Abstract
Robotic assisted unicompartmental knee arthroplasty (RAUKA) has emerged as a successful approach for optimizing implant positioning accuracy, minimizing soft tissue injury, and improving patient-reported outcomes. The application of RAUKA is expected to increase because of its advantages over conventional unicompartmental knee arthroplasty. This review article provides an overview of RAUKA, encompassing the historical development of the procedure, the features of the robotic arm and navigation systems, and the characteristics of contemporary RAUKA. The article also includes a comparison between conventional unicompartmental arthroplasty and RAUKA, as well as a discussion of current challenges and future advancements in the field of RAUKA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Unicompartmental knee arthroplasty (UKA) has demonstrated favorable surgical outcomes in patients with end-stage unicompartmental osteoarthritis with proper indications [1, 2]. These indications include painful osteoarthritis or osteonecrosis limited to a single compartment of the knee, accompanied by significant reduction in joint space on radiographs [3]. Compared to total knee arthroplasty (TKA), UKA offers several advantages including less bone loss, the possibility of ligament preservation, reduced blood loss, shorter hospital stays, fewer postoperative complications, the maintenance of native biomechanics, and cost-effectiveness [4]. However, prior studies showed that UKA had shorter longevity and a higher revision rate than TKA, indicating that careful patient selection is essential for optimal surgical outcomes [5, 6]. UKA can result in suboptimal surgical outcomes due to potential malpositioning and malalignment. According to Jenny et al., 30% of conventional UKAs showed inaccurate implantation [7], while Keene et al. reported that only 60% of conventional UKAs demonstrated alignment within 2 degrees of the preoperative plan [8]. Batailler et al. reported that in conventional UKAs, tibial baseplate positioning had an outlier exceeding 3 degrees in 35% of cases [9]. The annual surgical volume of UKAs conducted by a surgeon significantly influenced implant survivorship, with surgeons who performed fewer than 10 UKAs per year showing a mean 8-year survival rate of 87.9% and surgeons who performed more than 30 UKAs per year showing a 92.4% mean 8-year survival rate [10].
Robotic-assisted UKA (RAUKA) was introduced to overcome these limitations of conventional UKA [6]. RAUKA aims to enhance bone-cutting accuracy, improve implant positioning and alignment restoration, reduce human error, and minimize soft tissue injury to reduce complications [4, 11]. RAUKA is primarily based on navigation and robotic-arm technology [12]. While navigation contributes to surgical planning and the accuracy of the procedure, it does not safeguard against potential manual errors by the surgeon. Therefore, the use of a robotic arm can reduce manual errors and improve surgical accuracy by confining operations to the intended area through the utilization of haptic feedback [13].
Meanwhile, the relatively restrictive criteria for UKA patient selection, including age over 60 years, weight under 82 kg, varus deformity less than 5 degrees, a range of motion over 90 degrees, and flexion contracture less than 5 degrees, are undergoing changes [14, 15]. As reported by Gowd et al., when the patient selection criteria were broadened to disregard factors such as knee deformity, age, activity level, pain level, and heavyweight and instead focused solely on the narrowing of the unicompartmental joint space as the indication for RAUKA, a favorable 4-year survivorship rate of 92% was observed [16]. A recent study by Bayouomi et al. reported a 10-year survivorship of 91.7% for RAUKA, along with a satisfaction rate of 91% [17]. With the expanded indications, satisfactory outcomes are expected to lead to increased numbers of both UKA and RAUKA [18]. In addition, the utilization UKA is expanding through the integration of robotic assistance [19], especially by diminishing the steep learning curve, a significant challenge for low-volume surgeons [20, 21]. The use of robotic-assisted knee arthroplasty has surged from below 0.1% in 2008 to 4.3% in 2018 [22]. Currently, 15 to 20% of UKAs in the United States are performed with the assistance of robotic technology, and this percentage is projected to grow by over 37% in the next decade [23].
2 History of conventional and robotic-assisted UKA
The concept of single-compartment knee arthroplasty replacement emerged in the 1950s when McKeever devised the first metallic tibial plateau for resurfacing only the tibial plateau [24]. In 1972, Marmor performed the first modern UKA, simultaneously resurfacing the same compartment of the femur and tibia [25]. However, the initial results of UKA were not satisfactory, with a revision rate of more than 30% reported in follow-up studies over 10 years [26]. The main reasons for revision were implant malposition and malalignment of the lower extremities [27]. These poor surgical outcomes led to a decline in interest in UKA, and TKA was regarded as the gold standard [28]. A decade later, the application of more detailed and strict inclusion criteria for UKA by Kozinn and Scott led to an improvement in UKA outcomes [14]. The Oxford UKA, introduced in 1982, made significant advancements in modern UKA [29]. Its design considerations focused on maximizing component contact area while preserving and restoring the natural tension in the remaining soft tissues. The Oxford UKA has developed over time, offering a larger range of sizes and a minimally invasive approach, resulting in a reported 10-year survivorship exceeding 95% [30, 31]. Since the early 1990s, novel implant designs with instrumentation comparable to that of TKA have been introduced [32, 33]. In a systematic review by van der List et al., modern UKA implants demonstrated a 15-year survivorship of 88.9% for medial and 89.4% for lateral UKA [34].
