Abstract
The diagnosis and management of nontuberculous mycobacterial (NTM) lung infection remains difficult even among experienced clinicians. Both the incidence and prevalence are likely expected to rise with an aging population. Careful assessment of clinical, radiologic, and microbiologic studies is warranted before initiating therapy, which is often complicated by significant drug side effects and limited sputum conversion rates. Indications to treat are best based on a number of factors, including specific NTM species, NTM lung-infection–associated symptoms, advanced bronchiectatic or cavitary disease, expected tolerance of therapy, and related comorbidities. Surgical resection of localized disease or refractory infection may warrant consideration, but should be done by an experienced surgeon and mycobacterial program. Increased awareness and treatment of NTM lung infection in recent years is likely due to earlier recognition and improved treatment strategies, though the approach to the NTM lung infection patient most often involves chronic lung disease management. NTM lung infection is perhaps is best characterized as a treatable lung infection, even without universally curable outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nontuberculous mycobacterium lung infection remains a challenge to even the most experienced clinicians in terms of diagnostic and therapeutic management [1]. With an increased awareness of NTM lung infections and its characteristic chronicity, incidence and prevalence continue to rise, particularly with an aging population. Its association with chronic lung diseases such as emphysema, cystic fibrosis, and bronchiectasis further complicates its diagnosis and management. This article is an overview of current and recent advances in the diagnosis and management of NTM lung infection in immunocompetent patients, including indications for treatment, approaches to advanced or resistant pathogens, and associated long-term morbidity and mortality.
Epidemiology
The majority of nontuberculous mycobacteria (NTM) causing human lung disease in the United States (US) are comprised of three commonly presenting groups; 1) mycobacterium avium complex (MAC) consisting of mycobacterium intracellulare and mycobacterium avium; 2) the rapidly growing mycobacteria (RGM) (M. abscessus, M. fortuitum, and M. chelonae); and 3) M. kansasii. It should also be noted, however, that over 140 species of NTM have been reported and that geographic differences are substantial as to which NTM species are most likely to cause infection worldwide [2•]. Clinicians who evaluate and treat NTM lung infections should be familiar with potential NTM species that are likely to cause infection based on local, regional, and national epidemiologic trends. Found ubiquitously in water and soil, infection has been associated with exposure to tap water [3], as well as biofilm-containing elements such as shower heads, humidifiers, or saunas [4], the latter of which may also rarely provoke a type IV allergic hypersensitivity response known as ‘hot tub lung’ [5, 6]. Currently not considered a classic mycobacterial infection, NTM-associated hypersensitivity pneumonitis is characterized by poorly formed airway-centric granulomas on pathology, with frequent clinical improvement after removal from antigen exposure [7].
A recent Japanese case-controlled study suggested that immunocompetent MAC lung infected individuals had increased exposure to soil when compared to bronchiectatic controls, while other studies have not demonstrated an association with specific water exposure or social habits, including swimming or gardening [8, 9]. Despite such associations, little conclusive evidence is available regarding preventative environmental interventions and the reduction in risk of development of or recurrence of NTM lung infection [10, 11]. Some investigators have advocated for environmental modifications, including raising the temperature of hot water heaters to greater than 130 degrees Fahrenheit, replacing shower heads with large water droplet (>1 mm) rather than fine mist heads, moistening peat moss when working with gardening supplies to minimize dust exposure, and a variety of other household measures to potentially minimize exposure [11].
The incidence and prevalence of NTM infection appear to be increasing over the last decade or more, with the prevalence of NTM now greater than tuberculosis in the United States. Prevalences range from 1.4 to 6.6 per 100,000 [12] to 8.6 per 100,000, with increased prevalence in those aged greater than 50 years (20.4/100,000) [13]. A recent analysis of US Medicare beneficiaries aged 65 and older suggested an annual prevalence of 20–47 cases per 100,000, or 8.2 % per year increase in both women and men throughout all regions of the US [14•]. Recent studies from Asia [15–17] and Europe [2•] also suggest an increasing incidence and prevalence, with MAC [15] remaining the most frequent species in many areas of the world [2•]. The significance of recent findings by Dutch investigators noting 22 % of presenting individuals with sputum analysis in the setting of acute chronic obstructive pulmonary disease (COPD) exacerbation with a positive NTM culture remains unclear. [18]. A prior study evaluating the infectious etiology in COPD patients hospitalized with acute exacerbation noted lower frequency of NTM positive sputum, but applied only solid culture media [19]. The association of NTM lung infection with bronchiectasis [20] and cystic fibrosis (CF) is well known [21–23], and in patients with diffuse tree-in-bud or nodular infiltrates in the setting of CF, progressive lung deterioration may occur [24]. The incidence of NTM lung infection in patients with CF, which most often occurs in patients older than 15 years of age and increases with age, has been estimated to be between 13 and 20 % [1, 22, 25]. In a selected cohort of bronchiectasis patients, NTM was cultured in 10 % of patients [26], which, interestingly, is also similar to a 10 % isolation rate of NTM in primary ciliary dyskinesia patients [1]. One study has also suggested increased NTM prevalence among those with gastroesophageal reflux disease on proton pump inhibitor therapy [27]. Pre-existing structural lung disease as that seen with emphysema, CF, or bronchiectasis has been associated with NTM lung infection [28].
Most patients with NTM infection have bronchiectasis, while many patients with primary bronchiectasis do not have NTM lung infection. Whether NTM lung infection or bronchiectasis comes first in those without pre-existing lung disease as a “chicken or egg” scenario is uncertain. In any case, it is likely that there are many phenotypic pathways that lead to the development of bronchiectasis with or without NTM lung infection [29•]. Finally, in patients with chronic immunosuppression or immunocompromised states, NTM may manifest not only with lung infection, but also disseminated infection, as seen in infection associated with HIV/AIDS [30], transplant patients [31], or those on biologics (e.g., TNF-antagonists) [32].
