Abstract
The present study was aimed to compare the phenolic composition and the functional properties (antioxidant and anticancer) of phenolic-rich extracts of two different propolis collected from Collo (PREPC) and Oum El Bouaghi (PREPO) regions. The phenolic composition was determined using LC/MS–MS. The antioxidant activity of the extracts was evaluated using alkaline DMSO and β-carotene-linoleic acid tests. Anticancer effect on HepG2 human hepatocellular carcinoma cells was determined using CCK-8 assay. Twenty-two phenolic compounds in PREPC and twenty-three in PREPO were detected and quantified by LC–MS/MS. Ferulic and caffeic acids were found to be the predominant compounds. Both extracts were able to inhibit lipid peroxidation and demonstrated their ability to scavenge superoxide radicals more effectively than the standards. The highest lipid peroxidation inhibition (IC50 = 17.58 ± 1.98 μg/mL) and superoxide radical scavenging effects (IC50 = 6.19 ± 0.24 μg/mL) were exhibited by PREPO. However, PREPC showed stronger cytotoxic activity against HepG2 (IC50 = 12.22 ± 0.05 µg/mL) than PREPO (IC50 = 18.68 ± 0.33 µg/mL). These results demonstrate the potential of extracts from Algerian propolis to be used in functional formulations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver cancer is the 5th most common cancer type and was reported as the 3rd common deadly cancer worldwide [1]. It is occurred by the presence of hepatocellular damage through reactive oxygen species and the generation of chronic inflammation related to hepatocarcinogenesis [2]. Emerging evidence states that diet is recognized as a potential lifestyle-related risk factor for the development of liver cancer. Hence, a healthy diet may play a preventative role in the development of such a life-threatening disease [3]. Bioactive foods with anticancer potential not only provide nutritional benefits, but also inhibit cancer progression within the human body [4]. Propolis, a natural bee product, is extensively used as an ingredient in functional foods [5]. It is known to have many diverse biological properties such as antimicrobial, anti-inflammatory, anticancer, and antioxidant activities [6]. Its therapeutic properties are due to its chemical composition and are mainly associated with the presence of biologically active components including different flavonoids, polyphenolic esters, caffeic acids and their esters [7]. The chemical composition of propolis is affected by botanical and geographical factors leading to variations in propolis bioactivities [8]. Hence, propolis from different regions may contain different bioactive compounds and could exhibit different biological activities.
Research on the chemical composition and functional potential of Algerian propolis are still very scarce. At present, there are no published studies on the cytotoxic effect of Algerian propolis against human liver cancer cells. The goal of this investigation, therefore, was to analyze and compare the phenolic composition of two phenolic-rich extracts obtained from two different propolis. Their antioxidant and anticancer effects against HepG2 human hepatocellular carcinoma cells were also evaluated.
Materials and methods
Reagents and chemicals
Standard compounds (purity ≥ 99%) used for LC–MS/MS analysis were purchased from Sigma-Aldrich Chemical Co., Ltd (St. Louis, Missouri, USA). Standards and reagents used in antioxidant activity were purchased from Sigma Chemical Co. (Sigma-Aldrich GmbH, Stern-heim, Germany). Solvents used for extraction and analysis were of analytical and HPLC‐MS grades, respectively. Human hepatocellular carcinoma (HepG2) cell line was obtained from the American Type Culture Collection (USA). Cell Counting Kit-8 (CCK-8) was purchased from Abcam (UK). Fetal bovine serum (FBS), Penicillin and Streptomycin were purchased from PAN-Biotech (GmbH, Germany).
Propolis collection and phenolic-rich extracts preparation
Two propolis samples produced by the honey bee Apis mellifera, were collected by beekeepers from beehives. Propolis (PO) was collected from hives located in Oum El Bouaghi (35° 52′ 39″ N, 7° 06′ 49″ E), which is a semi-arid region and propolis (PC) was collected from Collo (37° 00′ 23″ N, 6° 33′ 39″ E), which is a humid region. The samples were collected by scraping frames, walls and the entrance of the beehive. After removing impurities such as parts of plants and insects, the crude propolis sample was kept in freezer and then the frozen propolis was powdered.