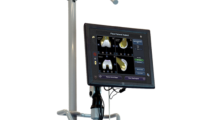
William Bargar introduced the first robotic orthopedic surgery system in the 1980s called ROBODOC, which was initially developed to improve the accuracy of femoral stem positioning in total hip arthroplasty [35]. The procedure involved the use of an external fixator to stabilize the femur, followed by the preparation of the femoral canal using a milling system. This approach greatly enhanced the accuracy of bone preparation and reduced the risk of fractures. Subsequently, this technology was adapted to enhance the precision of bony cuts in TKA and further extended to UKA [35]. Cobb and his team at Imperial College, London, developed the Acrobot (Acrobot Ltd., London, UK), which was the first haptic robotic technology designed for UKA. This system provided surgeons with tactile feedback to increase precision [36]. Subsequently, additional systems, such as Mako (Fig. 1) (MAKO Surgical Corporation, FL, USA), which uses a dynamic referencing system, and Navio (Smith & Nephew, PA, USA), which does not require preoperative imaging planning, were developed. These advancements in robotic technology have shown promising early clinical results, but further studies are necessary to evaluate their outcomes [4].
a The Mako system comprising an image-guided navigation system and a semi-active robotic arm. b A schematic representation of bony registration. (Image adapted from the "Mako Partial Knee Medial Unicondylar Resurfacing Surgical Reference Guide”, with permission from MAKO Surgical Corporation, FL, USA)
3 Types of robotic systems
3.1 Distinctive robotic platforms: Soft tissue intervention vs. Bony resection
Robotic surgical systems are broadly categorized into two primary types: those engineered for soft tissue intervention and those designed for bony resection. The Da Vinci robotic system (Intuitive Surgical Incorporation, CA, USA) is primarily designed for laparoscopic procedures on soft tissues. This system features multiple robotic articulations and a wide degree of freedom for its surgical instruments, enabling interventions on soft tissues through minimal entry portals [37, 38]. Unlike orthopedic robotic systems, the Da Vinci system emphasizes direct high-resolution 3D visualization, providing a magnified, 360-degree view of the surgical field. Furthermore, it enables the surgeon to operate remotely from a distant console [39].
Meanwhile, robotic systems in the field of orthopedics consist of navigation and robotic-arm technology (including handheld devices) [12]. These systems prioritize the precision of bone preparation, necessitating tools such as saws, burrs, and drills. Specifically, knee arthroplasty demands more than just the direct visualization of the surgical field; it necessitates the ability to attain accurate alignment and gap balancing through the utilization of navigation technology. The navigation system offers real-time, three-dimensional (3D) visual guidance to the surgeon during the procedure, while the robotic arm performs the actual surgery under the surgeon’s control (Fig. 2) [30].
3.2 Passive, semi-active, and active systems
Robotic systems can be categorized into passive, semi-active, and active types, depending on the level of autonomy provided to both the surgeon and the robot [40].
Passive systems involve the surgeon's continuous and direct control during surgery. The robot assists by placing a guide or jig in a predetermined location while the surgeon manually performs the bony resections [41]. These systems use computer-assisted or navigation technology to provide positional guidance, which is displayed on an overhead monitor. While these systems can improve implant alignment, the lack of safety constraints, such as haptic feedback, may increase the risk of human error. In addition, no superiority in implant survival or clinical outcomes compared to conventional manual arthroplasty has been reported [41, 42].
Semi-active robotic systems, often referred to as haptic systems, provide multimodal feedback (e.g., auditory, tactile, or visual) to enhance the surgeon's control over surgical tools, usually by restricting bony cuts within spatial boundaries [43]. Some semi-active technologies also control the speed and depth of surgical instruments, offering additional safety mechanisms [44]. For example, if the surgeon deviates from the established cutting margins, the system either retracts the saw/burr or reduces its speed to prevent deviation from the pre-defined surgical plan. Nonetheless, these systems still require the surgeon's manual manipulation of the cutting instrument [45, 46].
Active robotic systems operate autonomously, performing cutting or milling without the direct handling of the surgeon. Preoperative imaging is used to create a surgical plan [43]. The surgeon makes the surgical approach, positions the retractors, and secures the limb to a holding device. After calibration, the robotic arm independently conducts femoral and tibial bone resections, with the surgeon maintaining control through an emergency stop button [46, 47].
3.3 Navigation in robotic systems
Computer-assisted navigation has been developed in the last two decades to improve the accuracy and precision of component positioning in knee arthroplasty, leading to improved limb alignment [48]. These navigation systems enable the recording of intraoperative joint range of motion and kinematics, allowing for the evaluation of knee mechanics in advanced arthritis [49]. Navigation systems can be categorized into three types: image-based navigation, imageless navigation, and the more recent accelerometer-based navigation systems [48]. Many studies have provided evidence that navigation is more consistent in achieving the intended alignment compared to conventional intramedullary/extramedullary alignment methods [50,51,52].
3.3.1 Image-based navigation
Image-based navigation uses preoperative or intraoperative imaging to create a 3D model of the patient's knee [53]. Preoperative imaging involves acquiring high-resolution images of the knee using imaging modalities such as computed tomography (CT), magnetic resonance imaging (MRI), or X-rays [43]. These images are processed by software to create a 3D model of the knee joint, which is used for surgical planning. Surgeons can determine implant size and positioning and anticipate potential challenges during surgery. However, matching preoperative images with the actual 3D anatomy during surgery poses a challenge in image acquisition. For example, when surgeons aim to visualize their contact point within the surgical field on the navigation monitor, a synchronization process is essential. This matching process superimposes the real-time surgical view onto the knee model displayed on the monitor. Various registration methods are available to address this issue, including identifying corresponding landmarks on the images and the patient's body, aligning a surface on the images with the actual body surface, or a combination of both approaches [54]. During surgery, an image-based navigation system provides real-time guidance to the surgeon, displaying surgical instruments and the patient's anatomy on a computer screen overlaid on the preoperative 3D model.
In contrast, intraoperative imaging does not require preoperative registration. The camera captures both the reference frame on the patient's body and the fluoroscopic C-arm simultaneously, allowing for the superimposition of surgical instruments onto the fluoroscopic image, even if there are positional changes in the patient's body [54]. This technique, known as virtual fluoroscopy, enables navigation using multiple views simultaneously, a unique feature of navigation systems. The popularity of image-based navigation systems has grown with the emergence of robotic-assisted arthroplasty [55].
