Abstract
Achyranthes aspera is an omnipresent herb of medicinal herbal group, used traditionally as medicine to cure different types of diseases. The components of hydro-methanolic extract of Achyranthes aspera (HMEA), are screened and tentatively identified using UPLC-PDA and MALDI-TOF-MS techniques. The phenolic compounds tentatively identified are quinic acid (m/z 192.040), chlorogenic acid (m/z 354.0894), kaempferol (m/z 286.0917), quercetin (m/z 302.0895) and chrysin (m/z 254.0654) present in the various ratios in which concentration level of chlorogenic acid and kaempferol are determined considering their peak intensity. To the best of our knowledge this is the first report on the characterization of HMEA using UPLC and MALDI-TOF-MS techniques to demonstrate the presence of various phenolic compounds and their antioxidant enzymes activity in vivo. The enzymes which showed increased activity are GST, GR, CAT, and SOD while activity of LDH decreased. These phenolic compounds have potential to be used as anti-cancerous molecules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Achyranthes aspera Linn (Family Amaranthaceae), is an annual herb grown at waste places all over India, Tropical Asia, Africa and the southern United States (Hedberg et al. 1982). The Indian name in Hindi of this plant is Chirchita, latkana, Latjira or Apamarg and in English it is known as prickly chaff-flower. The ayurvedic preparation done from this plant is apamarga taila and agnimukha. The importance of medicinal value of this herb has been proved to treat various diseases, e.g., leprosy, bronchial asthma, heart disease, piles, dysentery blood diseases and whooping cough (Tripathi et al. 1963; Singh 1995; Suresh et al. 1985). The extract of this herbal plant has been reported for antimicrobial activity (Basu et al. 1957), immuno-stimulant activity in mice (Vasudeva et al. 2002), anti-peroxidative and anti-inflammatory activity in rats (Tahiliani and Kar 2000; Vetrichelvan and Jegadeesan 2003; Gokhale et al. 2002). The anti-carcinogenic activity has also been proved in vitro in Raji cell line (Chkraborty et al. 2002) and pancreatic cancer cell lines (MiPaCa-2 and PanC 10.05) (Subbarayan et al. 2010) but no reports are available so for in vivo. The seeds of this herbal plant are rich in protein and hence cooked and eaten. The plant’s powder is used in diarrhea, in diuretic problems, skin diseases, bleeding piles and externally during toxic bites. However, little information is available regarding the chemical composition and various components present in the hydro methanolic extract.
Due to diverse medicinal properties of A.aspera, chemical composition analysis of this wild herb has become exceedingly important so its various species could be utilized in the food industry as well as in the pharmaceutical industry. The presence of phenolic compounds in the medicinal plants including herbs due to their human health benefits has attracted the attention for the development of methods and application of several analytical techniques for their identification (Robards 2003). The analytical technique which is most commonly used for separation, identification and quantification of phenolic compounds are reverse-phase high performance liquid chromatography (RP-HPLC), with diode array detection (DAD) and mass spectrometry (MS) detection (Kähkönen et al. 2001; Häkkinen et al. 1999). In the present study, we used a rapid and reproducible UPLC method, which is based on RP-HPLC, with 1.7 μM particle size, together with a liquid system that can operate such column at much higher pressure (Nováková et al. 2006; Wang et al. 2007). UPLC technique has been preferred because it offers many advantages over the conventional HPLC method such as reducing run time and less-solvent consumption (Guan et al. 2007; Liu et al. 2007; Zhang et al. 2007). Here UPLC (ultra performance liquid chromatography) has been used for identification of the components based on their retention times, where as MALDI-TOF-MS (matrix assisted laser desorption ionization - time of flight – mass spectrometry) has been used to collect information on specific chemical composition on the basis of molecular mass (m/z) of compounds to extract significant information about structure. MALDI-TOF-MS is a competent tool for large biomolecules analysis (Karas et al. 1987) and extensively used in food analysis for oligomeric polyphenol and anthocyanin detection (Wang and Sporns 2000; Reed et al. 2005). It gives high speed of analysis, high sensitivity with good tolerance of contaminants and ability to analyze complex mixtures (Karas 1996).
