Abstract
The aim of this study was to elucidate the factors affecting the dispersibility and morphology of soy powders. The influences of the configuration of a spraying system (CSS) on particle size and dispersibility of soy powders were investigated. The dispersibility, morphology, and particle sizes of powders produced using a wheel and those obtained using a nozzle differed. A moderate increase in particle size (from 15 to 24 μm) improved powder dispersibility in most cases. At a total protein content in the powder (w/w%)/total solids in the concentrate (w/w%) ratio of between 30/20 and 35/20, using the wheel significantly increased particle size (from 23 to 40 μm) in comparison to nozzle (from 14 to 20 μm); moreover, this increase in particle size was correlated to increase in dispersibility (r 2 = 0.90). These results are in agreement with published findings showing that a surface area that is too large (small particles) can lead to poor dispersibility of a powder in the case of both whey protein and micellar casein. In addition, the wheel provided a smaller span of particle sizes than the nozzle. The powders obtained with the wheel were therefore more homogeneous than those produced with the nozzle, making them easier to characterize in terms of functional properties. In summary, the dispersibility of soy powders depends on CSS with the wheel providing the most dispersible particles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Soy products, with their high levels of proteins, isoflavones, omega-3-fatty acid, and dietary fibers, are potential functional and nutritional ingredients in food manufacturing. Soy protein has potential health benefits since its consumption may reduce cholesterol and/or the risk of heart disease (Imram et al. 2003). Soy protein is currently available in the form of soy milk or as additives to human foodstuffs. The relevance of producing soy powders is to increase shelf-life and to facilitate handling, storage, and transport, including the shipping costs. The quality and functionality of powders containing proteins have been reported to be dependent on many factors such as protein concentrations in the powder (Nazareth et al. 2009; Kinsella 1979), and/or the spray-drying conditions (Schuck et al. 2004). According to Kinsella (1979), the more denatured the protein, the poorer its solubility. High protein content in a rehydrated soy powder leads to the formation of a precipitate which is the main issue for developing soy powder with high functional properties. In summary, the quality of the final powder is depending on the raw materials that constitute it and/or of the technique used to obtain it. A well-known method to produce soy powders is spray drying and on the laboratory scale either wheel or nozzle can be used. However, the wheel is rarely used on an industrial scale because of the adhesion of the particles to the wall of the spray drier, and to overcome this problem maltodextrin is generally used as a drying carrier. It has been reported that even under the best spray-drying conditions, a certain amount of maltodextrin is required to reduce the wall deposition phenomenon (Bhandari et al. 1993; Gupta 1978; Langrish et al. 2007). Another way to minimize the problem of stickiness during spray drying is to modify the surface properties of the droplets/particles by using small amounts of protein (Adhikari et al. 2003). The glass transition theory is also well reported as an explanation of the stickiness issues during spray drying (Roos and Karel 1991a, b; Schuck et al. 2007). For instance, formulas with high molecular weight additives can increase the glass transition temperature (T g) and therefore reduce the risk of stickiness (Tonon et al. 2009). In certain circumstances, degree of freedom to change the powder formula is restricted by nutritional considerations, and therefore addition of ingredients such as maltodextrins could be very limited. In these situations, using the nozzle or wheel could be appropriate to elucidate the stickiness of powders within the drying towers. However, the quality and the functionality of the powders obtained by spray drying depends on the configuration of the spraying system (CSS) used (wheel/nozzle). As a result, the structural modifications of the particles that can occur when a nozzle is replaced by a wheel, may affect certain features of powders such as wettability, sinkability, dispersibility, and solubility. It has been reported in the literature that modification of protein structure by chemical methods such as hydrolysis, to allow greater conformational flexibility, may improve its surface behavior and therefore its functionality (Carp et al. 1997; Kim and Kinsella 1987a, b; Wagner and Gueguen 1999). Nevertheless, these structural modifications decrease the stability of foam, and the addition of polysaccharides as stabilizers may be required (Nakamura et al. 2006). The shape and density of the particles of the final powders are influenced mainly by the bulk formula, rheology, and stability. In addition to the bulk formula, spray-drying conditions have a critical impact on the shape and density of the final powder. Basically, the spray-dried powders obtained from small-scale dryers (laboratory scale) is often small particle size (<50 μm), with poor rehydration properties (e.g., soy protein powder). These powders require agglomeration to improve handling and reconstitution properties in order to produce instant products (Turchiuli et al. 2011). According to Hino et al. (2000), spray-dried particles produced on a laboratory scale are amorphous, while those produced on an industrial scale are sometimes crystalline. Crystallinity difference may be due to variation in the initial droplet size and subsequent drying rate (Hino et al. 2000).