The economic burden of managing NTM was recently assessed and found to be substantial, with a median cost of $19,786 per patient, including significant drug side effects seen in 50 % of patients receiving commonly used regimens treated over a median of 2,638 days [33].
Clinical presentation
Clinical symptoms of NTM lung infections vary and are nonspecific, but may include cough, fatigue, dyspnea, and weight loss [9, 34]. Low-grade fever and night sweats may also be noted, along with hemoptysis. Other associations have included mitral valve prolapse, pectus excavatum, and scoliosis [9]. Fulminant or acute lung injury resulting in respiratory failure or septicemia requiring hospitalization may occasionally occur as a first presentation of infection, although this remains rare [35]. Patients may also be asymptomatic, despite multiple positive sputum cultures with abnormal radiologic findings.
As NTM are found ubiquitously in the environment, specific host factors importantly play a pivotal role in increasing risk of infection [36]. Recent studies have supported a distinct phenotype of MAC lung infection patients, represented by taller and thinner postmenopausal women without pre-existing lung disease [9, 37••]. Abnormal immune phenotypes in these patients have been less consistently demonstrated. Specifically, measured serum levels of leptin and adiponectin involved in the regulation of body fat and cytokine production (IFN-γ and IL-10) were notably abnormal in those with NTM disease compared to non-infected controls [37••]. However, patients with NTM infection had no direct relationship between serum leptin levels and body fat, with decreased IFN-γ production and increased IL-10 levels measured by stimulated whole blood assays suggesting a blunted inflammatory response. In contrast, similar immune abnormalities were not observed when IFN-γ production was measure by stimulated peripheral blood monocytes in MAC lung-infected patients [9]. Patients were, however, thinner overall prior to infection, suggesting this as a possible risk factor for disease rather than infection causing subsequent weight loss [9]. Implications for this morphotypic profile in terms of prospective risk of developing disease and likelihood of relapse among treated patients is currently unknown, but remains an area under active investigation, including studies involving genetic mutations of fibrillin and related moieties [38].
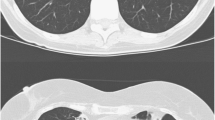
Radiologic patterns of disease are characterized by computed tomography (CT) as nodular or tree-in-bud infiltrates without underlying structural disease, bronchiectatic disease with focal or diffuse cylindrical bronchiectasis, or fibrocavitary disease often the result of prior emphysema or pre-existing structural abnormality. Cylindrical bronchiectasis with nodular infiltrates in the right middle lobe or lingula are characteristic of the presentation of NTM lung infection in phenotypically taller, thinner post-menopausal women historically labelled as having Lady Windemere’s syndrome. [39–41]. Recent data supports the long-standing clinical observation correlating more advanced infection or chronic fibrocavitary radiologic findings with worse prognosis and less response to therapy [42], as well as worse clinical scores and quality of life measures [43].
Diagnostic criteria
The 2007 American Thoracic Society / Infectious Disease Society of America (ATS/IDSA) guidelines proposed the following criteria for the diagnosis of nontuberculous mycobacterium lung infection: 1) Positive AFB culture sputum from at least two separate sputum samples, or one bronchoscopic washing or lavage; 2) Clinical symptoms (cough, dyspnea, fatigue, hemoptysis, fever, or unintended weight loss) and nodular or cavitary opacities or multifocal bronchiectasis on radiographic imaging; and 3) exclusion of other responsible or causative diagnoses [1]. Initial fluorochrome or acid fast staining of both fluid and tissue specimens with semiquantitative scaling of visible organisms per high power field is recommended when available. It should be emphasized that a prerequisite of microbiologic assessment of NTM lung infection is identification of organisms at the species level, prior to committing to a treatment program [1]. Likewise, abnormal microbiologic findings such as positive sputum or bronchoscopic specimen mycobacterial cultures in the setting of a lack of symptoms and radiologic abnormalities may not represent NTM lung infection. Respiratory symptoms and positive microbiologic studies in the absence of radiologic findings may also suggest other causes for current presentation, though no criteria exist in this setting to exclude potential infection, particularly if initial smear or culture burden is low. In such cases, repeat imaging and close follow-up are warranted. Isolation of a single NTM isolate without supporting criteria or a presumptive diagnosis based only on clinical and radiologic features is inappropriate for initiation of treatment [1].
The development more recently of a specific IgA serodiagnostic test for MAC lung infection demonstrated a strong specificity of 94 % and modest sensitivity ranging between 70 % and 82 % [44]. How this serologic diagnostic tool will impact clinical decision making and NTM lung infections remains to be determined.
General treatment considerations
Despite isolation of NTM organisms from multiple respiratory specimens, particularly among those with structural lung disease, a clear diagnosis of infection and assignment of respiratory symptoms to NTM lung infection are often confounded by concomitant respiratory disease such as COPD or bronchiectasis. In this setting, correlation of microbiologic data such as positive AFB smear or culture results with nonspecific clinical or radiologic features may be difficult. It is not uncommon for the clinician to require longitudinal follow-up and /or assessment of response to empiric therapy of underlying pre-existing lung disease to further assist in determining whether presenting symptoms are related to NTM lung infection or another process. In contrast, less common NTM species that usually represent infection if present include M. xenopi, M. szulgai, and M. kansasii. If these NTM are present in respiratory secretions with appropriate radiologic and clinical findings, directed therapy for NTM lung infection is often warranted. Geographic variability also impacts the likelihood of a specific NTM species causing NTM lung infection. For example, M kansasii in the US frequently represents NTM lung infection when isolated in respiratory secretions, whereas it is a less common pathogenic isolate when isolated in select areas of Western Europe [1, 45]. Other species such as M. fortuitum are uncommonly associated with significant NTM lung infection, although M. fortuitum is frequently associated with esophageal disease, which should be evaluated accordingly [46]. NTM species such M. terrae and M. gordonae generally do not cause lung infection.