The purpose of current study is detection, identification and characterization of various phenolic compounds present in the HMEA using UPLC and MALDI-TOF-MS techniques and evaluation of antioxidant activity of HMEA in vivo in cancerous mice model. The current research findings will helpful in further research on detailed characterization of these compounds, uses and applications to cure various diseases, including cancer.
Material and methods
Chemicals
Acetic acid, HPLC grade, purity ≥ 98 %, acetone, HPLC grade, purity ≥ 98 %, methanol, HPLC grade, purity ≥ 98 %, acetonitrile (ACN), HPLC grade, purity ≥ 98 %, trifluroacetic acid (TFA), HPLC grade, purity ≥ 98 %, α-cyano-4-hydroxy-cinnamic acid (CHCA), ethylene diamine tetra acetate (EDTA), sodium carbonate (Na2CO3), aluminum chloride (AlCl3), potassium carbonate (K2CO3), Folin-Ciocalteu reagent were purchased from Merk. Gallic acid, Quercetin, 0.22 μm nylon filtere,. 1-chloro-2,4-dinitrobenzene (CDNB); 5,5′-dithio-2-nitrobenzoic acid (DTNB); reduced glutathione (GSH); oxidized glutathione (GSSG), 2,6-dichlorophenol-indophenol (DCPIP), sodium pyruvate, reduced nicotinamide adenine dinucleotide phosphate (NADPH), reduced nicotinamide adenine (NADH), pyrogalol were purchased from Himedia.
Herbal material collection and extract preparation
A. aspera plants were collected from the BHU campus. Plants were washed thoroughly and air dried in shade at room temperature (RT) and dehydrated in an incubator at 37 °C for 16 h. The whole plants were made powder by milling, sieved through a fine mesh and stored at RT for further use. The total plant extract was prepared by first suspending 10 g of powdered A. aspera in 100 mL of extraction solution (water: methanol :: 30:70) gently agitating for 24 h at RT. The suspension was spun at 2,000 rpm for 10 min at RT. The clear supernatant was decanted into a fresh tube and was lyophilized, weighed and preserved at 4 °C, till further use. The lyophilized dry powder of hydromethanolic extract of A. aspera (HMEA) was dissolved in phosphate buffer saline (PBS) for experimental use.
Total flavonoid determination in HMEA
Total flavonoids were determined by aluminum chloride (AlCl3) colorimetric method (Chang et al. 2002). A dilute HMEA (0.5 mL) was separately mixed with 1.5 mL of methanol, 0.1 mL of 10 % aluminum chloride, 0.1 mL of 1.0 M potassium acetate and 2.8 mL of distilled water. The mixture was incubated for 30 min at RT and absorbance was recorded at 415 nm on a double beam JASCO V-630 spectrophotometer (Japan). The standard plot was prepared using quercetin solutions in methanol at a concentration of 10 to100 μg/mL.
Total phenols determination in HMEA
Phenolic compound determination was carried out using Folin ciocalteu reagent (McDonald et al. 2001). HMEA was mixed with 5 mL folin ciocalteu reagent (1:10 diluted) and aqueous 4 mL Na2CO3 (1.0 M). The mixture was left for 15 min and absorbance was taken at 760 nm by double beam JASCO V-630 spectrophotometer (Japan). The standard curve of gallic acid was prepared in methanol:water (50:50 v/v) at concentrations 50 μg/mL to 250 μg/mL.
UPLC-condition
UPLC (WATERS, USA) with photo diode array (PDA) was used for the detection of phenolic compounds. UPLC BEH C18 column with 100 × 2.1 mm/ID, and particle size 1.7 μm, was used. The column temperature was maintained at 40 °C. The binary system phases used were: eluent A (2 % acetic acid, 98 % water) and eluent B (100 % methanol), with a flow rate of 0.3 mL/min. 5 μL of 0.22 μm nylon filtered HMEA sample was used for separation with gradient started with 8 % B, reached 60 % B in 12 min and 97 % B after 15 min, 97 % B was kept for 2 min.
