CS, including those with underlying HF [79,80,81]. Lending further support to their use, all participants in a large cohort of 77 individuals with CS treated with TNF-α inhibitors had clinical improvement, without any reported cases of worsening HF [82••].
Rituximab, a chimeric monoclonal antibody against the protein CD20, has also been used for refractory disease, though evidence for its use is limited to case reports and small retrospective cohort studies [83, 84]. In a study of 5 CS individuals treated with rituximab, all had improvement in FDG-PET findings, and 3 patients (60%) demonstrated improvement in LV function [84].
Treatment of Conduction Abnormalities
AV block, sinus node dysfunction, and bundle branch blocks are common early findings in CS. AV block is related to granuloma formation or fibrosis along the conduction system, often in the basal septum [85]. Conduction abnormalities may be responsive to IST, and guidelines recommend IST for 2nd or 3rd degree heart block in addition to device implantation if there is an indication for pacing [11••, 86]. However, a study of 53 CS individuals with high-degree AV block found half of individuals developed fatal cardiac arrhythmias (sustained ventricular tachycardia (VT) or SCD) within a 3-year follow-up period regardless of receiving IST or the presence of concomitant HF [87]. These data strongly emphasize the 2017 American Heart Association (AHA)/American College of Cardiology (ACC)/HRS recommendations for concurrent implantable cardioverter defibrillator (ICD) placement in individuals with CS who otherwise have an indication for permanent pacemaker implantation [88].
Treatment of Cardiac Arrhythmias
Cardiac arrhythmia is a common manifestation of CS, and atrial fibrillation, atrial flutter, or VT may be seen in 20–32% of individuals [89, 90]. Arrhythmias are likely due to inflammation or fibrosis within the myocardium [91]. In electroanatomical mapping studies of individuals with ventricular arrhythmias, areas of abnormal EKG correlated to areas of LGE on CMR in 90% of cases [92].
Retrospective studies suggest a high incidence of atrial arrythmias (AA) in CS, with atrial fibrillation being most common [90]. However, in a more recent prospective study of 33 treatment-naïve individuals with CS who had implanted devices, 11 had device detected AA, 2 had clinically significant AA, and only 1 patient had AA as a presenting feature of CS [93]. Antiarrhythmic and/or nodal agents are often required and may include β-blockers, calcium-channel blockers, sotalol, dofetilide, and amiodarone. In cases of AF, anticoagulation may be considered based on the individuals CHA2DS2-VASc score [11••].
Ventricular arrhythmia is an independent predictor of mortality in individuals with CS [72, 94]. IST has been shown to be effective in reducing ventricular arrhythmias, especially when started before the onset of LV dysfunction [95]. Comprehensive recommendations for antiarrhythmic agent selection follow the 2017 AHA/ACC/HRS guidelines [88]. Additionally, HRS guidelines provide recommendations for ICD placement. Class I recommendations include individuals with LVEF < 35% despite optimal therapy and individuals with sustained ventricular arrhythmias or prior cardiac arrest. ICD placement is also suggested in individuals with unexplained syncope or inducible ventricular arrhythmia on electrophysiology study (EPS) (Class IIa recommendation) [11••].
Those with CS are at increased risk for SCD. Risk stratification is important in individuals with CS who otherwise do not meet criteria for ICD. In a meta-analysis describing the utility of electrophysiology studies (EPS) to predict ventricular arrhythmias, ICD therapy, need for advanced circulatory support, or death, EPS had sensitivity of 71% and specificity of 96% for predicting adverse outcomes in individuals with CS and no prior history of VT and LVEF > 35% [96]. Additionally, individuals with CS who had ICD placement for secondary prevention and had LVEF > 35% were still found to have a high rate of appropriate ICD therapy, further supporting that potentially fatal arrhythmias commonly occur in individuals who may not meet traditional criteria for ICD placement [97].
Treatment of Congestive HF
Treatment of HF in CS involves both IST and general management of HF. HF management follows guideline-directed medical therapy (GDMT) as laid out in the 2022 update to the AHA/ACC/HFSA guidelines [98]. Specific features of GDMT will not be reviewed in this article, though all individuals with CS should be stratified by AHA/ACC stage, and managed according to guideline recommendations.