Some patients may initially be relatively asymptomatic, despite concerning microbiologic burden or notable radiologic findings. In the setting of upper lobe cavitary disease, timely definitive specific identification of the responsible infection and initiation of directed therapy is recommended rather than delayed observation, even in the asymptomatic patient [47]. Other patients with less extensive disease may have minimal progression over time, reflecting the variability of the natural history of NTM lung infection. In the balance, the decision to treat often becomes difficult, as treatment is complex and prolonged over many months with a substantial proportion of patients experiencing medication-related side effects. Relapse may occur in up to 50 % of patients within 2 years of treatment completion [1]. Even among a range of clinicians, expert and non-expert opinion regarding diagnosis and aggressiveness of therapy varies. Non-experts are less likely to perceive microbiologic findings as significant infection or see opportunity for successful therapy [48]. This is presumably based on a biased understanding that prolonged courses of antibiotics often result in cumulative effects of drug toxicity and related symptoms adding to the risk of poor compliance. Patients should fully participate in this decision to treat or not to treat, being mindful of the balance of risks and benefits of treatment, as well as expectations of goals of therapy. A diagnosis of NTM lung infection does not in itself dictate the immediate start of treatment, although patients with known or suspected NTM lung infection must be monitored closely and reassessed proportionately to extent of disease and potential goals of therapy.
Treatment
MAC
Treatment of MAC lung infection involves macrolide-centered therapy, consisting of a macrolide, a rifamycin, and ethambutol with or without an aminoglycoside [1]. Differentiating M. avium from M. intracellular has not clearly resulted in different treatment responses, although a recent report suggests patients with M. intracellular had more severe clinical presentations and less response to therapy [49]. Susceptibility testing of MAC is only recommended for macrolides, given evidence that in vitro and in vivo correlation with other antimycobacterial agents is poor [50]. Reasons for this discordance remain complex, and are an area under active investigation [51]. A standard starting regimen for mild MAC lung infection consists of azithromycin (500 mg/day) or clarithromycin (1000 mg/day), ethambutol (25 mg/kg daily), and rifampin (600 mg daily), given in an intermittent fashion three times a week [1]. Additional dose adjustments for patients weighing less than 50 kg may be needed with the macrolide and rifamycin. Daily treatment is recommended in MAC lung infection patients with more advanced or diffuse bronchiectasis and fibrocavitary disease. In this situation, triple drug mycobacterial therapy includes azithromycin 250 mg daily or clarithromycin 500 mg twice daily; ethambutol 15 mg/kg daily, and rifampin 600 mg daily[1]. Finally, in those with more extensive infection, consideration may be given to the use of adjunctive intravenous amikacin (25 mg/kg three times a week) initiated for the first two to four months of therapy. The role of inhaled amikacin in the initial and / or maintenance treatment regimens remain uncertain, although recent preliminary data presented in abstract form suggests substantial potential [52, 53].
A recent retrospective analysis of 180 patients with nodular/bronchiectatic disease undergoing the recommended ATS/IDSA-guided macrolide-based therapy reported an overall sputum conversion rate of 86 %, including the use of a better-tolerated, thrice weekly regimen [54•]. Monotherapy with macrolides is not recommended, with limited evidence for efficacy using macrolide combination therapy with fluoroquinolones [55], which may also increase macrolide resistance when used as a single first-line therapy [56]. Given this increased risk for the development of macrolide resistance, the combination of macrolide and quinolone should be avoided [56]. The role of clofazimine as adjunctive therapy is equally unclear, with limited data available [57, 58].