MALDI-condition
Prior to MALDI analysis of 0.22 μm nylon filtered HMEA sample, the column was optimized in order to develop MALDI-TOF-MS techniques. An appropriate matrix selected was α-cyano-4-hydroxy-cinnamic acid (CHCA) as 10 mg/mL in 50 % acetonitrile (ACN) with 0.1 % TFA. Optimal mixing of sample and matrix (1:1) was spotted on MALDI plate and it was air-dried to obtain a homogenous co-crystal. The sample was analyzed using reflection mode on Applied Biosystem MALDI-TOF-MS (USA). MALDI-TOF-MS reproduces only a singly charged molecular ion for each parent molecule and allows detection of high mass with precision. Spectrum was collected in a positive ion linear mode as average from m/z 99 to 1,000 laser shots of predetermined or randomly positioned across a spot.
Animals and experimental design
Seven to eight week old inbred strains of Balb/c mice having approximately equal weight were taken. Mice were kept in hygienic condition at RT with a 12-h light/dark cycle, and provided with standard pellet laboratory animal feed and water ad libitum. The mice were randomly assigned into four groups; Group I: HMEA non feed and urethane non primed normal mice; Group II: HMEA feed i.e. HMEA control; Group III: Urethane primed i.e. urethane control; Group IV: Urethane primed and HMEA feed. Each group contained 6 mice. Animal ethical rules were followed as per the guide lines provided by the animal ethical committee, Institute of medical sciences, Banaras Hindu University, Varanasi, India.
Cancer (tumor) induction and HMEA treatment
Lung cancer (tumor) was induced by i.p. (intra peritoneal) injection of urethane 1 g/Kg body weight of mice for eight consecutive weeks with 24 week latency. After 24 week of latency period mice of group IV were orally feed with HMEA 100 mgKg−1 day−1 for 4 week (30 days). Mice of group III were given eight consecutive week i.p. injection of urethane (1 g/kg body weight of mice), than after 24 week latency period PBS was orally given for 4 week (30 days). Mice of group II were given i.p. injection of PBS for eight consecutive weeks there after 24 week of latency period HMEA 100 mgKg−1 day−1 were orally (30 days) given for 4 week. The mice of group I without urethane injection and HMEA feed were used as control. Urethane and lyophilized dry powder of HMEA were dissolved in phosphate buffer saline (PBS) for experimental use.
Biochemical assays
Mice were sacrificed by cervical dislocation and the entire lung was perfused immediately into ice cold PBS. The perfused lung tissue was carefully removed, made free from extraneous tissues, trimmed and rinsed in chilled 0.15 M Tris-NaCl buffer (0.15 M NaCl + 10 mM Tris–HCl, pH 7.4). The lung tissue was blotted dry, weighed quickly and homogenized in ice cold 0.15 M Tris-NaCl buffer to yield a 10 % (w/v) homogenate. Homogenate was centrifuged at 12,000 rpm for 45 min at 4 °C. The supernatant was retained and used for assaying antioxidant enzyme activity such as glutathione S-transferase (GST), glutathione reductase (GR), superoxide dismutase (SOD), catalase (CAT), and glycolytic enzyme lactate dehydrogenase (LDH). The GST activity was determined spectrophotometrically (JASCO V-630 spectrophotometer) at 37 °C by Habig’s method (Habig et al. 1974). The GR activity was measured by the method of Carlberg and Mannervik (Carlberg and Mannervik 1985). The CAT activity in the supernatant of lung tissue homogenate was measured using spectrophotometric method described by Aebi (1984). SOD assay was done utilizing the technique of Del Maestro and McDonald (Del Maestro and McDonald 1986). LDH assay was carried out by the method of Bergmeyer and Bernt (Bergmeyer and Brent 1971).
Statistical analysis
All the experiments were conducted thrice and were reproducible. Data were expressed as mean ± SEM. The pair-wise comparisons of all data were made by one way analysis of variance (ANOVA). The P values ≤ 0.05 were considered as significant.