The feasibility of manufacturing soy powders with high protein content and suitable rehydration properties was evaluated in this work. Soy powder improved with maltodextrin was used as a model food powder to be spray dried using a pilot plant spray dryer equipped with a wheel or a nozzle. As the functional properties of the powders depend on its structure, the aim of this study was to investigate the possible impact of CSS on the structure of the spray-dried powder.
2 Materials and methods
2.1 Soy supreme fiber reduced
Soy supreme fiber reduced (SSFR) with 45% w/w total protein was purchased from SunOpta Grains and Food Group (ST. Hope, MN 56046, USA) and was used as the starting material for preparing different concentrates. The moisture content in SSFR was 4% w/w.
2.2 Maltodextrin
The maltodextrin (MD) containing 5% w/w in moisture content used as drying carrier was purchased from Glucidex Roquette (France). Two types of MD with different dextrose equivalents (DE 17 and DE 39) were used.
2.3 Preparation of model concentrates
Four different concentrates for these experiments were prepared by using different maltodextrins (DE 17 and DE 39) to adjust the total protein content (TP, expressed in percentage of dry matter in the total solid in the concentrate) at different total solids (TS, expressed in percentage of dry matter in the concentrate) in the concentrates (10/40, 20/30, 30/20, and 35/20 (TP (w/w%)/TS (w/w%)) by dissolving SSFR mixed with MD in osmosis water. The concentrates were stirred using a R1300 dissolver stirrer (IKA power basic) at 500 rpm for less than 1 h at room temperature to form homogeneous viscous fluids. The homogeneous concentrated viscous fluids were subsequently used as feeds for spray drying. The viscosity measurements were performed using an AR 2000 rheometer (TA instruments, Guyancourt, France) equipped with coaxial cylindrical geometry (stator inner radius, 25 mm; rotor outer radius, 23 mm; immersed cylinder height, 30.00 mm; bottom cap, 4 mm). Measurements were realized at 20 °C by using a rotor at an operating shear rate increasing from 1 to 10 s−1, and then decreasing from 10 to 1 s−1 at 20 °C. Apparent viscosity was determined using the Herschel–Bulkley model and it was considered as the viscosity value at the shear rate of 1 s−1. Concentrates with viscosities between 100 and 200 mPa.s were selected as a feed for spray drying to avoid sticking to the dryer. The viscosity of each concentrate was 105 ± 4.5, 186 ± 30, 158 ± 33, and 194 ± 0.5 mPa.s for 10/40, 20/30, 30/20, and 35/20 ratio respectively using MD 17. For MD 39 the viscosity of each concentrate was not measured because preliminary studies performed on these concentrates (using MD DE 6 and 29) showed that the viscosity decreased with the increasing of the dextrose equivalent.
2.4 Spray drying
A pilot-scale spray dryer (GEA Niro A/S, Mobile Minor Dryer (MMD), Søeborg, Denmark) was used to spray dry the homogeneous viscous fluids. The MMD was equipped with a peristaltic pump and a spraying system consisting in a rotary atomizer (wheel) or a two-fluid spray nozzle (concentrate/compressed air). The dimensions of the drying chamber cylinder height were 0.62 m, diameter 0.80 m, and 60 °C conical base. The MMD was operated in co-current mode. The drying air was dehumidified (10% of relative humidity) using a Munters dehumidifier (Sollentuna, Sweden) heated by electric batteries (power 7.5 kW). At 20 °C, the relative humidity of the drying air was 7% and the water content was 1 g of water for 1 kg of air (1 g/1 kg, water/air). Two different types of atomizer (wheel and nozzle, from GEA, Niro atomizer, France) have been used for blowing off droplets (Fig. 1). The two-fluid nozzle atomizer is composed of concentric tubes. One had an inner and outer diameter of 17 and 26 mm, respectively, within was placed another tube that the inner and outer diameter was 5 and 10 mm, respectively. The liquid and compressed air are not intermixed before blowing off. Liquid blown off from the inner tube (orifice of the nozzle ≈ 0.82 mm) is sheared by the compressed air flow from the outer tube (orifice ≈ 5.5 mm) creating a kind of cone composed of fine droplets (Fig. 1a). The rotary atomizer (Wheel, type F01A) is composed of a rotary disk and a wheel which had several rectangular orifices of high ≈ 5.5 mm and width ≈ 3.3 mm. Compressed air is injected into the cone-shaped system (Fig. 1b) caused the rotation of the disk which leads to the rotation of the wheel. Liquid brown off the orifices of the wheel is sheared by the centrifugal force caused by the rotation of the wheel creating droplets in all directions of the field of rotation of the wheel.