3.3.2 Imageless navigation
Imageless navigation systems negate the need for preoperative imaging, thereby reducing costs and radiation exposure [40]. These systems generate a 3D model of the knee using intraoperative data input by the surgeon, including specific anatomical landmarks and the range of motion [49]. This 3D model of the knee is derived from a pre-existing knee CT database, incorporating anatomic references such as the center of the knee and landmarks on the proximal tibia and distal femur [56]. Optical tracking mechanisms are integrated into imageless navigation systems to measure the position and orientation of optical reference frames [57].
Imageless navigation requires three main components: a computer, a tracking system, and trackers. The tracking system, synchronized with a camera, monitors various components, including a stylus used by the surgeon to digitize anatomical landmarks, as well as an instrumented plate that registers the position and orientation of cutting blocks and bone surfaces [49]. Reflective balls or other devices serve as trackers, tracked by the camera through created light beams. Software algorithms are employed by the computer to track these trackers and create a virtual rendering of spatial orientation. The accuracy of tracking in imageless navigation systems depends on the integration of the tracking camera and the corresponding reference frames, with an accuracy range of 0.5 to 3 mm [58]. However, inherent inaccuracies may arise in imageless navigation referencing due to potential errors in identifying prescribed anatomical reference points by the surgeon [59].
3.3.3 Accelerometer-based navigation
Accelerometer-based navigation (ABN) is a recent technique that uses sensors to provide real-time data on the position, alignment, and trajectory of the knee during surgery, including angular degrees and translation distances [60]. Unlike conventional intramedullary alignment guides, ABN does not depend on assumptions derived from the anatomical axis. Instead, it registers the true mechanical axis [61]. One advantage of ABN is the elimination of large console systems, resulting in no initial setup costs. Additionally, ABN effectively resolves any intraoperative line-of-sight issues between the camera and the reference arrays [62]. ABN also avoids pin site complications by not requiring trans-osseous femoral and tibial tracker fixation, reducing surgical time. These features make ABN a less invasive and potentially more cost-effective option [63]. However, a limitation of ABN is the inability to verify the accuracy of the resection using a navigated array after tibial or femoral resection, which is a feature present in larger console navigation systems. Furthermore, ABN does not provide information on soft-tissue tension and lacks the capability to assist in setting tibial or femoral component positioning. The cost of single-use components required during surgery should also be considered [63].
3.4 Contemporary RAUKA systems
Contemporary RAUKA systems share common features in surgical workflow, including patient-specific planning, bone registration, and dynamic soft tissue balancing through real-time feedback (Fig. 3) [12]. Currently, the most widely used systems for RAUKA include those developed by Acrobot, Mako and Navio. However, there is a paucity of comparative analyses evaluating the accuracy and outcomes of these systems [4].
The Acrobot (active constraint robot) is a semi-active, image-based system that involves CT scans for preoperative planning. During surgery, the surgeon uses an active-constraint device mounted on a positioner to prepare the bone “free-hand” using the robotic handle. The patient's position and bony surface are registered and reconstructed using a CT-based surface matching algorithm. A safe zone is defined, which prevents unintended damage if the handle is moved beyond the designated area [36].
The Mako system also uses preoperative CT scans for planning, determining the position and size of the implant based on the captured scan. During surgery, arrays are attached to pins inserted into the femur and tibia. Unlike the Acrobot, which uses a static referencing system, requiring the leg to remain fixed during surgery, the Mako system uses a dynamic referencing system. The arrays enable tracking despite alterations in position throughout the operation and allows the measurement of gap data at multiple flexion angles. A reconstructed 3D image from the CT scan provides virtual visualization of knee structures, enabling adjustments of implant alignment or positioning based on gap data for optimal placement. Bone resections are performed within defined boundaries using a saw or burr.
The Navio system utilizes an imageless platform and a handheld burr for bone resections. Similar to the Mako system, it tracks movements during surgery through an array attached to checkpoint pins inserted into the tibia and femur. However, the Navio system does not require preoperative CT scans, reducing radiation exposure. Knee flexion and extension are performed to assess the range of motion, while varus/valgus stress is applied to evaluate soft tissue laxity. Reference points are verified, and direct surface mapping enables 3D reconstruction. Intraoperative surface mapping and bone registration allows the surgeon to plan bony cuts and choose implant sizes. Upon establishing the surgical plan, real-time bone cutting within the defined boundary is enabled using a handheld burr [64].
4 Comparison of outcomes between conventional UKA and RAUKA
Comparative studies assessing the outcomes of conventional UKA and RAUKA have been widely conducted. Comparisons of the two techniques have primarily concentrated on radiological results, including component positioning and alignment, as well as clinical results, such as patient-reported outcome measures and surgical complications [65,66,67,68].
In a cadaveric study by Lonner et al. using the Navio system, bone preparation and implant positioning were achieved within 1.3 mm and 2 degrees of the planned values, respectively [65]. Another cadaveric study by Citak et al. compared the outcomes of RAUKA and conventional UKA, with the robotic group demonstrating root-mean-square (RMS) errors for femoral and tibial component placements within 1.9 mm and 3.7 degrees and 1.4 mm and 5.0 degrees, respectively. In contrast, the conventional group exhibited RMS errors of 5.4 mm and 10.2 degrees for the femoral component and 5.7 mm and 19.2 degrees for the tibial component [66]. Similarly, Smith et al. reported an RMS error of 1.46 degrees for tibial and femoral implants using the NAVIO system [67].
In the first clinical series of robotic UKA using a semi-active robotic system (Mako), Pearle et al. demonstrated a disparity of within 1 degree between the pre-planned and intraoperative tibiofemoral angles. Postoperative long leg radiographs revealed a deviation of fewer than 1.6 degrees from the intraoperative values [69]. A randomized clinical trial by Rodriguez compared 15 conventional UKA patients and 13 RAUKA patients using the Acrobot system. The results showed an absence of outliers in implant positioning and lower extremity alignment in the RAUKA group, while 60% of the conventional group were considered outliers [70]. A systematic review conducted by Bouché et al. found that RAUKA exhibited superior precision in bone cuts for the femoral component in the coronal plane, the tibial component in the sagittal plane, and overall lower limb alignment compared to conventional UKA. However, the study did not identify any significant differences in functional or clinical outcomes up to 24 months postoperatively [71].