The frequent occurrence of drug intolerances can be addressed in part by initiating medications in a step-wise fashion so as to improve tolerance. Our practice is to initiate treatment with one oral MAC medication at a time, starting with a lower dose and sequentially and incrementally increasing to full dosing of all medications over a 2–3 week period. Close follow-up and monitoring with blood work and eye exams are recommended to ensure safety, tolerance, and patient compliance. The impact of the use of probiotics for drug tolerance during treatment of mycobacterial infection has not been studied, but is a common practice. Routine chest computed tomography (CT) scans during therapy are generally not recommended. Plain chest radiographs done on a 6–12 month basis provide adequate imaging information. A ‘new’ baseline chest CT imaging at the end of mycobacterial therapy may be useful as a point of reference, although there is no data to support this practice. Support and assistance delivered by a multidisciplinary team to facilitate management and monitoring of multidrug regimens for NTM lung infection, including pharmacy, nursing, and respiratory therapy, have been well received by patients. The general endpoint of successful MAC therapy is 12 months of sustained negative sputum culture. Sputum conversion, when it does occur, generally occurs after a period of no more than 3–6 months, assuming smear positive fibrocavitary disease is not present. Follow-up monthly sputum samples are generally collected starting after 2–3 months of therapy. As the duration of therapy is targeted for at least 12 months from the first of the sustained negative sputum culture assessments, most patients receive between 15 and 18 months of therapy. Patients with more extensive disease or fibrocavitary disease often require 18 to 24 months or longer of therapy; some of these patients never convert sputum cultures. Refractory disease is defined by failure to convert AFB sputum culture after receiving 6 months of recommended mycobacterial therapy. Although reasons for lack of conversion may include incomplete or suboptimal therapy due to patient non-compliance or medication intolerance, some MAC patients do not convert sputum cultures even without extensive or fibrocavitary disease. Repeat macrolide susceptibility should be performed on isolates from patients failing to convert sputum, to ensure the absence of development of macrolide resistance. If sputum conversion does not occur, transitioning from thrice weekly therapy to daily therapy may be appropriate, along with substitution of rifampin with rifabutin, which has less p450 cytochrome activation [59] and therefore less potential to reduce macrolide plasma concentrations. Care should be taken to appropriately dose rifabutin if a change from rifampin is made. Therapeutic drug monitoring has continued to generate interest for its potential role in establishing antimicrobial efficacy and drug modification. However, Koh and colleagues recently reviewed therapeutic drug levels of patients with MAC treated with combined therapy, and concluded that although lower levels of clarithromycin plasma concentrations were seen when given with rifampin, there was no association of lower plasma concentrations with worse treatment outcome [60]. In contrast, pharmacokinetic assessment of general regimens used to treat MAC as reviewed by van Ingen et al. suggested lower serum levels than expected for the bactericidal activity of several agents, including clarithromycin, azithromycin, and ethambutol; this deficiency was proposed as a mechanism for less than desired outcomes and prolonged therapeutic regimens with MAC treatment [61]. Macrolide resistant disease is treated with the addition of a parenteral agent (usually amikacin) added to a rifamycin and ethambutol. Although cessation of macrolide is historically done, there is no data in this situation to evaluate any potential benefit of continued macrolide use as an immunomodulatory agent. Treatment failure defined by lack of sputum conversion is highest in those with greater microbiologic burden, structural lung disease such as cavitation, previous use of macrolide monotherapy, and those in whom there is relapse or recurrence.
If focal disease is noted, the combination of a multidrug mycobacterial regimen in conjunction with surgical resection may yield the best opportunity for sustained sputum conversion. Surgical resection is often recommended in localized cavitary or bronchiectatic disease, and is often initiated after an attempt at optimal medical therapy (at least 3 to 6 months) [62]. Some centers recommend earlier assessment for surgical consideration in select patients. Medical therapy is required when surgical options are explored, and is generally started prior to surgery, continued in the perioperative period, and maintained postoperatively. Selection criteria for surgical resection have not been standardized and final recommendations toward eligibility for a surgical intervention often depend on a multidisciplinary mycobacterial approach [63], with the recommendation that resection be done by a surgeon and at an institution with robust mycobacterial surgical experience.
Rapidly growing mycobacterium (M. abscessus)
M. abscessus is the most common of the rapidly growing mycobacteria, comprising the majority of RGM-related lung infections and being the second most common NTM lung infection in the US [64]. M. abscessus is often also associated with extrapulmonary infection including skin, soft tissue or bone disease. Similar to MAC, the largest affected demographic with M. abscessus lung infection are postmenopausal Caucasian female non-smokers, often presenting with cylindrical bronchiectasis and nodular infiltrates. Discrimination between M. abscessus and MAC lung infection cannot be done based solely on phenotypic presentation or radiographic abnormalities, underscoring the importance of microbiologic confirmation before the consideration of the start of NTM treatment. Prior data suggests that in 15 % of patients with M. abscessus lung infection, MAC may also be present [64], while a more recent study suggests up to 55 % of patients may have coexistent or subsequent MAC cultured in respiratory secretions [65•]. Microbiologic susceptibility testing is recommended for all culture isolates of M. abscessus. Susceptibility is most often assessed for clarithromycin, amikacin, cefoxitin, ciprofloxacin, imipenem, linezolid, and tigecycline. Macrolides may play a central role in the treatment regimen of a select group of M. abscessus lung infections, in addition to multiple other oral and intravenous agents added based on in vitro susceptibility testing. The role of inhaled therapies, and in particular inhaled amikacin, remains uncertain, although preliminary results recently presented in abstract form suggest substantial promise. There is no proven regimen that regularly succeeds in sustained sputum conversion [1]. A recently demonstrated regimen with a sputum conversion rate of 68 % included clarithromycin in combination with parenteral cefoxitin and amikacin for a minimum of 1 month, followed by combination oral therapy with ciprofloxacin or doxycycline for 12 months [66]. The discordance between in vitro susceptibility results and in vivo clinical response for M. abscessus lung infection was recently explained in part by the recognition of an inducible erythromycin ribosomal methylase (erm) gene [67]. Specifically, M. abscessus has been further sub-classified in part based on the presence or absence of an inducible erm gene that reduces macrolide binding and imparts macrolide resistance. Gene sequencing analysis of the 16s rRNA as well as other genes outperforms conventional biochemical assays to differentiate M. abscessus ssp. abscessus, ssp. massiliense, and ssp. boletti. M. abscess ssp. abscessus is known to have an inducible erm gene, whereas M. abscessus ssp. massiliense and most isolates of M. boletti do not have an active erm gene and retain macrolide susceptibility. Assessment for the inducible erm gene is best determined by assessing macrolide with in vitro susceptibility after a 14 day period of incubation with macrolide. Other RGM such as M. fortuitum also express an inducible erm gene, whereas M. chelonae does not. The practical implication of the presence or absence of inducibility is that macrolides are retained as an important element of a multidrug mycobacterial regimen for those RGM without inducible erm genes. For M. abscessus ssp. abscessus, a typical multidrug mycobacterial regimen would therefore include amikacin with two additional agents such as cefoxitin or imipenem, as well as tigecycline or linezolid. For M. abscessus ssp. massiliense, a macrolide remains an important component to a similar multidrug treatment regimen. The clinical significance of clarithromycin inducing a greater expression of the erm gene than azithromycin is intriguing, but uncertain in terms of management and initial drug choice [68].