Whatever the type of atomizer used the compressed air was injected at a pressure of 2 bars. In consequence, the velocity of the wheel was ≈3,000 rpm. The peristaltic pump with a feeding rate of 5 to 300 mL.min−1 fed the concentrates into the atomizer; controlling the compressed air pressure made it possible to control both the rotary atomizer (wheel velocity at 30,000 rpm) and the two-fluid nozzle. The inlet temperature of the drying air was set at 160 °C and the outlet temperature was maintained at 60 °C by adjusting the feed flow rate via the peristaltic pump. Outlet powder temperature was controlled using two temperature sensors placed in the pipe that connected the drying chamber and the cyclone. During spray drying, dried powder was separated from air within a cyclone separator of the drier, the powder was collected from the base of the cyclone, and the exhaust air exited to the atmosphere via a pump.
2.5 Characterization of physico-chemical properties
The water activity (a w) of the powder samples was determined at 25 °C using an aw-meter (Novasina, AWC200, CH-8808, Switzerland). Measurement of dispersibility was performed according to the procedure described by Schuck et al. (2012). Particle size distribution and mass median diameter (d 0.5) of the different concentrates and powder samples obtained by spray-drying were characterized by the dry and wet method in a Mastersizer laser diffraction particle size analyzer (Mastersizer 2000; Malvern Instruments, UK) fitted with a Scirocco 2000 dry powder and wet liquid feeder unit. Refraction index for the solid (soy product) and liquid (water) materials were taken as 1.45 and 1.33, respectively. The mass median diameter (d 0.5) and the span ((d 0.9–d 0.1)/d 0.5) were recorded and expressed as the mean of several measurements. Wettability, solubility index (SI), and true density (ρ TR,) was measured after grinding a sample using a coffee grinder; bulk density (ρ B), tapped density (ρ T), interstitial air (IA), and total occluded air (OAT) of all powders were determined according to the methods of Schuck et al. (2012).
3 Results and discussion
3.1 Influence of nozzle/wheel on water activity (a w)
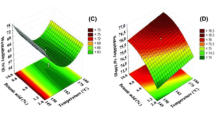
The water activity in the powder produced by the wheel (a w ≈ 0.18) was significantly higher than that produced by the nozzle (a w ≈ 0.14; Fig. 2). This difference in water activity was mainly due to the configuration of the spraying system. In fact, using the nozzle provides small droplets (d 0.5 ≈ 15 μm) thus their surface area were high and therefore the removal of water inside the droplet was fast. In contrast, the wheel provides large droplets (d 0.5 ≈ 24 μm) thus the removal of water inside the droplet was slow. In consequence, the water activity of the powder obtained using the wheel was higher than the powder obtained using the nozzle. In addition, according to Dacanal et al. (2010), smaller particles have greater surface area per mass, resulting in a higher drying rate and consequently in lower final moisture content (Dacanal and Menegalli 2010). For a given outlet and inlet temperature (160/60) the concentrate flow rate varied between 30 and 40 mL.min−1 for all experiments. At this temperature, the influence of the variation of the concentrate flow rate (30–40 mL.min−1) was not significant on the water activity measured.