The preparation of PREPO (phenolic-rich extract from PO) and PREPC (phenolic-rich extract from PC) was carried out according to Park and Ikegaki [9]. Briefly, 20 g of propolis was extracted three times with 200 mL hydroalcoholic solution (80% Methanol, 20% Distillated water) for 72 h. After filtration, the filtrate was evaporated by rotary evaporator (under 50 °C temperature) to obtain dry extract and stored under dry conditions at 4 °C until analyzed.
For chemical and antioxidant studies, 1 mg of each extract was dissolved in 1 mL of methanol. However, for anticancer assay, 2 mg of each extract was dissolved in 1% DMSO (50 µL DMSO, 4950 µL growth medium) and diluted with growth medium to the desired concentration prior to exposure.
Chemical composition analysis by LC–MS/MS
The phenolic component of the extracts was analyzed by using an LC (Agilent 1260 Infinity) system coupled to a triple quadrupole mass spectrometer (Agilent 6420 Triple Quadrupole LC–MS). The chromatographic separation of the phenolic compounds was carried out on a C18 reversed-phase ODS column (25 × 4.6 mm × 5 μm). The injection volume of the standards and the samples was 2 μL. The mobile phase consisted of water/0.1% formic acid (eluent A), methyl alcohol (eluent B) at a flow rate of 0.4 mL/min. The elution conditions were as follows: 2% B for 3 min, 25% B for 6 min, 50% B for 10 min, 95% B for 14 min, 2% B for 17.5 min. MS analysis was performed in both positive and negative ionization modes. The multiple reaction monitoring (MRM) mode was used to quantify the analytes. The LC–MS/MS data were collected and processed by Mass Hunter software (version B.07.01). The phenolic compounds of samples were identified by comparing their retention time, UV profile and mass spectra with those of authentic standards. All the phenolics detected were quantified using the calibration curves of corresponding standard solutions and the results were expressed as nanograms per milligram of dried propolis extract.
Antioxidant activities
Superoxide radical scavenging activity
The scavenging activity of extracts towards the superoxide radical (O2·−) was measured in terms of inhibition of generation of O2·−. The method was performed by using alkaline DMSO method as reported in Bensouici et al. [10]. Superoxide radical (O2· −) is generated by the addition of sodium hydroxide (NaOH) to air saturated DMSO. The generated superoxide remains stable in solution and reduces nitroblue tetrazolium (NBT) into formazan dye at room temperature, which can be measured at 560 nm [11]. Briefly, to the reaction mixture containing 40 μL of extract (or standard compound) at various concentrations and 130 µL of alkaline DMSO (100 mL DMSO containing, 20 mg NaOH in 1 mL distilled water), 30 µL NBT (1 mg/mL solution in distilled water) was added and absorbance was noted at 560 nm against blank samples. The decrease in the absorbance of the reaction sample indicated the increase in superoxide anion scavenging activity. The percent inhibition of superoxide anion generation was calculated using the following formula:
I%: inhibition percentage, Ac: absorbance in the presence of the control. As: Absorbance in the presence of the sample.
The results are expressed as IC50 value (μg/mL).
Lipid-peroxidation inhibitory activity
The lipid peroxidation inhibitory potential of the extracts was determined by the β-carotene-linoleic acid test system as mentioned in Bensouici et al. [10]. In this model, β-carotene undergoes rapid discoloration in the absence of an antioxidant because of the coupled oxidation of β-carotene and linoleic acid, which generates free radicals. The linoleic acid free radical (formed upon the withdrawal of a hydrogen atom from one of its diallylic methylene groups) attacks the highly unsaturated β-carotene molecules. As a result, β-carotene is oxidized and partly broken down; subsequently the system loses its chromophore [12]. The addition of an antioxidant inhibits lipid peroxidation and thus delays β-carotene bleaching. Briefly, β-carotene (0.5 mg) in 1 mL of chloroform and 25 µL of linoleic acid were dissolved in 200 µL of Tween 40 emulsifier mixture. After evaporation of chloroform under vacuum, 50 mL of distilled water saturated with oxygen, were added by vigorous shaking. The assay mixture, containing 160 µL β-carotene emulsion and 40 µL of extract solution, was incubated at 45 °C. After 120 min, the decrease in the absorbance of β-carotene was measured at 470. The antioxidant activity was expressed as percent inhibition relative to the control using the following equation:
where I% is the inhibition percentage, AS0 is the initial absorbance at time 0 in the presence of the sample, ASt is the absorbance at time 120 min in the presence of the sample, AC0 is the initial absorbance at time 0 in the presence of the control and ACt is the absorbance at time 120 min in the presence of the control.