Long-term mycobacterial therapy is recommended for most patients with M. abscessus. Recent published data suggest that prolonged use of a macrolide-based therapy using one or two additional parenteral agents (amikacin and cefoxitin or imipenem) led to an 80 % conversion rate in one series [69]. Those treated in combination with surgical resection also fare better than medical management alone. Recently, Jarand et al. reported the 7-year, single-center experience of 69 M. abscessus patients, noting similar outcomes in those with medical therapy alone and medical therapy in conjunction with surgical resection, though those with resection had less relapse [65]. Reported historical differences in treatment responses of M. abscessus are likely related in part to different proportions of patients with M. abscessus ssp. massiliense (no erm gene, better response) compared to M. abscessus ssp. abscessus (active erm gene, less response). Curative therapy for those unable to tolerate surgery and without localized disease may not be possible, although suppressive therapy, either with intermittent therapy for several months at a time or continuous therapy, may be possible in some patients.
M. kansasii
M. kansasii is a less common etiology of NTM lung infection in the US. The clinical presentation may mimic that of initial or reactivation tuberculosis. It is frequently associated with underlying structural lung disease or heavy smoking history, and radiologic features of upper lobe nodular or cavitary disease are present in about half [70] to 80 % of patients [71]. In the United States, there is a predilection for disease occurrence along the Southern and Central states [72]. The cornerstone of therapy is rifampin, with initial susceptibility testing recommended for rifampin alone. If rifampin resistance is detected, additional in vitro drug susceptibility is recommended. The traditional recommended regimen for rifampin-sensitive M. kansasii consists of daily therapy using isoniazid (INH), ethambutol, and rifampin, to complete at least 12 consecutive months of negative sputum culture while on drug therapy. Macrolides and fluoroquinolones generally have demonstrated good in vitro and in vivo activity against M. kansasii. In the setting of rifampin resistance, a multidrug regimen can be based on in vitro susceptibilities [73]. Intermittent therapy is feasible, though daily therapy is generally recommended. Overall, sputum conversion rates for M. kansasii with standard therapy approach 100 %, with approximately two-thirds requiring only initial treatment regimens [74] and a relapse rate of 5–10 % over the initial 5-year post-treatment period.
Other NTM
Less common NTM isolates contributing to lung infection include several subspecies of the other rapid growers, M. chelonae and M. fortuitum, the latter being less common in terms of clinically significant pulmonary infection. M. szulgai, M. malmoense, and M. xenopi in the correct clinical setting likely warrant therapy, as they often represent true NTM lung infection when isolated. Treatment regimens for M. xenopi commonly include a three-drug regimen similar to regimens used to treat MAC, while M. szulgai is approached in a similar fashion as M. kansasii [1]. In vitro susceptibility testing is helpful to assist guiding initial therapy for M. szulgai, but not for M. xenopi, which appears to have poor clinical correlation with in vivo response [1]. M. fortuitum less commonly contributes to pulmonary infection, though if treatment is initiated, limited use of macrolide therapy is appropriate, given the universal presence of the inducible erm gene mutation. M. gordonae and M. terrae complex rarely cause lung infection, though clinical disease has been reported [75, 76]. Major antibiotic strategies may be categorized as macrolide-based (M. chelonae, M. xenopi) or multidrug INH or rifampin-based regimens, according to drug susceptibilities (M. malmoense, M. szulgai,). An individualized approach is necessary for the less common NTM, as standardized or directed therapy remains elusive. Targeted end points of therapy are generally extrapolated from the experience with MAC lung infection, and include clinical and radiologic stabilization with sputum culture conversion for 12 consecutive months of therapy. Again, care must be taken to assess for underlying etiologies of lung disease before initiation of therapy, and where appropriate, consultation with an NTM expert is recommended.
Associated bronchiectasis
NTM lung infection is not uncommonly associated with bronchiectasis. The underlying pathophysiologic factors of structural lung disease that may contribute to a favorable milieu establishing concomitant NTM lung infection are not yet well understood. In some instances, NTM lung infection including nodular infiltrates clearly precede the development of bronchiectasis. Characteristically, most patients with NTM lung infection have a component of bronchiectasis, whereas only a fraction of those with bronchiectasis have NTM disease. Delineating symptoms of bronchiectasis from NTM infection may prove challenging, as patients often have both NTM and other bacteria present in the airways. Chronic macrolide use as an inflammatory or immunomodulatory agent in patients with cystic fibrosis or recurrent symptomatic non-CF bronchiectasis is an increasingly common practice based on recent studies [77–79]. The clinical imperative is to avoid the development of macrolide resistance through routine screening for NTM in patients with bronchiectasis and CF prior to the consideration of use of macrolide monotherapy for immunomodulatory purposes [23]. The benefits of concomitant bronchial hygiene as an adjunct to NTM infection has not been clearly defined, although bronchial hygiene remains an essential element of the management of bronchiectasis, independent of etiology. Treatment of other pathogenic bacteria in the setting of bronchiectasis is recommended and may clarify whether presenting respiratory symptoms are secondary to bronchiectasis, NTM infection, or both. If clinical or radiologic symptoms improve despite recurrent NTM cultures, close follow-up and observation may be appropriate in lieu of initiating directed NTM therapy. When present, attention to other comorbid conditions, including GERD and sinus disease, is equally essential.