Results
Phenolic and flavonoid estimation in HMEA
Amount of phenolic and flavonoid present in HMEA were estimated by spectrophotometric method. Total phenolic content in HMEA was expressed as mg gallic acid g−1 using the following equation based on calibration curve of gallic acid standard; y = 5.436x-0.048, where y is an absorbance and x is the mg gallic acid g−1. The total flavonoid content in HMEA was expressed as mg g−1. Using the straight line equation based on calibration curve of quercetin standard; y = 6.431x−0.057, where y is an absorbance and x is the mg quercetin g−1. Total phenolic content of HMEA are high, 100 mg dry weight of crude extract contains 2.4 mg gallic acid g−1 phenolic acid and 1.1 mg quercetin g−1 flavonoid.
UPLC analysis of HMEA
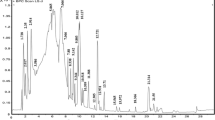
UPLC is used for the separation of phenolic compounds and other components from complex mixture of HMEA, identification of the compounds based on chromatographic behavior i.e. their different affinities for resin packed column. Figure 1 clearly indicates a good separation of flavonoids and phenolic acid etc. Chromatogram (Fig. 1) showed various phenolic compounds which were separated and tentatively identified by comparing retention time (tR) of known standard sample injected in same condition and reported earlier in the literature (Trautvetter et al. 2009). On the basis of retention time (tR), only nine compounds were identified out of sixteen known and unknown compounds present in HMEA are summarized in Table 1, where compounds are numbered according to their retention time (tR), obtained in the chromatogram (Fig. 1). The nine phenolic compounds tentatively namely, quinic acid, shikimic acid, phloroflucinol, gallic acid, chlorogenic acid, p-coumeric acid, acetylsalicylic acid, quercetin, kaempferol, and chrysin, with tR of 0.82, 0.84, 1.09, 3.67, 5.1, 6.56, 9.8, 10.40, and 13.41 min, respectively.
MALDI-TOF-MS analysis of HMEA
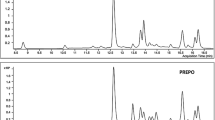
MALDI-TOF-MS could generate abundant positive ion mode, [M]+ and provide structural information for the identification and confirmation of phenolic compound in the HMEA (Fig. 2). Based on m/z value spectra of MALDI-TOF-MS, five compounds identified were quinic acid (m/z 192.0420), chlorogenic acid (m/z 354.0894), quercetin(m/z 302.0895), kaempferol (m/z 286.0917), and chrysin (m/z 254.0654) (Table 2). By MALDI-TOF-MS we verified and indentified five phenolic compounds (Table 2) out of nine phenolic compounds which were identified by UPLC based on tR (Table 1).. The m/z value of mass spectra of MALDI-TOF-MS m/z 192.0420, m/z 354.0894, m/z 302.0895, m/z 286.0917, and m/z 254.0654 (Fig. 2) were very similar to the molecular weight quinic acid, chlorogenic acid, kaempferol, quercetin and chrysin. Out of these m/z based characterized compounds, peak intensity of chlorogenic acid (m/z 354.0894) and kaempferol (m/z 286.0917) was found higher than other compounds. This showed that both compounds are in higher concentration in comparison to others (Fig. 2).
Effect of HMEA on antioxidant enzymes and lactate dehydrogenase enzyme activities
The specific activities of antioxidant GST, GR, CAT, SOD, and non-antioxidant LDH enzymes were measured to understand in the change of activities in lung tissue of mice group I, II, III, and IV (Fig. 3). The activity of GST (Fig. 3a), GR (Fig. 3b), CAT (Fig. 3c), and SOD (Fig. 3d) were significantly increased in both normal and urethane primed mice HMEA feed groups as compared to their respective HMEA non feed group (control). The increase in the activity of GST, GR, CAT, and SOD were 1.10, 1.25, 1.18, and 1.28 fold respectively in mice of group IV in comparison to mice of group III. While the activity of GST, GR, CAT, and SOD increased by 1.15, 1.16, 1.10 and 1.22 folds respectively in mice of group II in comparison to mice of group I. The activity of LDH decreased in the HMEA feed groups. The activity of LDH (Fig. 3e) decreased by 1.28 fold in mice of group IV in comparison to mice of group III where as the LDH activity decreased by 1.15 folds in mice of group II in comparison to mice of group I.