Follow-up Evaluation
Clinical follow-up is critical in CS, though data is limited in regards to duration of therapy or appropriate weaning strategies. FDG-PET can assess response to IST [55], though optimal timing for repeat imaging remains uncertain. In one review, repeat FDG-PET is suggested at 3 months after initiation of therapy followed by 3 months after cessation of IST. If imaging was improved, individuals entered a schedule of surveillance EKG and TTE, and any new abnormality prompted repeat FDG-PET [99]. Furthermore, close clinical surveillance is important in individuals who are weaned off IST. A retrospective study following individuals on IST found 88% of individuals who discontinued IST later developed radiologic evidence of active disease at an average of 8.4 months after discontinuation [100].
Our Approach to Management of CS
Our approach to management of CS is summarized in Fig. 2. We broadly separate initial treatment choices into steroid monotherapy and steroid plus steroid-sparing agent arms. Based on the literature to date, we feel either arm is appropriate and should be individualized based on potential risks associated with prolonged corticosteroid use as well as provider-patient shared-decision making. The results from the ongoing CHASM CS-RCT may help clarify initial treatment selection. Individuals should be regularly reassessed for ICD placement in both arms. Additionally, HF and arrhythmias should be managed per AHA/ACC guidelines and are not specifically addressed in our algorithm. Individuals with evidence of active CS on CMR or FDG-PET or have high-risk features are treated with IST. Initial evaluation for ICD occurs at the time of starting IST. Individuals with active CS treated with steroid monotherapy receive an initial dose of 0.5 mg/kg/day or a maximum daily dose of 30–40 mg prednisone or equivalent drug. This is continued for an induction period of 4 weeks and then gradually tapered to a maintenance dose of 5–10 mg/day. Repeat FDG-PET is performed at 3–6 months. In individuals with resolution of FDG uptake and favorable clinical response to IST, further weaning and discontinuation of steroids over additional 12–18 months is reasonable, with close clinical surveillance and repeat advanced imaging if suggestion of worsening disease. Unchanged FDG uptake warrants returning to the initial dose of steroid and consideration of methotrexate or alternative steroid-sparing agent. Increased FDG uptake or a clinical picture suggestive of disease progression is managed by increasing corticosteroid dose and initiating methotrexate simultaneously. In patients with presumed ICS and low-moderate diagnostic confidence, we will often preferentially start with steroid monotherapy and repeat FDG-PET to evaluate if the abnormal uptake is steroid responsive rather than starting multiple IST agents, though this is an institutional practice without significant supporting data. Our general institutional practice is to start those with active CS on an initial regimen of corticosteroid with simultaneous initiation of a steroid-sparing agent. Methotrexate is usually considered first-line unless there is a contraindication. In this approach, initial corticosteroid dose is 0.5 mg/kg/day or a maximum daily dose of 30–40 mg of prednisone, in combination with methotrexate 5–10 mg/week. Methotrexate dose is increased by 2.5 mg every 2 weeks, and once at a maintenance dose of 15–20 mg/week, the corticosteroid is weaned. This titration schedule provides a 4-week induction phase of corticosteroids, consistent with JCS guidelines. The difference in this approach is that prednisone is weaned more rapidly; we suggest 5 mg/week. At 3–6 months, repeat FDG-PET is repeated. In individuals with resolution of radiographic abnormalities and good clinical response, we recommend continuing steroid-sparing agent for total 12–18 months. Persistent and unchanged radiographic disease usually prompts addition of TNF-α inhibitor while maintaining the current corticosteroid dose. If FDG-PET or clinical response suggests disease progression, then corticosteroids are increased to initial dose, and a TNF-α inhibitor is added.
Conclusion
This review aims to summarize the growing body of evidence surrounding diagnostic and therapeutic options in CS, and to present a streamlined clinical approach to these individuals. Our review highlights the superiority of advanced imaging modalities both in establishing a diagnosis and guiding therapy. The role of steroid-sparing agents and biologic drugs is shifting the treatment paradigm of CS. Despite advances, there remain unanswered clinical questions for the best diagnostic approaches, initial treatment strategies, follow-up, and titration of treatments. More robust data will provide key information which will ultimately benefit individuals with CS.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Rivera NV, et al. High-density genetic mapping identifies new susceptibility variants in sarcoidosis phenotypes and shows genomic-driven phenotypic differences. Am J Respir Crit Care Med. 2016;193(9):1008–22.