NTM morbidity and mortality
Mortality data for NTM-related pulmonary disease is largely unknown. Mirsaeidi et al. recently reviewed NTM-related causes of death from 1999 through 2010 using mortality data from the National Center for Health Statistics, noting an age adjusted mortality rate of 0.1 per 100,000 person years [80]. Risk factors for increased risk of death included older age, female gender, and non-Hispanic white ethnicity. Structural lung disease associated with COPD or bronchiectasis was also more common in those with NTM as compared to those with tuberculous-related deaths. In a recent study from Japan, mortality from NTM appears to be increasing since the 1970s, with 5-year follow-up of patients seen from 2004–2005 suggesting a mortality rate of 1–2 % annually [17]. Ito and colleagues reported upper lobe cavitary disease in MAC infected patients as highly predictive of 5-year mortality, noting an all-cause mortality rate of 25.6 %, with no statistical difference between those were treated and untreated (22.3 % vs. 33.3 %, p = 0.30) [47]. Although further epidemiologic study is needed, the incidence of NTM lung infection is expected to climb with age and related comorbidities, disproportionately contributing to increased health care expenditures as well as increased morbidity and mortality.
Conclusion
NTM lung infection represents an increasingly adverse impact on lung health in an aging population in the US and other areas of the world. The most common NTM isolates causing lung infection in the US include MAC, M. abscessus, and M. kansasii. Geographic differences in epidemiology are notable and need to be taken into account when assessing relative prevalence and risk of NTM lung infection warranting therapy. As a disease of the elderly and those with underlying chronic structural lung disease, the incidence and prevalence of NTM lung infection is expected to rise with ongoing challenges in the recognition, diagnosis, and treatment of infection. Therapy remains complex and is often characterized by a prolonged and arduous multidrug treatment course complicated by significant drug side effects, limited sputum conversion, and substantial risk of relapse. The NTM lung infection patient may be best cared for through a multidisciplinary approach utilizing both medical and surgical options, along with a multidisciplinary team including pharmacy, respiratory therapy, and nursing. Combinations of oral, inhaled, and parenteral mycobacterial therapies that represent optimal treatment of NTM lung infection is an area of active investigation through ongoing clinical trials. With or without treatment, NTM lung infection patients need continued monitoring and comprehensive attention to other comorbid conditions, including bronchiectasis, GERD, and sinus disease, when present.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Griffith DE et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416.
Hoefsloot W et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J. 2013;42(6):1604–13. This study provides global epidemiologic data highlighting differences that have been observed for many years. It also provides a model and template for epidemiologists and clinicans to be mindful of the distribution of NTM specific species and solidify a microbiologic diagnosis prior to treatment when indicated.
Hernandez-Garduno E, Elwood K. Nontuberculous mycobacteria in tap water. Emerg Infect Dis. 2012;18(2):353.
Thomson R et al. Isolation of nontuberculous mycobacteria (NTM) from household water and shower aerosols in patients with pulmonary disease caused by NTM. J Clin Microbiol. 2013;51(9):3006–11.
Marras TK et al. Hypersensitivity pneumonitis reaction to Mycobacterium avium in household water. Chest. 2005;127(2):664–71.
Aksamit TR. Hot tub lung: infection, inflammation, or both? Semin Respir Infect. 2003;18(1):33–9.
Hanak V et al. Hot tub lung: presenting features and clinical course of 21 patients. Respir Med. 2006;100(4):610–5.
Maekawa K et al. Environmental risk factors for pulmonary Mycobacterium avium-intracellulare complex disease. Chest. 2011;140(3):723–9.
Kim RD et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med. 2008;178(10):1066–74.
Dirac MA et al. Environment or host?: A case–control study of risk factors for Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2012;186(7):684–91.
Falkinham 3rd JO. Reducing human exposure to Mycobacterium avium. Ann Am Thoracic Soc. 2013;10(4):378–82.
Prevots DR et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182(7):970–6.
Winthrop KL et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182(7):977–82.
\Adjemian J et al. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185(8):881–6. Investigators very nicely capture an overview of United States NTM distribution using a Medicare data base, in patients aged 65 years and older, derived from billing diagnoses. Although there are inherent biases and limitations using this type of data base, the analysis provides a more comprehensive look at the distribution of NTM disease across the US than has previously been available. It provides a basis for asking additional probing research questions into the distribution of NTM lung infection and potential links to environmental exposures.
Jing H et al. Prevalence of nontuberculous mycobacteria infection, China, 2004–2009. Emerg Infect Dis. 2012;18(3):527–8.
Kim JK, Rheem I. Identification and distribution of nontuberculous mycobacteria from 2005 to 2011 in cheonan. Korea Tubercul Respir Dis. 2013;74(5):215–21.
Morimoto K et al. A steady increase in nontuberculous mycobacteriosis mortality and estimated prevalence in Japan. Ann Am Thoracic Soc. 2014;11(1):1–8.
Hoefsloot W et al. Prevalence of nontuberculous mycobacteria in COPD patients with exacerbations. J Infect. 2013;66(6):542–5.
Ko FW et al. A 1-year prospective study of the infectious etiology in patients hospitalized with acute exacerbations of COPD. Chest. 2007;131(1):44–52.
Griffith DE, Aksamit TR. Bronchiectasis and nontuberculous mycobacterial disease. Clin Chest Med. 2012;33(2):283–95.
Martiniano SL et al. Clinical significance of a first positive nontuberculous mycobacteria culture in cystic fibrosis. Ann Am Thoracic Soc. 2014;11(1):36–44.
Roux AL et al. Multicenter study of prevalence of nontuberculous mycobacteria in patients with cystic fibrosis in france. J Clin Microbiol. 2009;47(12):4124–8.