Antioxidant enzymes specific activity in the mice lungs tissue of urethane primed and non-primed groups. (a) Glutathione S-transferase specific activity expressed as μmole CDNB-GSH conjugate formed/min/mg protein (b) Glutathione reductase specific activity expressed as μmole of NADPH consumed/min/mg protein (c) Catalase specific activity expressed as μmole of H2O2 consumed/min/mg protein (d) Super oxide dismutase specific activity expressed as μmole/min/mg protein (e) Lactate dehydrogenase specific activity expressed as μmole of NADH oxidation/min/mg protein (values are expressed as mean ± SEM for n = 6 per group and compared against respective control by ANOVA p < 0.05 were considered significant)
Discussion
The herbs occupied a distinct place in the life of human being and animals’ right from pre-historic period till date. A. aspera has become one of the most important medicinal herbs due to presence of large number of medicinal properties. The use of medicinal herb in the various illnesses is due to their phytochemical constituents (Chahlia 2009) which has antioxidant, antimicrobial and anti-inflammatory activities. Preliminary phytochemical study and findings made by earlier scientific groups of A. aspera root and inflorescence showed that it contains alkaloids, flavonoids, saponins, steroid and terpenoids (Sinha and Dogra 1985; Agrawal et al. 1989). In the present work we carried out the further characterization of phenolic compounds i.e. flavonoids and phenolic acid, its concentration level in HMEA (crude extract of aerial part of A. aspera) and assay of antioxidant activity in vivo. Phenolic compounds have a broad spectrum of biochemical activities; such as antioxidant, free-radical scavengers, hinders oxidative degradation of biomolecules thereby improve the quality and nutritive value of foods (Leenen et al. 2000; Ng et al. 2000; Kahkonen et al. 1999; Rice-Evans et al. 1995), however no report till date is available on the characterization of phenolic constituents of crude extract in the aerial parts of A. aspera. We are first to report in the present study that HMEA is a rich source of phenolic compounds based on identification and characterization by using UPLC-PDA (Fig. 1) and MALDI-TOF-MS (Fig. 2) techniques and have antioxidant activity that could be used to mitigate the various diseases.
These phenolic compounds scavenge produced free radicals either by binding and neutralizing them or by forming quinine methide intermediate known to be excreted via bile (Pan et al. 1999), reducing the chances of occurring cancer and other inflammatory diseases. Reduction in free radical genesis by HMEA could be preventing free radical mediated cell damages, thus enhances the oxidative immune system and reducing the chances of cancer development. As earlier report suggests that lung cancer in the urethane primed mice model is due to generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) (Beckman et al. 1990), subsequently uneven production of certain inflammatory cytokines creating appropriate micro environment for cancer development (Narayan and Kumar 2012 ). The ROS causes oxidative damage to normal cells and produces oxidative stress (Dean et al. 1997; Kundu and Surh 2008). The oxidative stress suppresses the intracellular defense system by decreasing the activity of antioxidant enzymes (Sun 1990).
HMEA enhances the activity of antioxidant enzymes and lowers the activity of LDH. The antioxidant enzyme GST is known to detoxify electrophilic compounds (Hemachand et al. 2002) and plays crucial role in protecting the cells from chemical carcinogens by scavenging free radical molecules and finally reducing the oxidative stress. This is experimentally well proven facts that a person with low activity of GST enzyme are highly prone to the cancer disease (Mitrunen et al. 2001) and this is categorically supported by our results of GST (Fig. 3a), which showed the lower or decrease in the GST activity in the cancerous lung tissues that later got increased in the HMEA feed cancerous mice lung tissues (Fig. 3a). The activity of antioxidant enzyme GR (Fig. 3b) shows similar effect as GST. On the other hand CAT and SOD protect the lipid peroxidation thus preventing and protecting the deleterious effects of generated ROS-metabolites (Ray and Husain 2002). The earlier experimental results showed that CAT activity decreases in various cancerous tissues for example in Morris hepatoma, Lewis lung carcinoma and in tumorigenic hamster kidney including other human cancers (Corrocher et al. 1986; Kahlos et al. 2001). Comparable to earlier findings we observed decreased CAT (Fig. 3c) and SOD (Fig. 3d) activities in urethane primed cancerous lung tissues.