Grunewald J, et al. Immunogenetics of disease-causing inflammation in sarcoidosis. Clin Rev Allergy Immunol. 2015;49(1):19–35.
Newman LS, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170(12):1324–30.
Cozier YC, et al. Sarcoidosis in black women in the United States: data from the Black Women’s Health Study. Chest. 2011;139(1):144–50.
Rybicki BA, et al. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145(3):234–41.
Arkema EV, Cozier YC. Sarcoidosis epidemiology: recent estimates of incidence, prevalence and risk factors. Curr Opin Pulm Med. 2020;26(5):527–34.
Sève P, et al. Sarcoidosis: a clinical overview from symptoms to diagnosis. Cells. 2021;10(4).
Cacoub P, et al. Cardiac sarcoidosis: a long term follow up study. PLoS One. 2020;15(9):e0238391.
Hamzeh N, et al. Pathophysiology and clinical management of cardiac sarcoidosis. Nat Rev Cardiol. 2015;12(5):278–88.
Uusimaa P, et al. Ventricular tachyarrhythmia as a primary presentation of sarcoidosis. Europace. 2008;10(6):760–6.
•• Birnie DH, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11(7):305–23. HRS Guidelines for cardiac sarcoidosis.
Webb M, et al. Cardiac involvement in sarcoidosis deaths in Wayne County, Michigan: a 20-year retrospective study. Acad Forensic Pathol. 2018;8(3):718–28.
Iwai K, et al. Pathological studies on sarcoidosis autopsy. I. Epidemiological features of 320 cases in Japan. Acta Pathol Jpn. 1993;43(7–8):372–6.
Matsui Y, et al. Clinicopathological study of fatal myocardial sarcoidosis. Ann N Y Acad Sci. 1976;278:455–69.
Iwai K, et al. Racial difference in cardiac sarcoidosis incidence observed at autopsy. Sarcoidosis. 1994;11(1):26–31.
Okada DR, et al. Isolated cardiac sarcoidosis: a focused review of an under-recognized entity. J Nucl Cardiol. 2018;25(4):1136–46.
•• Terasaki F, et al. JCS 2016 guideline on diagnosis and treatment of cardiac sarcoidosis - digest version. Circ J, 2019;83(11):2329–2388. JCS Guidelines for cardiac sarcoidosis.
Judson MA, et al. The WASOG Sarcoidosis Organ Assessment Instrument: an update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31(1):19–27.
Nagao S, et al. Electrocardiographic abnormalities and risk of developing cardiac events in extracardiac sarcoidosis. Int J Cardiol. 2015;189:1–5.
Crouser ED, et al. Diagnosis and detection of sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;201(8):e26–e51.
Ohira H, et al. Underdiagnosis of cardiac sarcoidosis by ECG and echocardiography in cases of extracardiac sarcoidosis. ERJ Open Res. 2022;8(2).
Kurmann R, Mankad SV, Mankad R. Echocardiography in Sarcoidosis. Curr Cardiol Rep. 2018;20(11):118.
Darlington P, et al. Diagnostic approach for cardiac involvement in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2019;36(1):11–7.
Di Stefano C, et al. Diagnostic and predictive value of speckle tracking echocardiography in cardiac sarcoidosis. BMC Cardiovasc Disord. 2020;20(1):21.
Schouver ED, et al. Early detection of cardiac involvement in sarcoidosis with 2-dimensional speckle-tracking echocardiography. Int J Cardiol. 2017;227:711–6.
Bera D, et al. Serum angiotensin converting enzyme, erythrocyte sedimentation rate and high sensitive-C reactive protein levels in diagnosis of cardiac sarcoidosis- where do we stand? Indian Pacing Electrophysiol J. 2020;20(5):184–8.
Handa T, et al. Significance of plasma NT-proBNP levels as a biomarker in the assessment of cardiac involvement and pulmonary hypertension in patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2010;27(1):27–35.
Kiko T, et al. A multiple biomarker approach in patients with cardiac sarcoidosis. Int Heart J. 2018;59(5):996–1001.
Kobayashi Y, et al. Association of high serum soluble interleukin 2 receptor levels with risk of adverse events in cardiac sarcoidosis. ESC Heart Fail. 2021;8(6):5282–92.