Binder AM et al. Epidemiology of nontuberculous mycobacterial infections and associated chronic macrolide use among persons with cystic fibrosis. Am J Respir Crit Care Med. 2013;188(7):807–12.
Olivier KN. The natural history of nontuberculous mycobacteria in patients with cystic fibrosis. Paediatr Respir Rev. 2004;5(Suppl A):S213–6.
Olivier KN et al. Nontuberculous mycobacteria. I: multicenter prevalence study in cystic fibrosis. Am J Respir Crit Care Med. 2003;167(6):828–34.
Fowler SJ et al. Nontuberculous mycobacteria in bronchiectasis: Prevalence and patient characteristics. Eur Respir J. 2006;28(6):1204–10.
Koh WJ et al. Prevalence of gastroesophageal reflux disease in patients with nontuberculous mycobacterial lung disease. Chest. 2007;131(6):1825–30.
Aksamit TR. Mycobacterium avium complex pulmonary disease in patients with pre-existing lung disease. Clin Chest Med. 2002;23(3):643–53.
Aksamit TR, Philley JV, Griffith DE. Nontuberculous mycobacterial (NTM) lung disease: the top ten essentials. Respir Med. 2014;108(3):417–25. This summary article further highlights many of the controversies that NTM lung infection patients and clinicians caring for them frequently encounter. Although answers to the controversies are not yet available, this reference highlights the context from which additional research questions are to be posed.
Mayskaya MU et al. Morphological manifestations of the atypical mycobacteriosis caused by nontuberculous mycobacteria in the HIV infected patients. Ann Clin Lab Sci. 2014;44(2):131–3.
Piersimoni C. Nontuberculous mycobacteria infection in solid organ transplant recipients. Eur J Clin Microbiol Infect Dis. 2012;31(4):397–403.
Winthrop KL et al. Nontuberculous mycobacteria infections and anti-tumor necrosis factor-alpha therapy. Emerg Infect Dis. 2009;15(10):1556–61.
Ballarino GJ et al. Pulmonary nontuberculous mycobacterial infections: antibiotic treatment and associated costs. Respir Med. 2009;103(10):1448–55.
Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease. Incidence, presentation, and response to therapy in a community setting. Am Rev Respir Dis. 1991;143(6):1381–5.
Billinger ME et al. Nontuberculous mycobacteria-associated lung disease in hospitalized persons, United States, 1998–2005. Emerg Infect Dis. 2009;15(10):1562–9.
Chan ED, Iseman MD. Underlying host risk factors for nontuberculous mycobacterial lung disease. Semin Respir Crit Care Med. 2013;34(1):110–23.
Kartalija M et al. Patients with nontuberculous mycobacterial lung disease exhibit unique body and immune phenotypes. Am J Respir Crit Care Med. 2013;187(2):197–205. This is a very important reference along with its predecessor from the NIH (ref 9 above Kim et al.) that captures the distinctive phenotype of the most common presentation and features of NTM lung infection in the United States. Moreover, these two papers highlight the subtle differences in measurement of immunotypes that may impact the link between ubiquitous exposure to NTM in the environment and development of lung infection. In a similar fashion, subtle differences in methodology of measurement of immune responses may heavily influence whether abnormal responses can be differentiated from normal responses.
Paulson ML, Olivier KN, Holland SM. Pulmonary non-tuberculous mycobacterial infection in congenital contractural arachnodactyly. Int J Tubercul Lung Dis. 2012;16(4):561–3.
Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern. Lady Windermere Syndr Chest. 1992;101(6):1605–9.
Iseman MD et al. Disease due to Mycobacterium avium-intracellulare. Chest. 1985;87(2 Suppl):139S–49.
Prince DS et al. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989;321(13):863–8.
Kuroishi S et al. Mycobacterium avium complex disease: prognostic implication of high-resolution computed tomography findings. Eur Respir J. 2008;32(1):147–52.
Maekawa K et al. High-resolution computed tomography and health-related quality of life in Mycobacterium avium complex disease. Int J Tubercul Lung Dis. 2013;17(6):829–35.
Kitada S et al. Serodiagnosis of Mycobacterium avium complex pulmonary disease in the USA. Eur Respir J. 2013;42(2):454–60.
van Ingen J et al. Chapter 3. Pulmonary diseases caused by non-tuberculous mycobacteria. Eur Respir Monogr. 2012;58:25–37.
Hadjiliadis D, Adlakha A, Prakash UB. Rapidly growing mycobacterial lung infection in association with esophageal disorders. Mayo Clin Proc. 1999;74(1):45–51.
Ito Y et al. Predictors of 5-year mortality in pulmonary Mycobacterium avium-intracellulare complex disease. Int J Tubercul Lung Dis. 2012;16(3):408–14.
Marras TK et al. Opinions differ by expertise in Mycobacterium avium complex disease. Ann Am Thoracic Soc. 2014;11(1):17–22.
Koh WJ et al. Clinical significance of the differentiation between Mycobacterium avium and Mycobacterium intracellulare in M avium complex lung disease. Chest. 2012;142(6):1482–8.
Kobashi Y et al. Relationship between clinical efficacy of treatment of pulmonary Mycobacterium avium complex disease and drug-sensitivity testing of Mycobacterium avium complex isolates. J Infect Chemother. 2006;12(4):195–202.
van Ingen J et al. Resistance mechanisms and drug susceptibility testing of nontuberculous mycobacteria. Drug Resist Updates. 2012;15(3):149–61.