5 Current limitations of RAUKA
Despite the promising advantages of RAUKA, the current RAUKA system is still in the process of development and has several limitations. First, it remains under the direct control of the surgeon, lacking autonomous decision-making capabilities. Additionally, it is unable to detect injuries occurring during surgery. Essential aspects such as planning, implant positioning and sizing, as well as the execution of bony cuts, rely entirely on the surgeon's manual input.
5.1 Learning curve
Similar to various other surgical procedures, RAUKA exhibits a learning curve. In a prospective study conducted by Kayani et al., involving 60 cases of conventional UKA followed by 60 cases of RAUKA, a learning curve of six cases was identified as the threshold for achieving proficiency equivalent to conventional UKA in terms of surgical time and comfort levels. Notably, no such learning curve was observed for implant positioning, joint line restoration, and postoperative alignment [20]. Another study by Tay et al. reported an 11-case learning curve. Interestingly, no differences were found in learning curves or patient outcomes when comparing high-volume and low-volume UKA surgeons, suggesting that RAUKA could be advantageous for surgeons with lower procedural volumes [21]. Still, the learning curve for RAUKA is comparatively shorter than that of conventional UKA, with previous research suggesting that 25 cases are required to achieve a satisfactory outcome, while the initial 10 cases may yield inferior results [72, 73].
5.2 Surgical time
Compared to conventional UKA, RAUKA is associated with increased surgical time. Goh et al. reported a 32 min increase in operative time in RAUKA compared to conventional UKA [74]. Similarly, Hansen et al. reported a 20 min increase of tourniquet time during RAUKA compared to conventional UKA [75]. In addition, MacCallum et al. reported a 16 min increase for RAUKA [76].
5.3 Economic issues
The cost of RAUKA is subject to variation based on factors such as the healthcare system where the surgery is performed and perioperative considerations. For instance, the initial ROBODOC robotic system, introduced in the 1990s, was priced at $635,000 in Europe [77]. The utilization of RAUKA requires expenses for purchasing the robotic device, conducting preoperative imaging, training surgical personnel, and increased operative time [20, 78]. Moschetti et al. reported that RAUKA becomes more cost-effective than conventional UKA when over 94 RAUKA surgeries are performed yearly and two-year revision rates are kept under 1.2% [79]. Although RAUKA incurred higher costs, the increased implant longevity justified the additional expenditure associated with the robotic system [79, 80]. In a study by Christen et al., the image-based robotic arthroplasty had the highest supplementary charge of $2,600 compared to conventional procedures. It was followed by the imageless robotic arthroplasty (an added $1,530), with navigation-only methods being the most affordable [81]. Swank et al. reported that steady growth in robotic operations might offer a return on investment in two years [82]. Goh et al., using a time-driven activity-based costing method, showed that overall facility costs were lower for RAUKA, despite longer operation time and higher personnel costs. Savings in implant costs accounted for this decrease, though contractual dealings between implant manufacturers and medical institutions may influence these differences [74].
6 The future of RAUKA
In the future, the focus in RAUKA will be on enhancing precision and accuracy and developing more automated surgical planning algorithms. Technological advancements are expected to reduce surgical time, improve cost-effectiveness, reducing the economic burden on patients, hospitals, and society [80]. Efforts will also be made to reduce the learning curve and achieve consistent surgical outcomes, regardless of the surgeon's experience or surgical volume [20, 21]. Technological advancements, including refined landmark recognition, could provide precise implant position, through enhanced alignment and gap balancing. These innovations can present multiple options tailored to the patient's specific anatomy and the surgeon's surgical preferences. In addition, these advancements may eliminate the need for bony pins during registration and facilitate bone resection without complete joint visualization. This could lead to shorter surgical time and less postoperative complications. Additionally, RAUKA may allow surgeons to restore alignment to the pre-arthritic state, addressing a limitation in conventional UKA and potentially restoring natural knee movements [83].
6.1 Full automation of RAUKA
While current RAUKA procedures utilize semi-active systems, the future may see a shift toward fully active systems. This transition could streamline the surgical process by reducing the personnel count. Currently, surgeons manually manipulate soft tissue retraction, ensuring retractors are aptly placed within the surgical field. To achieve full automation in RAUKA, the development of automated retraction technology and a robotic "eye" capable of soft tissue recognition is necessary. Also, the presence of additional robotic arms capable of performing retraction tasks, and a reduction in the size of bulky robotic arms, are required. Such technology should incorporate a sensor that monitors soft tissue tension, adjusting retraction forces to avoid surpassing damage thresholds. Furthermore, the current bulky design of robotic arms adds to the inconvenience of moving the device in and out of the operating room. Downsizing these devices and integrating them into surgical booms would mitigate this issue.
6.2 Registration methods
Accurate bone registration is crucial for computer navigation and robotic surgery. However, the current registration systems can be expensive, cumbersome, and have limitations in accuracy or require intraoperative radiation [84]. To address these drawbacks, Liu et al. proposed spatial surface registration using a preoperative knee MRI and CT image-derived fusion model point cloud and an intraoperative laser-scanned cartilage surface point cloud [56]. Similarly, He et al. utilized impression molding with a structured-light 3D scanner, eliminating the need for X-rays and allowing efficient data capture in a single scan [84]. These technological advancements have the potential to improve the speed and accuracy of registration for RAUKA.
6.3 Artificial intelligence in RAUKA
The integration of artificial intelligence (AI) into RAUKA is expected to advance in the future. AI models and algorithms have been developed to emulate human intelligence and perform specific tasks [85, 86]. AI has the potential to enhance the capabilities of robotic surgical systems in understanding complex in vivo environments, preoperative planning, making decisions, predicting outcomes, and executing tasks with superior precision, safety, and efficiency, either autonomously or under human supervision [85, 87]. The incorporation of AI into RAUKA could minimize human errors and reduce operating time [88]. AI technology is also anticipated to improve the precise planning of UKA, leading to better surgical outcomes and patient satisfaction [89].