Kolluri N, et al. Routine laboratory biomarkers as prognostic indicators of cardiac sarcoidosis outcomes. Sarcoidosis Vasc Diffuse Lung Dis. 2022;39(3):e2022023.
Markatis E, et al. Cardiac sarcoidosis: diagnosis and management. Rev Cardiovasc Med. 2020;21(3):321–38.
Freeman AM, et al. Predictors of cardiac sarcoidosis using commonly available cardiac studies. Am J Cardiol. 2013;112(2):280–5.
Mehta D, et al. Cardiac involvement in patients with sarcoidosis: diagnostic and prognostic value of outpatient testing. Chest. 2008;133(6):1426–35.
Padala SK, et al. Impact of early initiation of corticosteroid therapy on cardiac function and rhythm in patients with cardiac sarcoidosis. Int J Cardiol. 2017;227:565–70.
Elwazir MY, Bois JP, Chareonthaitawee P. Utilization of cardiac imaging in sarcoidosis. Expert Rev Cardiovasc Ther. 2022;20(4):253–66.
Smedema JP, et al. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol. 2005;45(10):1683–90.
•• Greulich S, et al. Hybrid cardiac magnetic resonance/fluorodeoxyglucose positron emission tomography to differentiate active from chronic cardiac sarcoidosis. JACC Cardiovasc Imaging. 2022;15(3):445–456. Study showing diagnostic utility in hybrid CMR/FDG-PET in evaluation of cardiac sarcoidosis.
Aitken M, et al. Diagnostic accuracy of cardiac MRI versus FDG PET for cardiac sarcoidosis: a systematic review and meta-analysis. Radiology. 2022;304(3):566–79.
Greulich S, et al. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging. 2013;6(4):501–11.
Yoshida A, et al. Direct comparison of the diagnostic capability of cardiac magnetic resonance and endomyocardial biopsy in patients with heart failure. Eur J Heart Fail. 2013;15(2):166–75.
Kouranos V, et al. Complementary role of CMR to conventional screening in the diagnosis and prognosis of cardiac sarcoidosis. JACC Cardiovasc Imaging. 2017;10(12):1437–47.
Sobol I, et al. Assessment of unexplained cardiomyopathy: clinical utility of delayed-enhancement cardiac magnetic resonance compared to endomyocardial biopsy. J Am Coll Cardiol. 2012;59(13, Supplement):E1553.
Flamée L, et al. Prognostic value of cardiovascular magnetic resonance in patients with biopsy-proven systemic sarcoidosis. Eur Radiol. 2020;30(7):3702–10.
Coleman GC, et al. Prognostic value of myocardial scarring on CMR in patients with cardiac sarcoidosis. JACC Cardiovasc Imaging. 2017;10(4):411–20.
Hulten E, et al. Presence of late gadolinium enhancement by cardiac magnetic resonance among patients with suspected cardiac sarcoidosis is associated with adverse cardiovascular prognosis: a systematic review and meta-analysis. Circ Cardiovasc Imaging. 2016;9(9):e005001.
Crouser ED, et al. Improved detection of cardiac sarcoidosis using magnetic resonance with myocardial T2 mapping. Am J Respir Crit Care Med. 2014;189(1):109–12.
Greulich S, et al. Comprehensive cardiovascular magnetic resonance assessment in patients with sarcoidosis and preserved left ventricular ejection fraction. Circ Cardiovasc Imaging. 2016;9(11).
Dill T. Contraindications to magnetic resonance imaging. Heart. 2008;94(7):943–8.
Sobic-Saranovic D, Artiko V, Obradovic V. FDG PET imaging in sarcoidosis. Semin Nucl Med. 2013;43(6):404–11.
Soussan M, et al. Functional imaging in extrapulmonary sarcoidosis: FDG-PET/CT and MR features. Clin Nucl Med. 2014;39(2):e146–59.
Agrawal T, et al. Diagnosis of cardiac sarcoidosis: a primer for non-imagers. Heart Fail Rev. 2022;27(4):1223–33.
Kim SJ, Pak K, Kim K. Diagnostic performance of F-18 FDG PET for detection of cardiac sarcoidosis; a systematic review and meta-analysis. J Nucl Cardiol. 2020;27(6):2103–15.
Youssef G, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med. 2012;53(2):241–8.