The results are expressed as IC50 value (μg/mL).
Anticancer study
Cell culture
The human hepatocellular carcinoma (HepG2) cell line was maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with l-glutamine, 10% (v/v) heat-inactivated fetal bovine serum (FBS), 100 IU/mL penicillin and 100 μg/mL streptomycin at 37 °C in a humidified incubator with 5%CO2. Cells were checked under Zeiss PrimoVert inverted microscope, and subculturing was performed when cells reached 80% confluency.
Cytotoxicity assay
The cytotoxicity of the extracts on HepG2 cells was determined by using the Cell Counting Kit-8 (CCK-8) assay according to the manufacturer’s instructions. Briefly, the cells were counted using a trypan blue solution. Next, 100 µL of cell suspension (1 × 105 cells per well) was plated into 96-well plate and incubated at 37 °C in a CO2 incubator (5%) for 24 h. Then, cells were treated with serial concentrations (3.125, 6.25, 12.5, 25, 50, 100 and 200 µg/mL) of each extract (100 µL) and incubated for 72 h. Thereafter, cells were washed and 100 µL of fresh medium was added. Then, 10 μL of CCK-8 solution was added to each well and incubated at 37 °C for 3 h. Absorbance at 450 nm was determined using a microplate reader. The cytotoxic activity was measured using the following equation and the results were given as IC50 value.
Cell morphology analysis
The morphological changes in HepG2 cells exposed to increasing concentrations (3.125–200 μg/mL) of PREPC and PREPO were investigated using an inverted phase microscope (PrimoVert, Zeiss) at 40 X magnification and compared with control cells.
Statistical analysis
Except LC–MS/MS, the results were illustrated as means ± standard deviation of three measurements. The IC50 values were calculated by linear regression analysis. Data were analyzed by Student t-test using GraphPad Prism software (version 6.0.1). Results were considered statistically significant at p < 0.05.
Results
Identification and quantification of phenolic compounds by LC–MS/MS analysis
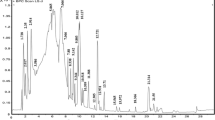
The phenolic profiles of the extracts were analyzed by LC–MS/MS triple quadrupole. Figure 1 shows the chromatograms while Table 1 shows the content of each propolis extract. Twenty-two phenolic compounds were detected in PREPC, whereas twenty-three compounds were identified in PREPO. Twenty-two phenolics were common between the two propolis but with different amounts. One compound was detected only in PREPO, which is 3,4-dihydroxyphenylacetic acid. The molecules identified in the extracts belong to four phenolic sub-classes including phenolic acids, flavonoids, lignans and other polyphenols. Phenolic acids were the major phenolic sub-class found in both extracts followed by flavonoids. The predominant individual phenolic compounds in PREPC were ferulic and caffeic acids followed by apigenin and kaempferol. Gallic acid, p-coumaric acid and quercetin were also found in high amount. The most abundant components in PREPO, however, were ferulic and caffeic acids followed by verbascoside and quercetin. Kaempferol and apigenin were also determined in high quantity.