7 Conclusion
The application of RAUKA has effectively addressed the limitations of conventional UKA, enhancing surgical precision and accuracy. The integration of diverse robotic systems and navigation has improved component positioning and patient outcomes in UKA. Contemporary RAUKA systems have introduced innovative features, such as patient-specific planning and real-time feedback. The future of RAUKA is expected to advance through enhanced surgical precision, increased cost-effectiveness, the utilization of advanced registration methods, the development of soft tissue recognition technology, and the integration of AI
References
Baker P, Jameson S, Critchley R, Reed M, Gregg P, Deehan D. Center and surgeon volume influence the revision rate following unicondylar knee replacement: an analysis of 23,400 medial cemented unicondylar knee replacements. J Bone Jt Surg Am. 2013;95(8):702–9. https://doi.org/10.2106/jbjs.L.00520.
Berger RA, Meneghini RM, Jacobs JJ, Sheinkop MB, Della Valle CJ, Rosenberg AG, et al. Results of unicompartmental knee arthroplasty at a minimum of ten years of follow-up. J Bone Jt Surg Am. 2005;87(5):999–1006. https://doi.org/10.2106/jbjs.C.00568.
Jennings JM, Kleeman-Forsthuber LT, Bolognesi MP. Medial Unicompartmental Arthroplasty of the Knee. J Am Acad Orthop Surg. 2019;27(5):166–76. https://doi.org/10.5435/jaaos-d-17-00690.
Liu P, Lu FF, Liu GJ, Mu XH, Sun YQ, Zhang QD, et al. Robotic-assisted unicompartmental knee arthroplasty: a review. Arthroplasty. 2021;3(1):15. https://doi.org/10.1186/s42836-021-00071-x.
Niinimäki T, Eskelinen A, Mäkelä K, Ohtonen P, Puhto AP, Remes V. Unicompartmental knee arthroplasty survivorship is lower than TKA survivorship: a 27-year Finnish registry study. Clin Orthop Relat Res. 2014;472(5):1496–501. https://doi.org/10.1007/s11999-013-3347-2.
Lyons MC, MacDonald SJ, Somerville LE, Naudie DD, McCalden RW. Unicompartmental versus total knee arthroplasty database analysis: is there a winner? Clin Orthop Relat Res. 2012;470(1):84–90. https://doi.org/10.1007/s11999-011-2144-z.
Jenny JY, Boeri C. Unicompartmental knee prosthesis implantation with a non-image-based navigation system: rationale, technique, case-control comparative study with a conventional instrumented implantation. Knee Surg Sports Traumatol Arthrosc. 2003;11(1):40–5. https://doi.org/10.1007/s00167-002-0333-8.
Keene G, Simpson D, Kalairajah Y. Limb alignment in computer-assisted minimally-invasive unicompartmental knee replacement. J Bone Joint Surg Br. 2006;88(1):44–8. https://doi.org/10.1302/0301-620X.88B1.16266.
Batailler C, White N, Ranaldi FM, Neyret P, Servien E, Lustig S. Improved implant position and lower revision rate with robotic-assisted unicompartmental knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2019;27(4):1232–40. https://doi.org/10.1007/s00167-018-5081-5.
Liddle AD, Pandit H, Judge A, Murray DW. Effect of surgical caseload on revision rate following total and unicompartmental knee replacement. J Bone Joint Surg Am. 2016;98(1):1–8. https://doi.org/10.2106/JBJS.N.00487.
Negrín R, Duboy J, Iñiguez M, Reyes NO, Barahona M, Ferrer G, et al. Robotic-assisted vs conventional surgery in medial unicompartmental knee arthroplasty: a clinical and radiological study. Knee Surg Relat Res. 2021;33(1):5. https://doi.org/10.1186/s43019-021-00087-2.
Zhang J, Ng N, Scott CEH, Blyth MJG, Haddad FS, Macpherson GJ, et al. Robotic arm-assisted versus manual unicompartmental knee arthroplasty : a systematic review and meta-analysis of the MAKO robotic system. Bone Jt J. 2022;104-b(5):541–8. https://doi.org/10.1302/0301-620x.104b5.Bjj-2021-1506.R1.
Chen X, Deng S, Sun ML, He R. Robotic arm-assisted arthroplasty: the latest developments. Chin J Traumatol. 2022;25(3):125–31. https://doi.org/10.1016/j.cjtee.2021.09.001.
Kozinn SC, Scott R. Unicondylar knee arthroplasty. J Bone Jt Surg Am. 1989;71(1):145–50.
Hiranaka T, Furuhashi R, Takashiba K, Kodama T, Michishita K, Inui H, et al. Agreement and accuracy of radiographic assessment using a decision aid for medial Oxford partial knee replacement: multicentre study. Knee Surg Relat Res. 2022;34(1):13. https://doi.org/10.1186/s43019-022-00140-8.
Gowd AK, Plate JF, Lichtig A, Gencer A, Yanmis O, D’Agostino R, et al. Favourable mid-term outcomes following unicompartmental knee arthroplasty with wider patient selection: A single-centre experience. J Isakos. 2023. https://doi.org/10.1016/j.jisako.2023.03.002.
Bayoumi T, Kleeblad LJ, Borus TA, Coon TM, Dounchis J, Nguyen JT, et al. Ten-year survivorship and patient satisfaction following robotic-arm-assisted medial unicompartmental knee arthroplasty: a prospective multicenter study. J Bone Jt Surg Am. 2023. https://doi.org/10.2106/jbjs.22.01104.