Bekki M, et al. Localization of myocardial FDG uptake for prognostic risk stratification in corticosteroid-naïve cardiac sarcoidosis. J Nucl Cardiol. 2022;29(5):2132–44.
Ning N, et al. Serial cardiac FDG-PET for the diagnosis and therapeutic guidance of patients with cardiac sarcoidosis. J Card Fail. 2019;25(4):307–11.
Coulden RA, et al. Utility of FDG PET and cardiac MRI in diagnosis and monitoring of immunosuppressive treatment in cardiac sarcoidosis. Radiol Cardiothorac Imaging. 2020;2(4):e190140.
Saric P, et al. PET imaging in cardiac sarcoidosis: a narrative review with focus on novel PET tracers. Pharmaceuticals (Basel). 2021;14(12).
Christopoulos G, et al. Suppressing physiologic 18-fluorodeoxyglucose uptake in patients undergoing positron emission tomography for cardiac sarcoidosis: the effect of a structured patient preparation protocol. J Nucl Cardiol. 2021;28(2):661–71.
Osborne MT, et al. Patient preparation for cardiac fluorine-18 fluorodeoxyglucose positron emission tomography imaging of inflammation. J Nucl Cardiol. 2017;24(1):86–99.
Quinn B, et al. Radiation dosimetry of 18F-FDG PET/CT: incorporating exam-specific parameters in dose estimates. BMC Med Imaging. 2016;16(1):41.
Divakaran S, et al. Diagnostic accuracy of advanced imaging in cardiac sarcoidosis. Circ Cardiovasc Imaging. 2019;12(6):e008975.
Dweck MR, et al. Hybrid magnetic resonance imaging and positron emission tomography with fluorodeoxyglucose to diagnose active cardiac sarcoidosis. JACC Cardiovasc Imaging. 2018;11(1):94–107.
Trivieri MG, et al. Challenges in cardiac and pulmonary sarcoidosis: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76(16):1878–901.
Ramirez R, et al. Advanced imaging in cardiac sarcoidosis. J Nucl Med. 2019;60(7):892–8.
Subramanian K, et al. Access to cardiac PET/CT by sarcoidosis patients and cost-effectiveness analysis of cardiac PET/MR compared to the standard of care. Clin Imaging. 2023;94:50–5.
Ezzeddine FM, et al. Electrogram-guided endomyocardial biopsy yield in patients with suspected cardiac sarcoidosis and relation to outcomes. J Cardiovasc Electrophysiol. 2021;32(9):2486–95.
Yilmaz A, et al. Comparative evaluation of left and right ventricular endomyocardial biopsy: differences in complication rate and diagnostic performance. Circulation. 2010;122(9):900–9.
Rosario KF, et al. Cardiac sarcoidosis: current approaches to diagnosis and management. Curr Allergy Asthma Rep. 2022;22(12):171–82.
Fazelpour S, et al. Corticosteroid and immunosuppressant therapy for cardiac sarcoidosis: a systematic review. J Am Heart Assoc. 2021;10(17).e021183.
Stievenart J, et al. Cardiac sarcoidosis: systematic review of the literature on corticosteroid and immunosuppressive therapies. Eur Respir J. 2022;59(5).
Subramanian M, et al. Pre-treatment myocardial (18)FDG uptake predicts response to immunosuppression in patients with cardiac sarcoidosis. JACC Cardiovasc Imaging. 2021;14(10):2008–16.
Yazaki Y, et al. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88(9):1006–10.
Griffin JM, et al. Management of cardiac sarcoidosis using mycophenolate mofetil as a steroid-sparing agent. J Card Fail. 2021;27(12):1348–58.
Gallegos C, et al. Non-steroidal treatment of cardiac sarcoidosis: a systematic review. Int J Cardiol Heart Vasc. 2021;34:100782.
•• Vis R, et al. Prednisone vs methotrexate in treatment naïve cardiac sarcoidosis. J Nucl Cardiol. 2023. Study comparing prednisone, methotrexate, or combination therapy showing effective suppression of FDG-PET imaging in all groups with no significant difference in clinical outcomes. Supports use of prednisone plus methotrexate or methotrexate monotherapy in treatment of CS.
Nagai S, et al. Treatment with methotrexate and low-dose corticosteroids in sarcoidosis patients with cardiac lesions. Intern Med. 2014;53(23):2761.