We also observed increased activity of LDH enzyme in urethane primed lung tissues of Balb/c mice in comparison to non-urethane primed (control) lung tissues but activity decreased after HMEA feeding. The higher activity of LDH was due to damage of the lung cells/tissues caused by urethane mediated generation of reactive free radicals e.g. ROS and RNS, which further created microenvironment to enhance the damaging effects of lung cells/tissues. It is pertinent to mention here that enhanced LDH enzyme activity has become one of the most useful markers to demonstrate cell damage. We observed decrease in the activity of LDH in cancerous lung tissues of urethane primed mice and feed with HEMA which showed restoration or minimization of lung tissue damage caused due to urethane
Conclusions
The current study showed that HMEA possesses high diversity of the phenolic compounds containing phenolic acids and flavonoids, which synergistically implies their antioxidant, free-radical scavenging activity and hinders oxidative degradation of cells, tissues and organs. Crude extract of aerial parts of A. aspera rich in phenolic compounds are increasingly have interest in the field of nutrition, health and medicine due to its significant high antioxidant activities. The quantification of total phenolic acids and flavonoids suggests that 100 mg dry weight (aerial crude extract) contains 2.4 mg gallic acid g−1 phenolic acid and 1.1 mg quercetin g−1 flavonoid. Overall antioxidant properties of HMEA are due to its ability to enhance the activities of various antioxidant enzymes GST, GR, CAT, and SOD present in the lung cells especially could be other organ cells to improve the antioxidant defense system to prevent and cure diseases. As suggested by our results, the anti-oxidant system of the cells gets further boost by the decreased activity of non-antioxidant enzyme LDH. Moreover, since HMEA enhances anti-oxidant properties even in normal control as experimental results suggests, it could be beneficial for both non-cancerous (healthy) individuals, demonstrating its preventive values as well as for cancerous patient showing its ability to cure the cancer disease and might be for many other unknown diseases. Further research is required to evaluate and elucidate the more rational molecular approach to be used for targeted therapy to combat various diseases including lung cancer, other cancer types.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Agrawal RG, Pant P, Tewari LC, Singh J, Pandey MJ, Tiwary DN (1989) Preliminary phytochemical screening of medicinal plants of hilly district of U.P. Bull Med Ethnobot Res 10:176–86
Basu NK, Neogi NC, Srivastava VP (1957) Biological investigation of Achyranthes aspera Linn and its constituent achyrathine. J Proc Inst Chem 29:433–437
Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA (1990) Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 87:1620–1624
Bergmeyer HU, Brent E (1971) In: Methods of enzymatic analysis, vol. II. Verlag Academic Press, New York, pp 5574–5579
Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113:484–490
Chahlia N (2009) Effect of Capparis decidua on hypolipidemic activity in rats. J Med Plants Res 3:481–484
Chang C, Yang M, Wen H, Chern J (2002) Estimation of total flavonoid content in propolis by two complememtary colorimetric methods. J Food Drug Anal 10:178–182
Chkraborty A, Brantner A, Mukainaka T, Nobukuni Y, Kuchide M, Konoshima T, Tokuda H, Nishino H (2002) Cancer chemopreventive activity of Achyranthes aspera leaves on Epstein –Barr virus activation and two-stage mouse skin carcinogenesis. Cancer Lett 177:1–5
Corrocher R, Casaril M, Bellisola G, Gabrielli GB, Nicoli N, Guidi GC, De Sandre G (1986) Severe impairment of antioxidant system in human hepatoma. Cancer 58:1658–1662
Dean RT, Fu S, Stocker R, Davies MJ (1997) Biochemistry and pathology of radical-mediated protein oxidation. Biochem J 324:1–18
Del Maestro RF, McDonald W (1986) Oxidative enzymes in tissue homogenates. In: Greenwald RA (ed) CRC handbook of methods of oxygen radical research, CRC press, Boca Raton, Florida, pp 291–296.