Change in water activity in the dried powders obtained using the wheel or nozzle as an atomizer with TP/TS ratios of 10/40 and 35/20. Dried powder was obtained by spray drying the tertiary mixture (soy powder–maltodextrin–water). Maltodextrin powder was used to adjust the protein content in the powder
3.2 Influence of nozzle/wheel on powder particle size
3.2.1 Particle size distribution: determination of span and mass median diameter of particles
Table 1 summarizes the data obtained by measuring the particle sizes of the powders produced using the nozzle and the wheel as spray-drying system. As can be seen in Table 1, the particle size depended on the configuration of the spraying system (wheel/nozzle). Indeed, these findings show that it is possible to increase the particle size slightly by using a wheel rather than a nozzle. At TP/TS ratio of 30/20 and 35/20, using the wheel slightly increased particle sizes (from 19 to 24 μm) in comparison to using the nozzle (from 14 to 20 μm). The span corresponding to the wheel (span ≈ 1.5) was lower than that of the nozzle in (span ≈ 3.3) which means that the powders obtained with the wheel were more homogeneous than those produced with the nozzle, making them easier to characterize in terms of functional properties. In addition of the granulation phenomenon, particle size was more homogeneous with the wheel (span = 1.5) than with nozzle (span >3.3; Table 1). However, stickiness was more of an issue when a wheel was used rather than a nozzle. The greatest amount of wall deposition was seen on the inner wall of the minor pilot-scale spray drier because of the circular motion of the droplets at the beginning of atomization.
3.2.2 Scanning electron microscopy: surface morphology
Figure 3 shows scanning electron micrographs of some powders. It can be seen clearly that the powders produced by the wheel were different from those produced by the nozzle whatever the type of DE and TP/TS. The difference involved both the particle size and the surface morphology of the particles. Furthermore, the powders produced by the wheel were more homogeneous than those obtained by the nozzle. These results are in agreement with those obtained for span values (Table 1) and particle size distribution. Indeed, all these results show that the particle size of the powders produced by the wheel was greater than that obtained by the nozzle. Moreover, fine particles of irregular shapes were produced when the nozzle was used. In both cases (wheel or nozzle) the particles were microspheres with round external surfaces and some concavities which characterized spray-dried powders. The presence of concavities on the surface of spray-dried particles could result from the rapid evaporation of water during the spray-drying process, leading to the formation of a tough shell of solid polymer (Rosenberg et al. 1985; Ting et al. 1992). The drying conditions and/or the effects of the carriers that hinder the formation of a tight matrix during water evaporation may cause the development of pores (Fig. 3). Indeed, solvent diffusion may be much slower than the heat transfer to the inside of the droplets, and thus pressure can build up within the shell, causing expansion of the droplet (Favaro-Trindade et al. 2010). Similarly, porosity is mainly due to the diffusion of water from inside to the surface of the droplet during spray drying, leading to formation of entrapped air within the droplet. From our results, it seems that the particles obtained using the wheel, were more porous than those produced using the nozzle (Fig. 3d). In fact, the droplet volume during the detachment period depends on the orifice of the spraying system, the force acting on the droplet and the detachment time. The final droplet size can be correlated by the final droplet volume at the moment it breaks away. As the orifice of the nozzle is very thin, the volume of a droplet formed through a nozzle was smaller than those from the wheel. The smaller drop size of the sprayed liquid is, the larger specific surface area is provided and therefore the mass transfer between the hot air and the droplet was fast. In consequence, the occluded and interstitial airs of powder particles produced by the nozzle were higher than those produced by the wheel (Table 2).
The morphology of the powders illustrated in Fig. 3 suggests that drying was rapid from the surface to the internal parts and evaporation of the water inside caused shrinking of the shell. According to Hino et al. 2000, if the droplet is large, it takes a long time to dry the whole particle. In such a case, a crack might be formed on the particle surface or the droplets may be fractured because of the difference in vapor pressure between the inside and the surrounding area. The cracking and fracture phenomena and the appearance of holes during the transfer of water from the interior of the particle to the outside were more visible on the powders produced using the wheel than on those obtained using the nozzle. In this case, the absence of cracking, hollowing, and fracturing was probably due to the fact that the particles were smaller, making the water evaporation quick. This is one of the disadvantages of the nozzle. The pore size of the powders inevitably influences the dispersibility of soy powder.