Tolk JJ, Janssen RPA, Haanstra TM, Bierma-Zeinstra SMA, Reijman M. The EKSPECT study: the influence of Expectation modification in Knee arthroplasty on Satisfaction of patients: study protocol for a randomized controlled Trial. Trials. 2018;19(1):437. https://doi.org/10.1186/s13063-018-2821-2.
Kim KT. Unicompartmental knee Arthroplasty. Knee Surg Relat Res. 2018;30(1):1–2. https://doi.org/10.5792/ksrr.18.014.
Kayani B, Konan S, Pietrzak JRT, Huq SS, Tahmassebi J, Haddad FS. The learning curve associated with robotic-arm assisted unicompartmental knee arthroplasty: a prospective cohort study. Bone Jt J. 2018;100(8):1033–42. https://doi.org/10.1302/0301-620x.100b8.Bjj-2018-0040.R1.
Tay ML, Carter M, Bolam SM, Zeng N, Young SW. Robotic-arm assisted unicompartmental knee arthroplasty system has a learning curve of 11 cases and increased operating time. Knee Surg Sports Traumatol Arthrosc. 2023;31(3):793–802. https://doi.org/10.1007/s00167-021-06814-2.
Emara AK, Zhou G, Klika AK, Koroukian SM, Schiltz NK, Krebs VE, et al. Robotic-arm-assisted knee arthroplasty associated with favorable in-hospital metrics and exponentially rising adoption compared with manual knee arthroplasty. J Am Acad Orthop Surg. 2021;29(24):e1328–42. https://doi.org/10.5435/jaaos-d-21-00146.
Lonner JH, Moretti VM. The evolution of image-free robotic assistance in unicompartmental knee arthroplasty. Am J Orthop. 2016;45(4):249–54.
McKeever DC. The choice of prosthetic materials and evaluation of results. Clin Orthop. 1955;6:17–21.
Marmor L. The modular knee. Clin Orthop Relat Res. 1973;94:242–8. https://doi.org/10.1097/00003086-197307000-00029.
Marmor L. Unicompartmental knee arthroplasty. ten- to 13-year follow-up study. Clin Orthop Relat Res. 1988;226:14–20.
Insall J, Aglietti P. A five to seven-year follow-up of unicondylar arthroplasty. J Bone Jt Surg Am. 1980;62(8):1329–37.
Laskin RS. Unicompartmental tibiofemoral resurfacing arthroplasty. J Bone Joint Surg Am. 1978;60(2):182–5.
Goodfellow J, O’Connor J. The mechanics of the knee and prosthesis design. J Bone Joint Surg Br. 1978;60-b(3):358–69. https://doi.org/10.1302/0301-620x.60b3.581081.
Johal S, Nakano N, Baxter M, Hujazi I, Pandit H, Khanduja V. Unicompartmental knee arthroplasty: the past, current controversies, and future perspectives. J Knee Surg. 2018;31(10):992–8. https://doi.org/10.1055/s-0038-1625961.
Murray DW, Goodfellow JW, O’Connor JJ. The Oxford medial unicompartmental arthroplasty: a ten-year survival study. J Bone Joint Surg Br. 1998;80(6):983–9. https://doi.org/10.1302/0301-620x.80b6.8177.
Argenson JN, Chevrol-Benkeddache Y, Aubaniac JM. Modern unicompartmental knee arthroplasty with cement: a three to ten-year follow-up study. J Bone Jt Surg Am. 2002;84(12):2235–9.
Robertsson O, Dunbar M, Pehrsson T, Knutson K, Lidgren L. Patient satisfaction after knee arthroplasty: a report on 27,372 knees operated on between 1981 and 1995 in Sweden. Acta Orthop Scand. 2000;71(3):262–7. https://doi.org/10.1080/000164700317411852.
van der List JP, McDonald LS, Pearle AD. Systematic review of medial versus lateral survivorship in unicompartmental knee arthroplasty. Knee. 2015;22(6):454–60. https://doi.org/10.1016/j.knee.2015.09.011.
Bargar WL, Bauer A, Börner M. Primary and revision total hip replacement using the Robodoc system. Clin Orthop Relat Res. 1998;354:82–91. https://doi.org/10.1097/00003086-199809000-00011.
Cobb J, Henckel J, Gomes P, Harris S, Jakopec M, Rodriguez F, et al. Hands-on robotic unicompartmental knee replacement: a prospective, randomised controlled study of the acrobot system. J Bone Joint Surg Br. 2006;88(2):188–97. https://doi.org/10.1302/0301-620x.88b2.17220.
Gioutsos K, Kocher GJ, Schmid RA. Robotics in pulmonology and thoracic surgery: what, why and when? Panminerva Med. 2016;58(4):318–28.
Wedmid A, Llukani E, Lee DI. Future perspectives in robotic surgery. BJU Int. 2011;108(6 Pt 2):1028–36. https://doi.org/10.1111/j.1464-410X.2011.10458.x.
Hubens G, Coveliers H, Balliu L, Ruppert M, Vaneerdeweg W. A performance study comparing manual and robotically assisted laparoscopic surgery using the da Vinci system. Surg Endosc. 2003;17(10):1595–9. https://doi.org/10.1007/s00464-002-9248-1.
Sousa PL, Sculco PK, Mayman DJ, Jerabek SA, Ast MP, Chalmers BP. Robots in the operating room during hip and knee arthroplasty. Curr Rev Musculoskelet Med. 2020;13(3):309–17. https://doi.org/10.1007/s12178-020-09625-z.
Netravali NA, Shen F, Park Y, Bargar WL. A perspective on robotic assistance for knee arthroplasty. Adv Orthop. 2013;2013: 970703. https://doi.org/10.1155/2013/970703.
Konyves A, Willis-Owen CA, Spriggins AJ. The long-term benefit of computer-assisted surgical navigation in unicompartmental knee arthroplasty. J Orthop Surg Res. 2010;5:94. https://doi.org/10.1186/1749-799x-5-94.