Antioxidant activities
Superoxide radical scavenging activity
The scavenging of superoxide radical by PREPO and PREPC was evaluated by Alkaline DMSO method, in which O2·− was produced chemically without the presence of enzymes. In non-enzymatic system, compounds can exert an antioxidant activity by reducing the production of O2·−, or by a stabilizing action of the radical when donating or receiving electrons to the O2·− radical [13]. As shown in Fig. 2, both extracts showed a concentration-dependent increase in inhibition of superoxide generation. Maximum O2·− scavenging activity was observed at a final concentration of 200 μg/mL. Both extracts, however, exhibited a higher radical scavenging activity than the positive standards BHT and BHA (Table 2). Based on the IC50 values, the scavenging potential of the extracts was in the following order: PREPO > PREPC > BHT > BHA.
Lipid-peroxidation inhibitory activity
The effect of extracts on lipid peroxidation inhibition was determined by β-carotene/linoleic acid system. Both extracts showed good ability in this respect and inhibited the bleaching of β-carotene in a concentration-dependent manner (Fig. 3). The maximum inhibition percentages (99.76 ± 0.14%, 95.45 ± 1.70%, 95.28 ± 3.25% and 94.06 ± 1.22%) were reached at the final assay concentration (200 µg/mL) of BHA, PREPC, BHT and PREPO, respectively. Based on the IC50 values (Table 2), the samples can be classified in their effectiveness against β-carotene bleaching as follows: BHA > BHT > PREPO > PREPC.
Anticancer study
Cytotoxicity
The results of cytotoxicity assay revealed a clear dose-dependent cytotoxicity response against HepG2 cells 72-h posttreatment with PREPC and PREPO (Fig. 4). The maximum inhibition percentages 81.98 ± 0.15% and 81.83 ± 0.22% were reached at the final assay concentration (200 µg/mL) of PREPO and PREPC, respectively. These values, however, did not show any significant difference (p > 0.05). The IC50 values were found to be 12.22 ± 0.05 µg/mL for PREPC and 18.68 ± 0.33 µg/mL for PREPO, indicating a stronger cytotoxic effect of PREPC on HepG2 cells compared with that of PREPO.
Cell morphology analysis
The cytotoxic effects of PREPC and PREPO were further studied by morphological cellular imaging (Figs. 5 and 6). It was observed that untreated and 1%DMSO‑treated cells maintained a normal morphology. However, HepG2 cells treated with PREPC and PREPO within 72-h period lost the typical morphology in a concentration dependent manner. At lower concentrations (12.5 μg/mL and less) of PREPC and PREPO, the changes were less significant while at 25 μg/mL and higher concentrations, the changes were much more severe including loss of normal morphology and cellular junctions, reduction in cell volume and formation of apoptotic bodies. Most cells at higher concentrations lost contact with adjacent cells and acquired a spherical shape compared to untreated cells. These morphological alterations induced by PREPC and PREPO in HepG2 cells could be attributed to an apoptotic mechanism.
Discussion
The phenolic profiles of PREPC and PREPO were analyzed by LC–MS/MS and have shown the presence of several components (Table 1). There were some qualitative and quantitative differences between the two extracts, which could be explained by the difference in geographical origin of propolis. In line with the current results, Soltani et al. [14] also studied extracts from propolis samples collected from different locations in Algeria and confirmed the influence of geographical origin on the variation of the chemical profile of this material. Similar to other Algerian propolis, gallic acid, caffeic acid, p-coumaric acid, ferulic acid, chlorogenic acid, quercetin, apigenin, kaempferol have also been identified in PREPC and PREPO [15,16,17,18], which could be used for quality determination and standardization of Algerian propolis. However, the other compounds reported in the current study have never been identified in Algerian propolis but have been detected in propolis from other countries. Apigenin 7-glucoside, hesperidin, hyperoside and verbascoside have been detected in Cypriot propolis [19]. Protocatechic acid, p-hydroxybenzoic acid, syringic acid, eriodictiol, luteolin and vanillin have been found in Turkish propolis [20]. Homovanillic acid and pinorisenol have been characterized in Brazilian propolis [21, 22]. Taxifolin has been found in Moroccan propolis [23]. Pyrocatechol has been identified in Trigona laeviceps stingless bee propolis from Indonesia [24]. 3,4-Dihydroxyphenylacetic acid, however, has not been reported in propolis samples to the best of our knowledge.