•• Birnie D, et al. Cardiac sarcoidosis multi-center randomized controlled trial (CHASM CS- RCT). Am Heart J. 2020;220:246–252. Ongoing multicenter randomized clinical trial comparing prednisone and prednisone/methotrexate combination therapy as initial treatment in CS.
Page RL 2nd, et al. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134(6):e32–69.
Bakker ALM, et al. Effectiveness and safety of infliximab in cardiac sarcoidosis. Int J Cardiol. 2021;330:179–85.
Judson MA, et al. Outcomes of prednisone-tapering regimens for cardiac sarcoidosis: a retrospective analysis demonstrating a benefit of infliximab. Respir Med. 2022;203:107004.
Gilotra NA, et al. Clinical and imaging response to tumor necrosis factor alpha inhibitors in treatment of cardiac sarcoidosis: a multicenter experience. J Card Fail. 2021;27(1):83–91.
•• Baker MC, et al. TNF-alpha inhibition for the treatment of cardiac sarcoidosis. Semin Arthritis Rheum. 2020;50(3):546–552. Retrospective study showing effective treatment of CS with TNF-alpha inhibitors with good cardiac safety profile.
Krause ML, et al. Successful use of rituximab in refractory cardiac sarcoidosis. Rheumatology (Oxford). 2016;55(1):189–91.
Elwazir M, et al. Rituximab for the treatment of refractory cardiac sarcoidosis: a single-center experience. J Card Fail. 2022;28(2):247–58.
Sekhri V, et al. Cardiac sarcoidosis: a comprehensive review. Arch Med Sci. 2011;7(4):546–54.
Sadek MM, et al. Corticosteroid therapy for cardiac sarcoidosis: a systematic review. Can J Cardiol. 2013;29(9):1034–41.
Takaya Y, et al. Outcomes in patients with high-degree atrioventricular block as the initial manifestation of cardiac sarcoidosis. Am J Cardiol. 2015;115(4):505–9.
Al-Khatib SM, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018;15(10):e190–252.
Desai R, et al. The burden of cardiac arrhythmias in sarcoidosis: a population-based inpatient analysis. Ann Transl Med. 2018;6(17):330.
Viles-Gonzalez JF, et al. Supraventricular arrhythmias in patients with cardiac sarcoidosis prevalence, predictors, and clinical implications. Chest. 2013;143(4):1085–90.
Locke AH, et al. Arrhythmia in cardiac sarcoidosis. Cardiol Rev. 2021;29(3):131–42.
Muser D, et al. Characterization of the electroanatomic substrate in cardiac sarcoidosis: correlation with imaging findings of scar and inflammation. JACC Clin Electrophysiol. 2018;4(3):291–303.
Weng W, et al. Atrial arrhythmias in clinically manifest cardiac sarcoidosis: incidence, burden, predictors, and outcomes. J Am Heart Assoc. 2020;9(17):e017086.
Zipse MM, Sauer WH. Cardiac sarcoidosis and consequent arrhythmias. Card Electrophysiol Clin. 2015;7(2):235–49.
Yodogawa K, et al. Effect of corticosteroid therapy on ventricular arrhythmias in patients with cardiac sarcoidosis. Ann Noninvasive Electrocardiol. 2011;16(2):140–7.
Adhaduk M, et al. The role of electrophysiology study in risk stratification of cardiac sarcoidosis patients: meta-analyses and systemic review. Int J Cardiol. 2022;349:55–61.
Kron J, et al. Efficacy and safety of implantable cardiac defibrillators for treatment of ventricular arrhythmias in patients with cardiac sarcoidosis. Europace. 2013;15(3):347–54.
AHA/ACC/HFSA Guideline for the Management of Heart Failure. J Card Fail. 2022;28(5):e1–167.
Birnie DH, et al. Cardiac sarcoidosis. J Am Coll Cardiol. 2016;68(4):411–21.
Rosenthal DG, et al. Long-term corticosteroid-sparing immunosuppression for cardiac sarcoidosis. J Am Heart Assoc. 2019;8(18):e010952.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shaver, A., Schwartz, A., Bhatt, K. et al. Diagnostic Approach and Management of Cardiac Sarcoidosis. Curr Pulmonol Rep 12, 70–79 (2023). https://doi.org/10.1007/s13665-023-00309-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13665-023-00309-w