Davis KK et al. Aerosolized amikacin for treatment of pulmonary Mycobacterium avium infections: an observational case series. BMC Pulmonary Med. 2007;7:2.
Colin AA, Ali-Dinar T. Aerosolized amikacin and oral clarithromycin to eradicate Mycobacterium abscessus in a patient with cystic fibrosis: an 8-year follow-up. Pediatr Pulmonol. 2010;45(6):626–7.
Wallace, R.J., Jr., et al., Macrolide/Azalide Therapy for Nodular/Bronchiectatic: Mycobacterium avium Complex Lung Disease. Chest, 2014. This is an important recent large series of 180 patients with MAC nodular/bronchiectatic MAC lung infection. It highlights the efficacy of the ATS recommended thrice weekly mycobactial regimen with favorable sputum conversion rates yet with substantially less side effects and intolerance compared to a daily mycobacterial regimen.
Koh WJ et al. Treatment of refractory Mycobacterium avium complex lung disease with a moxifloxacin-containing regimen. Antimicrob Agents Chemother. 2013;57(5):2281–5.
Griffith DE et al. Clinical and molecular analysis of macrolide resistance in Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2006;174(8):928–34.
Field SK, Cowie RL. Treatment of Mycobacterium avium-intracellulare complex lung disease with a macrolide, ethambutol, and clofazimine. Chest. 2003;124(4):1482–6.
Kemper CA et al. The individual microbiologic effect of three antimycobacterial agents, clofazimine, ethambutol, and rifampin, on Mycobacterium avium complex bacteremia in patients with AIDS. J Infect Dis. 1994;170(1):157–64.
Wallace Jr RJ et al. Reduced serum levels of clarithromycin in patients treated with multidrug regimens including rifampin or rifabutin for Mycobacterium avium-M. intracellulare infection. J Infect Dis. 1995;171(3):747–50.
Koh WJ et al. Therapeutic drug monitoring in the treatment of Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2012;186(8):797–802.
van Ingen J et al. The pharmacokinetics and pharmacodynamics of pulmonary Mycobacterium avium complex disease treatment. Am J Respir Crit Care Med. 2012;186(6):559–65.
van Ingen J et al. Surgical treatment of non-tuberculous mycobacterial lung disease: strike in time. Int J Tubercul Lung Dis. 2010;14(1):99–105.
Mitchell JD et al. Anatomic lung resection for nontuberculous mycobacterial disease. Ann Thorac Surg. 2008;85(6):1887–92. discussion 1892–3.
Griffith DE, Girard WM, Wallace Jr RJ. Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am Rev Respir Dis. 1993;147(5):1271–8.
Jarand J et al. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis. 2011;52(5):565–71. This study offers clinicians some encouragement, along with studies of Jeon et al. ref 66 and Lyu et ref 69, that modest sputum culture rates may be possible in select M. abscessus lung infection patients. Differentiation of M. abscessus ssp. abscessus from ssp. massiliense is not included. These studies offer a look into the complexity of the variety of multidrug regimens often used. Side effects and drug intolerance remain substantial and further highlight the need for the development of more effective, easier, and better tolerated treatment regimens.
Jeon K et al. Antibiotic treatment of Mycobacterium abscessus lung disease: a retrospective analysis of 65 patients. Am J Respir Crit Care Med. 2009;180(9):896–902.
Nash KA, Brown-Elliott BA, Wallace Jr RJ. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother. 2009;53(4):1367–76.
Choi GE et al. Macrolide treatment for Mycobacterium abscessus and Mycobacterium massiliense infection and inducible resistance. Am J Respir Crit Care Med. 2012;186(9):917–25.
Lyu J et al. Outcomes in patients with Mycobacterium abscessus pulmonary disease treated with long-term injectable drugs. Respir Med. 2011;105(5):781–7.
Shitrit D et al. Pulmonary Mycobacterium kansasii infection in Israel, 1999–2004: clinical features, drug susceptibility, and outcome. Chest. 2006;129(3):771–6.
Takahashi M et al. Mycobacterium kansasii pulmonary infection: CT findings in 29 cases. Jpn J Radiol. 2012;30(5):398–406.
Bittner MJ et al. Emergence of Mycobacterium kansasii as the leading mycobacterial pathogen isolated over a 20-year period at a midwestern Veterans Affairs hospital. Clin Infect Dis. 1996;22(6):1109–10.
Wallace Jr RJ et al. Rifampin-resistant Mycobacterium kansasii. Clin Infect Dis. 1994;18(5):736–43.
Maliwan N, Zvetina JR. Clinical features and follow up of 302 patients with Mycobacterium kansasii pulmonary infection: a 50 year experience. Postgrad Med J. 2005;81(958):530–3.
Krisher KK, Kallay MC, Nolte FS. Primary pulmonary infection caused by Mycobacterium terrae complex. Diagn Microbiol Infect Dis. 1988;11(3):171–5.
Mazumder SA, Hicks A, Norwood J. Mycobacterium gordonae pulmonary infection in an immunocompetent adult. N Am J Med Sci. 2010;2(4):205–7.
Wong C et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):660–7.
Altenburg J et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA. 2013;309(12):1251–9.
Serisier DJ et al. Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA. 2013;309(12):1260–7.
Mirsaeidi M et al. Nontuberculous mycobacterial disease mortality in the United States, 1999–2010: a population-based comparative study. PLoS ONE. 2014;9(3):e91879.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Teng Moua and Timothy R. Aksamit each declare that there are no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moua, T., Aksamit, T.R. Diagnosis and management of nontuberculous mycobacterial lung infections. Curr Respir Care Rep 3, 161–169 (2014). https://doi.org/10.1007/s13665-014-0090-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13665-014-0090-4