The antioxidative potential of PREPC and PREPO was determined by their ability to scavenge superoxide anion radicals and to inhibit lipid peroxidation. Both extracts were found to be more effective in scavenging superoxide anion radical than the standard antioxidants, which could be related to their contents of phenolic compounds that have been recognized as powerful antioxidant agents, mainly due to their hydroxyl groups [25]. Phenolic compounds can play an important role in absorbing and neutralizing free radicals, quenching singlet and triplet oxygen, or decomposing peroxides [26]. In this study, PREPO showed higher superoxide radical scavenging and lipid peroxidation inhibition potentials than PREPC. This could be explained by the chemical analysis that revealed that PREPO is qualitatively and quantitatively richer in phenolic content especially flavonoids than PREPC. These findings are in good accordance with previous studies that have correlated the phenolic and flavonoid composition of propolis extracts with its antioxidant properties [16, 27]. However, studies on superoxide radical scavenging and lipid peroxidation inhibition properties of propolis methanolic extracts from different geographical origins showed variable effects. Miguel et al. [28] stated that Portuguese propolis exhibited superoxide radical scavenging with IC50 values ranged from 0.001 ± 0.003 to 0.053 ± 0.003 mg/mL, while Ichikawa et al. [29] indicated an IC50 value of 6.2 mg/mL of Brazilian propolis. Bouaroura et al. [30] who investigated the capacity of some Algerian propolis methanolic extracts to inhibit lipid peroxidation in β-carotene-linoleic acid system indicated IC50 values ranged from 11.34 ± 0.17 to 40.38 ± 0.39 µg/mL. In another study, propolis from Mila has been reported to inhibit lipid peroxidation with an IC50 value of 43.46 ± 0.03 µg/mL [31]. These differences in the effects of propolis from different collection sites could be due to the variation in propolis composition [32].
Propolis is a widely used bee product with broad biological activities including antitumor properties [33]. Many reports have demonstrated the cytotoxic effects of Algerian propolis in several human cancer cell lines including breast adenocarcinoma MCF-7, mammary gland adenocarcinoma MDA-MB-231, epithelial adenocarcinoma HeLa, prostate cancer PC3, myelogenous leukemia K562, pancreatic PANC-1 cancer and lung adenocarcinoma A549 cell lines [34,35,36,37]. However, no scientific study using liver cancer cell lines has ever been carried out to confirm its potential in the management of liver cancer. This study, therefore, was performed to evaluate the cytotoxic effect of PREPC and PREPO against human liver cancer HepG2 cell line. The obtained results revealed that PREPC and PREPO possess significant potential to inhibit the HepG2 cancer cells. Previous studies on propolis extracts from various regions of the world have stated its anticancer potential on human hepatocellular carcinoma cells. Turan et al. [38] and Gokduman [39] investigated the cytotoxic effect of Turkish propolis and reported IC50 values of 27.0 ± 0.8 µg/mL and 25.62 ± 1.50 µg/mL, however, these values are higher than those obtained in the current study. Abu Shady et al. [40] and Abd El-Hady et al. [41] indicated that Egyptian and Sudanese propolis exhibited anticancer potentials against HepG2 with IC50 values within the range of 62.5–70.9 µg/mL and 57–60 µg/mL, respectively. Such results were also higher than our findings. In another study by Sadeghi-Aliabadi et al. [42], Iranian propolis was found to exhibit a potent cytotoxicity with an IC50 value of 15 µg/mL, which is closer to our results. The anticancer activity of PREPC and PREPO in this study was very interesting because of the possible cytotoxic effects of their phytoconstituents. Their chemical profiles revealed the presence of several bioactive compounds that have been reported to exhibit anticancer activity such as ferulic acid, cafeic acid, apigenin and quercetin [43,44,45,46].
Induction of cancer cell apoptosis is a beneficial mechanism for cancer treatment [47]. The morphological cellular imaging in this study revealed that PREPC and PREPO mediated cytotoxic effect against HepG2 cells possibly via induction of apoptosis, which is in agreement with previous works that demonstrated the apoptotic effect of extracts from Algerian propolis in cancer cells [34, 35]. However, the precise mechanisms of action remain to be elucidated.