Gokhale AB, Damre AS, Kulkami KR, Saraf MN (2002) Preliminary evaluation of anti-inflammatory and anti-arthritic activity of S. lappa, A. speciosa and A. aspera. Phytomedicine 9:433–437
Guan J, Lai CM, Li SP (2007) A rapid method for the simultaneous determination of 11 saponins in Panax notoginseng using ultra performance liquid chromatography. J Pharmaceut Biomed Anal 44:996–1000
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferase, the first step in mercapturic acid formation. J Biol Chem 249:7130–7139
Häkkinen S, Heinonen M, Kärenlampi S, Mykkänen H, Ruuskanen J, Törrönen R (1999) Screening of selected flavonoids and phenolic acids in 19 berries. Food Res Int 32:345–353
Hedberg I, Hedberg O, Madati PJ, Mshigeni KE, Mshiu EN, Samuelsson G (1982) Inventory of plants used in traditional medicine in Tanzania I plants of the families Acanthaceae-Cucurbitaceae. J Ethnopharmacol 6:29–60
Hemachand T, Gopalakrishnan B, Salunke DM, Totey SM, Shaha C (2002) Sperm plasmamembrane-associated glutathione S- transferases as gamete recognition molecules. J Cell Sci 115:2053–2065
Kahkonen MP, Hopia AL, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, Heinonen M (1999) Antioxidant activity of plant extracts containing phenolic compounds. J Agri Food Chem 47:3954–3962
Kähkönen MP, Hopia AI, Heinonen M (2001) Berry phenolics and their antioxidant activity. J Agri Food Chem 49:4076–4082
Kahlos K, Soini Y, Sormunen R, Kaarteenaho-Wiik R, Paakko P, Linnainmaa K, Kinnula VL (2001) Expression and prognostic significance of catalase in malignant mesothelioma. Cancer 91:1349–1357
Karas M (1996) Matrix-assisted laser desorption ionization MS: a progress report. Biochem SocTrans 24:897–900
Karas M, Bachmann D, Bahr U, Hillenkamp F (1987) Matrix-assisted ultraviolet laser desorption of non-volatile compounds. Int J Mass Spectrom Ion Process 78:53–68
Kundu JK, Surh YJ (2008) Inflammatiom: gearing the journey to cancer. Mutat Res 659:15–30
Leenen R, Roodenburg AJ, Tijburg LB, Wiseman SA (2000) A single dose of tea with or without milk increases plasma antioxidant activity in humans. Eur J Clin Nutr 54:87–92
Liu M, Li Y, Chou G, Cheng X, Zhang M, Wang Z (2007) Extraction and ultra-performance liquid chromatography of hydrophilic and lipophilic bioactive components in a Chinese herb Radix Salviae Miltiorrhizae. J Chromatogr A 1157:51–55
McDonald S, Prenzler PD, Antolovich M, Robards K (2001) Phenolic content and antioxidant activity of olive extracts. Food Chem 73:73–84
Mitrunen K, Jourenkova N, Kataja V, Eskelinen M, Kosma VM, Benhamou S, Vainio H, Uusitupa M, Hirvonen A (2001) Glutathione S-transferase, M1, M3, P1 and T1 genetic polymorphism and susceptibility to breast cancer. Cancer Epidemiol Biomark Prev 10:229–236
Narayan C, Kumar A (2012) Constitutive over-expression of IL-1β, IL-6, NF-κB and Stat3 is a potential cause of lung tumor-genesis in ethyl carbamate induced Balb/c mice. J Carcinog 11:9
Ng TB, Liu F, Wang ZT (2000) Antioxidative activity of natural products from plants. Life Sci 66:709–723
Nováková L, Matysová L, Solich P (2006) Advantages of application of UPLC in pharmaceutical analysis. Talanta 68:908–918
Pan GX, Spencer L, Leary GJ (1999) Reactivity of ferulic acid and its derivatives towards hydrogen peroxide and peracetic acid. J Agri Food Chem 47:3325–3331
Ray G, Husain SA (2002) Oxidants, antioxidants and carcinogenesis. Indian J Exp Biol 40:1213–1232