3.3 Influence of nozzle/wheel on dispersibility
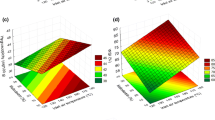
Whatever the TP/TS ratio, the powders produced using the wheel presented a significantly higher dispersibility index (DI; 64%) than those from the nozzle (52%; Fig. 4). The increase in dispersibility was associated with an increase in particle size (Table 1). A moderate increase in particle size (from 15 to 24 μm) improved the dispersibility of powders in most cases. In fact, during the process of rehydration of the small particle powders, the large surface area per mass lower the wetting ability, which impacts on dispersibility. According to Depreter et al. (2010) the formation of powder aggregates can be explained by the fact that inter-particle interactions are very high because of the increase in specific surface area. The wheel led to a smaller span (Table 1) of particle size than the nozzle, and furthermore, pore size was greater with the wheel than with the nozzle (Fig. 3). Therefore, the improved dispersibility was related to both an increase in particle size (Table 1) and a decrease in total occluded and interstitial air (Table 2). However, poor dispersibility of powders can be linked to the cohesion (stickiness) of powder particles to form aggregates and the following steps are required for good dissolution:
-
Wetting of the agglomerates
-
Solvation transfer to the core of the agglomerates and breaking the connection between particles
-
Dissolution of particles
If the cohesion between particles was too strong (as for powders produced by a nozzle) it may be that there was no breaking of the connections between the particles. In this case, large aggregates were solvated so that the exchange surface between the solvent and the solute is smaller and therefore leads to poor dispersion of the powder. According to Depreter et al. (2010), an irregular surface could increase inter-particle distances and therefore reduce Van der Waals interactions, resulting in increased powder dispersibility. The presence of occluded and interstitial air (see Table 2) and the formation of concavities in powder particles affect powder dispersibility. Cohesion between particles may therefore partly explain why fine particles (15 μm, Fig. 3) dispersed more slowly than larger particles (22 μm, Fig. 3). Furthermore, it can be seen by comparing Figs. 3c and d that the powder surface obtained by the wheel was rough and presented vacuoles and concavities, whereas the surface of powders obtained by nozzle was smooth.
3.4 Relationship between dispersibility and particle size
The aim of this study was to establish a correlation between particle size and the dispersibility of soy powder. To go further, an attempt of estimating the dispersibility of soy-based powders from the measurements of their particle size was realized. A correlation between d 0.5 and the dispersibility index was therefore investigated (Fig. 5).
Two types of powders can be distinguished on Fig. 5, according to their particle size and dispersibility index: (1) average sizes of particles between 15 and 25 μm corresponding to a dispersibility index between 50 and 70%, and (2) average sizes of particles between 35 and 45 μm corresponding to a dispersibility index of between 90 and 100%. The differences in particle size and dispersibility for each category were mainly due to the CSS.
4 Conclusions
The impact of the configuration of the spraying system on the dispersibility and particle size of soy powders produced using a pilot-scale spray dryer were investigated. The results showed that using the wheel rather than the nozzle resulted in a slightly improved dispersibility of the powder and this was explained mainly by the increase in particle size. The higher water activity of the powders obtained by the wheel was mainly attributed to the total surface area of the particles, which itself depends on the CSS. Porosity and occluded air results were consistent with the increase in dispersibility according to CSS: evidence was made that the powders obtained with the wheel configuration entrapped less occluded air, while direct observations suggested that their surface had many pores. This study thus demonstrated that modifying the CSS could be an alternative way to fine-tune the dispersibility of soy powders.
Abbreviations
- CSS:
-
Configuration of the spraying system
- DE:
-
Dextrose equivalent
- DI:
-
Dispersibility index
- IA:
-
Interstitial air
- OAT :
-
Occluded air
- MD:
-
Maltodextrin
- MMD:
-
Mobile minor dryer
- SSFR:
-
Soy supreme fiber reduced
- Span:
-
Variation coefficient
- TP:
-
Total protein
- TS:
-
Total solid
- a w :
-