Conclusion
This work is the first report about the chemical profile and anticancer activity of phenolic-rich extracts from propolis obtained from Collo and Oum El Bouaghi regions (Algeria). The extracts were found to be rich in phenolic compounds especially PREPO, in which a new phenolic compound 3,4-Dihydroxyphenylacetic acid was identified for the first time in propolis. The results showed also the antioxidant and anticancer importance of Algerian propolis, which support the potential health benefits of propolis as a potential candidate for developing functional food products. Further studies, however, are needed to determine their mechanisms of action and their safety.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
R.G. Aktas, Trak. Univ. J. Nat. Sci. 20, S55–S61 (2019). https://doi.org/10.23902/trkjnat.486558
J. Thusyanthan, N.S. Wickramaratne, K.S. Senathilake, U. Rajagopalan, K.H. Tennekoon, I. Thabrew et al., Adv. Pharmacol. Pharm. Sci. (2022). https://doi.org/10.1155/2022/6407688
E.S. George, S. Sood, A. Broughton, G. Cogan, M. Hickey, W.S. Chan et al., Nutrients 13(1), 172 (2021). https://doi.org/10.3390/nu13010172
P. Gupta, Functional Foods in Cancer Prevention and Therapy (Elsevier, Amsterdam, 2020), pp.33–46
F.C. Danert, C. Zampini, R. Ordoñez, L. Maldonado, E. Bedascarrasbure, M.I. Isla, Nat. Prod. Commun. 5, 2 (2014). https://doi.org/10.1177/1934578X1400900209
N. Abutaha, M. Al-Zharani, A. Alotaibi, M.A.W. Cordero, A. Bepari, S. Alarifi, Open Chem. (2021). https://doi.org/10.1515/chem-2021-0076
R.D. Bruce, Report, Australia Publication (2005). http://www.rirdc.gov.au/reports/HBE/05-040.pdf
V. Lagouri, D. Prasianaki, F. Krysta, Int. J. Food Prop. 17(3), 511–522 (2014)
Y.K. Park, M. Ikegaki, Biosci. Biotechnol. Biochem. 62(11), 2230–2232 (1998). https://doi.org/10.1271/bbb.62.2230
C. Bensouici, T. Boudiar, I. Kashi, K. Bouhedjar, A. Boumechhour, L. Khatabi, H. Larguet, J. Food Meas. Charact. (2019). https://doi.org/10.1007/s11694-019-00309-y
S. Bendjabeur, O. Benchabane, C. Bensouici, M. Hazzit, A. Baaliouamer, A. Bitam, J. Food Meas. Charact. 12, 2278–2288 (2018)
M. Amensour, E. Sendra, J. Abrini, S. Bouhdid, J.A. Pérez-Alvarez, J. Fernández-López, Nat. Prod. Commun. 4(6), 819–824 (2009). https://doi.org/10.1177/1934578x0900400616
S. Pombal, A.M. Roncero, I.E. Tobal, N. García, L. Silva, D. Diez et al., Nat. Prod. Commun. 15(2), 1–6 (2020). https://doi.org/10.1177/1934578X20908081
E.K. Soltani, K. Mokhnache, S. Mezaache-Aichour, N. Charef, J.P. De Haro, M.M. Zerroug et al., J. Drug Deliv. Ther. 10(2), 12–19 (2020)
A.G. Hegazi, F.K. Abd El Hady, K.H. Shaker, N. Modear, S. Houssin, Mellifera 12(23), 2–11 (2012)
N. Segueni, D. Evren, S. Akkal, B.O. Ozcelik, S. Rhouati, Pak. J. Pharm. Sci. 30(4 Suppl), 1417–1423 (2017)
S. Chaa, M.Y. Boufadi, S. Keddari, A.H. Benchaib, J. Soubhye, P. Van Antwerpen et al., Rev. Bras. Farmacogn. 29, 294–300 (2019). https://doi.org/10.1016/j.bjp.2019.01.005