Reed JD, Krueger CG, Vestling MM (2005) MALDI-TOF mass spectrometry of oligomeric food polyphenols. Phytochemistry 66:2248–2263
Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB (1995) The relative antioxidant activities of plant derived polyphenolic flavonoids. Free Radic Res 22:375–383
Robards K (2003) Strategies for the determination of bioactice phenols in plants, fruit and vegetables. J Chromatogr A 1000:657–691
Singh V (1995) Traditional remedies to treat asthma in north west and trans himalayan regions in J. and K. state. Fitoterapia 66:507–509
Sinha SKP, Dogra JVV (1985) A survey of plants of Bhagalpur and Santhal pargana for saponin, flavonoids and alkaloids. Int J Crude Drug Res 23:77–86
Subbarayan PR, Sarkar M, Impellizzeri S, Raymo F, Lokeshwar BL, Kumar P, Agarwal RP, Ardalan B (2010) Anti-proliferative and anti-cancer properties of Achyranthes aspera: specific inhibitory activity against pancreatic cells. J Enthnopharmacol 131:78–82
Sun Y (1990) Free radicals, antioxidant enzyme and carcinogenesis. Free Radic Biol Med 8:583–99
Suresh A, Anandan T, Sivanandam G, Veluchamy G (1985) A pilot study of Naayyuruvi kuzhi Thailam in Eraippunoi (bronchial asthma). J Res Ayur Sidha 6:171–176
Tahiliani P, Kar A (2000) Achyranthes aspera elevates thyroid hormone levels and decreases hepatic lipid peroxidation in male rats. J Enthanopharmacol 71:527–532
Trautvetter S, Koelling-Speer I, Speer K (2009) Confirmation of phenolic acid and flavonoids in honey by UPLC-MS. Apidologie 40:140–150
Tripathi SN, Chaturvedi GN, Dube GP (1963) Effect of Achyranthes aspera in the treatment of leprosy. J Med Sci (BHU) 4:103–112
Vasudeva RY, Duddukuri GR, Sunil BG, Athota RR (2002) Immunomodulatory activity of Achyranthes aspera on the elicittion of antigen-specific murine antibody response. Pharma Bio 40:175–178
Vetrichelvan T, Jegadeesan M (2003) Effect of alcohol extract of Achyranthes aspera Linn.on acute and subacute inflammation. Phytother Res 17:77–79
Wang J, Sporns P (2000) MALDI-TOF MS analysis of food flavonol glycosides. J Agri Food Chem 48:1657–1662
Wang X, Zhao T, Gao X, Dan M, Zhou M, Jia W (2007) Simultaneous determination of 17 ginsenosides in rat urine by ultra performance liquid chromatography-mass spectrometry with solid-phase extraction. Anal Chim Acta 594:265–273
Zhang Y, Jiao J, Cai Z, Zhang Y, Ren Y (2007) An improved method validation for rapid determination of acrylamide in foods by ultra-performance liquid chromatography combined with tandem mass spectrometry. J Chromatogr A 1142:194–198
Acknowledgments
C Narayan is thankful to University Grant Commission (UGC), New Delhi for awarding Senior Research Fellowship (SRF), providing financial support to conduct this research. We extend our sincere thanks also to Sunil K. Yadav, animal attendant to handle animals and Nirpendra Singh, University of Delhi South Campus for technical assistant to perform UPLC and MALDI-TOF-MS experiment.
Conflict of interest
There is no conflict of interest
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Narayan, C., Kumar, A. Identification and characterization of phenolic compounds in hydro methanolic extract of Achyranthes aspera (HMEA) by UPLC and MALDI-TOF-MS and in vivo antioxidant activity. Orient Pharm Exp Med 13, 51–59 (2013). https://doi.org/10.1007/s13596-012-0085-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-012-0085-z