Water activity
- d 0.5 :
-
Mass median diameter 50% of the distribution is above and 50% is below
- d 0.1 :
-
10% of the volume distribution is below this value
- d 0.9 :
-
90% of the volume distribution is below this value
- ρ B :
-
Bulk density
- ρ T :
-
Tapped density
- ρ TR :
-
True density
References
Adhikari B, Howes T, Bhandari BR, Truong V (2003) Characterization of the surface stickiness of fructose–maltodextrin solutions during drying. Dry Technol 21:17–34
Bhandari BR, Senoussi A, Dumoulin ED, Lebert A (1993) Spray drying of concentrated fruit juices. Dry Technol 11:1081–1092
Carp DJ, Wagner GB, Bartholomai GB, Pilosof AMR (1997) Rheological method for kinetics of drainage and disproportionation of soy proteins foams. J Food Sci 62:1105–1109
Dacanal GC, Menegalli FC (2010) Selection of operational parameters for the production of instant soy protein isolate by pulsed fluid bed agglomeration. Powder Technol 203:565–573
Depreter F, Amighi K (2010) Formulation and in vitro evaluation of highly dispersive insulin dry powder. Eur J Pharm Biopharm 76:454–463
Favaro-Trindade CS, Santana AS, Monterrey-Quintero ES, Trindade MA, Netto FM (2010) The use of spray drying technology to reduce bitter taste of casein hydrolysate. Food Hydrocolloids 24:336–340
Gupta AS (1978) Spray drying orange juice. US Patent n° US4112130 (A)
Hino T, Shimabayashi S, Ohnishi N, Fujisaki M, Mori H, Watanabe O, Kawashima K, Nagao K (2000) Development of a new type nozzle and spray-drier for industrial production of fine powders. Eur J Pharm Biopharm 49:79–85
Imram N, Gomez I, Soh V (2003) In: Imram N (ed) The soya handbook. Tetra Pak Centre of Expertise Soya, Singapore
Kim SH, Kinsella JE (1987a) Surface active properties of food proteins: effects of reduction of disulfide bonds on film properties and foams stability of glycinin. J Food Sci 52:128–131
Kim SH, Kinsella JE (1987b) Surface active properties of proteins: effects of progressive succinylation on film properties and foams stability of glycinin. J Food Sci 52:1341–1352
Kinsella JE (1979) Functional properties of soy proteins. J Am Oil Chem Soc 56:242–258
Langrish TAG, Chan WC, Kota K (2007) Comparison of maltodextrin and skim milk wall deposition rates in a pilot-scale spray dryer. Powder Technol 179:84–89
Nakamura A, Yoshidab R, Maedab H, Corredig M (2006) Soy soluble polysaccharide stabilization at oil–water interfaces. Food Hydrocolloids 20:277–283
Nazareth ZM, Deak NA, Johnson LA (2009) Functional properties of soy protein isolates prepared from gas-supported screw-pressed soybean meal. J Am Oil Chem Soc 86:315–321
Roos YH, Karel M (1991a) Plasticizing effect of water on thermal behaviour and crystallization of amorphous food models. J Food Sci 56:38–43
Roos YH, Karel M (1991b) Water and molecular weight effects on glass transitions in amorphous carbohydrates and carbohydrate solutions. J Food Sci 56:1676–1681
Rosenberg M, Kopelman IJ, Talmon Y (1985) A scanning electron microscopy study of microencapsulation. J Food Sci 50:139–144
Schuck P, Bouhallab S, Durupt D, Vareille P, Humbert JP, Marin M (2004) Séchage des lactosérums et dérivés: rôle du lactose et de la dynamique de l'eau. Lait 84:243–268
Schuck P, Méjean S, Dolivet A, Jeantet R, Bhandari B (2007) Keeping quality of dairy ingredients. Lait 87:481–488
Schuck P, Dolivet A, Jeantet R (2012) Analytical methods for food and dairy powders. Wiley, Hoboken
Ting TY, Gonda I, Gipps EM (1992) Microparticles of polyvinyl alcohol for nasal delivery. I. Generation by spray-drying and spray-desolvation. Pharm Res 9:1330–1335
Tonon RV, Baroni AF, Brabet C, Gibert O, Pallet D, Hubinger M (2009) Water sorption and glass transition temperature of spray dried. açai (Euterpe oleracea Mart.) juice. J Food Eng 94:215–221
Turchiuli C, Jimenèz T, Dumoulin E (2011) Identification of thermal zones and population balance modelling of fluidized bed spray granulation. Powder Technol 208:542–552
Wagner JR, Gueguen J (1999) Surface functional properties of native, acid treated, and reduced soy glycinin. 1. Foaming properties. J Agric Food Chem 47:2173–2187
Acknowledgments
The authors would like to thank Peter Nootenboom and Patricia Heussen (Unilever) for the scanning electron micrograph images.
This research was supported by Unilever (R&D Vlaardingen, Netherlands).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Syll, O., Khalloufi, S. & Schuck, P. Dispersibility and morphology of spray-dried soy powders depending on the spraying system. Dairy Sci. & Technol. 93, 431–442 (2013). https://doi.org/10.1007/s13594-013-0112-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13594-013-0112-y