A. Daikh, N. Segueni, N.M. Dogan, S. Arslan, D. Mutlu, I. Kivrak et al., J. Apic. Res. (2019). https://doi.org/10.1080/00218839.2019.1701777
A. Nalbantsoy, N.B. Sarıkahya, C.S. Ozverel, A.B. Barlas, D. Kirci, I.H. Akgun et al., J. Apic. Res. 61, 233–245 (2022). https://doi.org/10.1080/00218839.2021.1977028
H.A. Kahraman, H. Tutun, M.M. Kaya, M.S. Usluer, S. Tutun, C. Yaman et al., Biotechnol. Biotechnol. Equip. 36(1), 44 (2022). https://doi.org/10.1080/13102818.2022.2045217
A.A. Righi, G. Negri, A. Salatino, Evid Based Complement. Alternat. Med. (2013). https://doi.org/10.1155/2013/267878
V.P. Ribeiro, C. Arruda, J.A. Aldana-Mejia, J.K. Bastos, S.K. Tripathi, S.I. Khan et al., Chem. Biodivers. 18, 9 (2021). https://doi.org/10.1002/cbdv.202100288
O. Belmehdi, A. Bouyahya, J. Jeko, Z. Cziaky, G. Zengin, G. Sotko et al., Int. J. Second. Metab. 8(3), 195–213 (2021). https://doi.org/10.21448/ijsm.947033
I. Wibowo, N. Utami, T. Anggraeni, A. Barlian, R.E. Putra, A.D. Indriani et al., Zebrafish (2021). https://doi.org/10.1089/zeb.2020.1969
M. Ozturk, F. Aydogmus-Ozturk, M.E. Duru, G. Topçu, Food Chem. 103(2), 623–630 (2007). https://doi.org/10.1016/j.foodchem.2006.09.005
N.T. Manojlovic, P.J. Vasiljevic, P.Z. Maskovic, Rev. Bras. Farmacogn. 22(2), 291–298 (2012). https://doi.org/10.1590/s0102-695x2011005000184
T. Hamasaka, S. Kumazawa, T. Fujimoto, T. Nakayama, Food Sci. Technol. Res. 10(1), 86–92 (2004). https://doi.org/10.3136/fstr.10.86
M.G. Miguel, S. Nunes, S.A. Dandlen, A.M. Cavaco, M.D. Antunes, Food Sci. Technol. 34(1), 16–23 (2014)
H. Ichikawa, K. Satoh, T. Tobe, I. Yasuda, F. Ushio, K. Matsumoto et al., Redox Rep. 7(5), 347–350 (2002). https://doi.org/10.1179/135100002125000965
A. Bouaroura, N. Segueni, R. Erenler, A. May, C. Bensouici, S. Akkal et al., Curr. Bioact. Compd. 17(8), 79–87 (2021). https://doi.org/10.2174/1573407216999201231200041
S. Boulechfar, A. Zellagui, A.E. Chemsa, C. Bensouici, N. Segueni, M. Lahouel et al., J. Biol. Act. Prod. Nat. 9(6), 434–444 (2019). https://doi.org/10.1080/22311866.2019.1703816
L.L. Chaillou, M.A. Nazareno, J. Sci. Food Agric. 89, 978–983 (2009). https://doi.org/10.1002/jsfa.3542
D. Sawicka, H. Car, M.H. Borawska, J. Nikliński, Folia Histochem. Cytobiol. 50(1), 25–37 (2012)
W. Kebsa, M. Lahouel, H. Rouibah, M. Zihlif, M. Ahram, B. Aburmeleih et al., Anticancer Agents Med. Chem. 18(9), 1330–1337 (2018). https://doi.org/10.2174/1871520618666180808100800
H. Rouibah, W. Kebsa, M. Lahouel, M. Zihlif, M. Ahram, B. Aburmeleih et al., Anti-Cancer Agents Med. Chem. 18(3), 375–387 (2018). https://doi.org/10.2174/1871520618666180110143239
H. Brihoum, M. Maiza, H. Sahali, M. Boulmeltout, G. Barratt, L. Benguedouar et al., Braz. J. Pharm. Sci. 54(1), e17396 (2018). https://doi.org/10.1590/s2175-97902018000117396
B. Bakchiche, I.K. Temizer, A. Güder, O.G. Çelemli, S.C. Yegin, S.K. Bardaweel et al., J. Appl. Biotechnol. Rep. 7(2), 93–103 (2020). https://doi.org/10.30491/JABR.2020.109498