Figueroa F, Parker D, Fritsch B, Oussedik S. New and evolving technologies for knee arthroplasty—computer navigation and robotics: state of the art. J of ISAKOS. 2018;3(1):46–54.
Chen AF, Kazarian GS, Jessop GW, Makhdom A. Robotic technology in orthopaedic surgery. J Bone Joint Surg Am. 2018;100(22):1984–92. https://doi.org/10.2106/jbjs.17.01397.
Banks SA. Haptic robotics enable a systems approach to design of a minimally invasive modular knee arthroplasty. Am J Orthop (Belle Mead NJ). 2009;38(2 Suppl):23–7.
St Mart JP, Goh EL. The current state of robotics in total knee arthroplasty. EFORT Open Rev. 2021;6(4):270–9. https://doi.org/10.1302/2058-5241.6.200052.
Stulberg BN, Zadzilka JD. Active robotic technologies for total knee arthroplasty. Arch Orthop Trauma Surg. 2021;141(12):2069–75. https://doi.org/10.1007/s00402-021-04044-2.
Jones CW, Jerabek SA. Current role of computer navigation in total knee arthroplasty. J Arthroplasty. 2018;33(7):1989–93. https://doi.org/10.1016/j.arth.2018.01.027.
Siston RA, Giori NJ, Goodman SB, Delp SL. Surgical navigation for total knee arthroplasty: a perspective. J Biomech. 2007;40(4):728–35. https://doi.org/10.1016/j.jbiomech.2007.01.006.
Bäthis H, Perlick L, Tingart M, Lüring C, Zurakowski D, Grifka J. Alignment in total knee arthroplasty. A comparison of computer-assisted surgery with the conventional technique. J Bone Joint Surg Br. 2004;86(5):682–7.
Keyes BJ, Markel DC, Meneghini RM. Evaluation of limb alignment, component positioning, and function in primary total knee arthroplasty using a pinless navigation technique compared with conventional methods. J Knee Surg. 2013;26(2):127–32. https://doi.org/10.1055/s-0032-1319788.
Nair R, Tripathy G, Deysine GR. Computer navigation systems in unicompartmental knee arthroplasty: a systematic review. Am J Orthop (Belle Mead NJ). 2014;43(6):256–61.
Lang JE, Mannava S, Floyd AJ, Goddard MS, Smith BP, Mofidi A, et al. Robotic systems in orthopaedic surgery. J Bone Jt Surg Br. 2011;93(10):1296–9. https://doi.org/10.1302/0301-620x.93b10.27418.
Murphy S, Gobezie R. Image-guided surgical navigation: basic principles and applications to reconstructive surgery. Orthop J Harvard Med School. 2002;4:68–70.
Shatrov J, Parker D. Computer and robotic - assisted total knee arthroplasty: a review of outcomes. J Exp Orthop. 2020;7(1):70. https://doi.org/10.1186/s40634-020-00278-y.
Liu Y, Yao D, Zhai Z, Wang H, Chen J, Wu C, et al. Fusion of multimodality image and point cloud for spatial surface registration for knee arthroplasty. Int J Med Robot. 2022;18(5): e2426. https://doi.org/10.1002/rcs.2426.
Corbett J, Khan WS. Advances in navigation and robot-assisted surgery. Orthop Upper Lower Limb. 2020;2020:553–63.
Khadem R, Yeh CC, Sadeghi-Tehrani M, Bax MR, Johnson JA, Welch JN, et al. Comparative tracking error analysis of five different optical tracking systems. Comput Aided Surg. 2000;5(2):98–107. https://doi.org/10.1002/1097-0150(2000)5:2.
Yau WP, Leung A, Chiu KY, Tang WM, Ng TP. Intraobserver errors in obtaining visually selected anatomic landmarks during registration process in nonimage-based navigation-assisted total knee arthroplasty: a cadaveric experiment. J Arthroplasty. 2005;20(5):591–601. https://doi.org/10.1016/j.arth.2005.02.011.
Su E. Handheld navigation in total knee arthroplasty. Seminars Arthroplas. 2015;26(2):47–50. https://doi.org/10.1053/j.sart.2015.08.003.
Nam D, Weeks KD, Reinhardt KR, Nawabi DH, Cross MB, Mayman DJ. Accelerometer-based, portable navigation vs imageless, large-console computer-assisted navigation in total knee arthroplasty: a comparison of radiographic results. J Arthroplasty. 2013;28(2):255–61. https://doi.org/10.1016/j.arth.2012.04.023.
Rattanaprichavej P, Laoruengthana A. Accelerometer-based navigation versus conventional total knee arthroplasty for posttraumatic knee osteoarthritis. Clin Orthop Surg. 2022;14(4):522–9. https://doi.org/10.4055/cios21147.
Shah SM. After 25 years of computer-navigated total knee arthroplasty, where do we stand today? Arthroplasty. 2021;3(1):41. https://doi.org/10.1186/s42836-021-00100-9.
Jaramaz B, Nikou C. Precision freehand sculpting for unicondylar knee replacement: design and experimental validation. Biomed Tech (Berl). 2012;57(4):293–9. https://doi.org/10.1515/bmt-2011-0098.
Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206–12. https://doi.org/10.1007/s11999-014-3764-x.
Citak M, Suero EM, Citak M, Dunbar NJ, Branch SH, Conditt MA, et al. Unicompartmental knee arthroplasty: is robotic technology more accurate than conventional technique? Knee. 2013;20(4):268–71. https://doi.org/10.1016/j.knee.2012.11.001.
Smith JR, Riches PE, Rowe PJ. Accuracy of a freehand sculpting tool for unicondylar knee replacement. Int J Med Robot. 2014;10(2):162–9. https://doi.org/10.1002/rcs.1522.
Malhotra R, Gupta S, Gupta V, Manhas V. navigated unicompartmental knee arthroplasty: a different perspective. Clin Orthop Surg. 2021;13(4):491–8. https://doi.org/10.4055/cios20166.
Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplas. 2010;25(2):230–7. https://doi.org/10.1016/j.arth.2008.09.024.