I. Turan, S. Demir, S. Misir, K. Kilinc, A. Mentese, Y. Aliyazicioglu et al., Trop. J. Pharm. Res. 14(5), 777 (2015). https://doi.org/10.4314/tjpr.v14i5.5
K. Gokduman, Med. Sci. 8(3), 557–562 (2019)
H.M. Abu Shady, W.F. Mohamed, E.F. Sayed-Ahmed, S.A. Amer, Int. J. Curr. Microbiol. Appl. Sci. 5(3), 397–414 (2016). https://doi.org/10.20546/ijcmas.2016.503.047
F.K. Abd El-Hady, A.M.A. Souleman, I.G. Ibrahim, M.S. Abdel-Aziz, Z.A. El-Shahid, E.A.M. Ali, M.S.A. Elsarrag, Der Pharm. Lett. 8(19), 339–350 (2016)
H. Sadeghi-Aliabadi, J. Hamzeh, M. Mirian, Adv. Biomed. Res. 4(1), 42 (2015). https://doi.org/10.4103/2277-9175.151251
S. Ou, K.C. Kwok, J. Sci. Food. Agric. 84(11), 1261–1269 (2004). https://doi.org/10.1002/jsfa.1873
N.R. Prasad, A. Karthikeyan, S. Karthikeyan, B.V. Reddy, Mol. Cell. Biochem. 349, 11–19 (2011)
J. Madunic, I.V. Madunic, G. Gajski, J. Popic, V. Garaj-Vrhovac, Cancer Lett. 413, 11–22 (2018). https://doi.org/10.1016/j.canlet.2017.10.041
M. Azeem, M. Hanif, K. Mahmood, N. Ameer, F.R.S. Chughtai, U. Abid, Polym. Bull. (2022). https://doi.org/10.1007/s00289-022-04091-8
T. Senawong, S. Khaopha, S. Misuna, J. Komaikul, G. Senawong, P. Wongphakham et al., Sci. Asia 40, 420–427 (2014). https://doi.org/10.2306/scienceasia1513-1874.2014.40.420
Acknowledgements
Authors are grateful and thank the beekeepers Yassine Hadjem and Laidi Zaier who supplied Apis mellifera propolis samples from Oum el Bouaghi and Collo regions, respectively. The financial support from Algerian Ministry of Higher Education and Scientific Research and DGRSDT (PRFU Project, Code: D01N01UN040120180002) is gratefully acknowledged.
Funding
This work was supported by Algerian Ministry of Higher Education and Scientific Research and DGRSDT (PRFU Project, Code: D01N01UN040120180002).
Author information
Authors and Affiliations
Contributions
SB: Data curation, Formal analysis, Investigation, Visualization, Writing-original draft, Writing-review and editing. ZA: Data curation, Formal analysis, Investigation, Methodology. HDT: Data curation, Formal analysis, Investigation, Methodology. AZ: Project administration, Supervision, Validation, Visualization, Writing-review and editing. RGA: Supervision, Methodology, Resources, Validation, Writing-review and editing. CB: Supervision, Methodology, Resources, Data curation, Validation. Writing-review and editing. FD: Formal analysis, Data curation. LK: Resources, Data curation. GD: Resources, Data curation. ML: Writing-review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boulechfar, S., Akbulut, Z., Tepe, H.D. et al. LC–MS/MS analysis, antioxidant and anticancer effects of phenolic-rich extracts from Algerian propolis: a comparative study. Food Measure 17, 564–575 (2023). https://doi.org/10.1007/s11694-022-01652-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01652-3