Rodriguez F, Harris S, Jakopec M, Barrett A, Gomes P, Henckel J, et al. Robotic clinical trials of uni-condylar arthroplasty. Int J Med Robot. 2005;1(4):20–8. https://doi.org/10.1002/rcs.52.
Bouché PA, Corsia S, Hallé A, Gaujac N, Nizard R. Comparative efficacy of the different cutting guides in unicompartmental knee arthroplasty: a systematic-review and network meta-analysis. Knee. 2023;41:72–82. https://doi.org/10.1016/j.knee.2023.01.003.
Zhang Q, Zhang Q, Guo W, Liu Z, Cheng L, Yue D, et al. The learning curve for minimally invasive Oxford phase 3 unicompartmental knee arthroplasty: cumulative summation test for learning curve (LC-CUSUM). J Orthop Surg Res. 2014;9:81. https://doi.org/10.1186/s13018-014-0081-8.
Rees JL, Price AJ, Beard DJ, Dodd CA, Murray DW. Minimally invasive Oxford unicompartmental knee arthroplasty: functional results at 1 year and the effect of surgical inexperience. Knee. 2004;11(5):363–7. https://doi.org/10.1016/j.knee.2003.12.006.
Goh GS, Haffar A, Tarabichi S, Courtney PM, Krueger CA, Lonner JH. Robotic-assisted versus manual unicompartmental knee arthroplasty: a time-driven activity-based cost analysis. J Arthroplas. 2022;37(6):1023–8. https://doi.org/10.1016/j.arth.2022.02.029.
Hansen DC, Kusuma SK, Palmer RM, Harris KB. Robotic guidance does not improve component position or short-term outcome in medial unicompartmental knee arthroplasty. J Arthroplasty. 2014;29(9):1784–9. https://doi.org/10.1016/j.arth.2014.04.012.
MacCallum KP, Danoff JR, Geller JA. Tibial baseplate positioning in robotic-assisted and conventional unicompartmental knee arthroplasty. Eur J Orthop Surg Traumatol. 2016;26(1):93–8. https://doi.org/10.1007/s00590-015-1708-0.
Bargar WL. Robots in orthopaedic surgery: past, present, and future. Clin Orthop Relat Res. 2007;463:31–6.
Begum FA, Kayani B, Morgan SDJ, Ahmed SS, Singh S, Haddad FS. Robotic technology: current concepts, operative techniques and emerging uses in unicompartmental knee arthroplasty. EFORT Open Rev. 2020;5(5):312–8. https://doi.org/10.1302/2058-5241.5.190089.
Moschetti WE, Konopka JF, Rubash HE, Genuario JW. Can Robot-assisted unicompartmental knee arthroplasty be cost-effective? A Markov Decision Anal J Arthroplas. 2016;31(4):759–65. https://doi.org/10.1016/j.arth.2015.10.018.
Clement ND, Deehan DJ, Patton JT. Robot-assisted unicompartmental knee arthroplasty for patients with isolated medial compartment osteoarthritis is cost-effective: a markov decision analysis. Bone Joint J. 2019;101-b(9):1063–70. https://doi.org/10.1302/0301-620x.101b9.Bjj-2018-1658.R1.
Christen B, Tanner L, Ettinger M, Bonnin MP, Koch PP, Calliess T. Comparative cost analysis of four different computer-assisted technologies to implant a total knee arthroplasty over conventional instrumentation. J Pers Med. 2022;12(2):184. https://doi.org/10.3390/jpm12020184.
Swank ML, Alkire M, Conditt M, Lonner JH. Technology and cost-effectiveness in knee arthroplasty: computer navigation and robotics. Am J Orthop (Belle Mead NJ). 2009;38(2 Suppl):32–6.
Favroul C, Batailler C, Canetti R, Shatrov J, Zambianchi F, Catani F, et al. Image-based robotic unicompartmental knee arthroplasty allowed to match the rotation of the tibial implant with the native kinematic knee alignment. Int Orthop. 2023;47(2):519–26. https://doi.org/10.1007/s00264-022-05637-1.
He G, Ricca JM, Dai AZ, Mustahsan VM, Cai Y, Bielski MR, et al. A novel bone registration method using impression molding and structured-light 3D scanning technology. J Orthop Res. 2022;40(10):2340–9. https://doi.org/10.1002/jor.25275.
Panchmatia JR, Visenio MR, Panch T. The role of artificial intelligence in orthopaedic surgery. Br J Hosp Med. 2018;79(12):676–81. https://doi.org/10.12968/hmed.2018.79.12.676.
Khan RA, Jawaid M, Khan AR, Sajjad M. ChatGPT - Reshaping medical education and clinical management. Pak J Med Sci. 2023;39(2):605–7. https://doi.org/10.12669/pjms.39.2.7653.
Zhou XY, Guo Y, Shen M, Yang GZ. Application of artificial intelligence in surgery. Front Med. 2020;14(4):417–30. https://doi.org/10.1007/s11684-020-0770-0.
Panesar S, Cagle Y, Chander D, Morey J, Fernandez-Miranda J, Kliot M. Artificial Intelligence and the Future of Surgical Robotics. Ann Surg. 2019;270(2):223–6. https://doi.org/10.1097/sla.0000000000003262.
Li W, Xu SM, Zhang DB, Bi HY, Gu GS. Research advances in the application of AI for preoperative measurements in total knee arthroplasty. Life (Basel). 2023;13(2):451.
Acknowledgements
None
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to this review. The idea was conceived by Hyuk-Soo Han. Literature search and data analysis, drafting of the manuscript was performed by Sung Eun Kim and Hyuk-Soo Han. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, S.E., Han, HS. Robotic-assisted unicompartmental knee arthroplasty: historical perspectives and current innovations. Biomed. Eng. Lett. 13, 543–552 (2023). https://doi.org/10.1007/s13534-023-00323-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13534-023-00323-6