Abstract
Objective
Serum 1, 5-AG is a glycaemic marker reflecting control and fluctuations of short-term glucose. To reveal the relationship between 1, 5-AG with β-cell function, we investigated a certain number of Chinese adults with different glucose metabolisms.
Methods
In clinical cross-sectional study, 2184 subjects with no prior diabetes history are included and underwent an OGTT. HOMA-IR and HOMA-β were calculated. Correlations between 1, 5-AG and HOMA-IR or HOMA-β were observed, correcting for interference factors, and independent factors for HOMA-β and HOMA-IR were analysed. Subjects were divided into three groups according to OGTT results, and 1, 5-AG levels differed significantly between them.
Results
A significant positive correlation existed between 1, 5-AG and HOMA-β only in the diabetes mellitus group (r = 0.265, p < 0.001). Above phenomenon remained after adjusting for indicators, however, disappeared after considering serum uric acid. Both 1, 5-AG and HbA1c were independent factors for HOMA-β (1, 5-AG: β = 0.772, p = 0.023; HbA1c: β = -7.52, p = 0.003). Conclusion: 1, 5-AG remained a factor for HOMA-β only for those with NUA. 1, 5-AG reflects different glucose metabolism statuses and is an auxiliary observation reflecting the secretory function of β cells in NUA patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to statistics for 2021 from the International Diabetes Federation (IDF), China is ranked first in the world for number of diabetes mellitus patients [1]. It is well known that islet β-cell dysfunction and insulin resistance are important pathological factors in T2DM [2]. Furthermore, Chinese subjects with T2DM are characterized by a worse secretory function of β cells and a more serious early phase secretion defect [3]. Therefore, a timely understanding of the functional status of islets in diabetic patients is helpful for adjusting treatment plans and determining prognosis. Although an OGIRT and hyperinsulinaemic-euglycaemic clamp can be used to reflect insulin function, their strict operational methods limit random and flexible application. Therefore, a simple and easy way to estimate the status of β-cell function in diabetic patients is needed. Recently, a new glucose indicator, serum 1,5-AG, has gradually become known to the public [4]. It is a naturally occurring 1-deoxy form of glucose that can reflect dynamic changes in blood glucose through the mechanism of renal reabsorption [5,6,7]. A considerable number of studies in the literature have confirmed its superiority in monitoring short-term glycaemic levels (3–7 days) and glycaemic variability [8,9,10,11,12]. However, few studies in China or abroad have reported on the relationship between 1, 5-AG and the pathophysiological mechanisms of diabetes [13, 14]. So far, no study has been performed on the relationship between 1, 5-AG and β-cell function among people with different glucose metabolism levels, especially among people with normal glucose metabolism.
Therefore, a natural adult population without a history of prior diabetes was assembled from Jiangsu Province, China as research subjects for this study. Each subject underwent an OGTT and OGIRT to explore the relationship between 1, 5-AG and β-cell function in those with differing glucose metabolism levels and to see possible influencing factors.
Methods
Study design
The multi-center cross-sectional study was conducted in six cities in Jiangsu Province over a 1 year period (November 2015 to June 2016).
Selection of subjects
2500 individuals (18 to 65 years) were selected using a multistage, stratified sampling method. Patients with known diabetes/on treatment, liver dysfunction, or those with chronic kidney disease stage 4–5 were excluded. Non-resident population, pregnant subjects and those with incomplete data were also excluded. Ultimately, 2184 subjects were included in the final analysis.
Anthropometry and biochemical measurements
Each subject underwent OGTT, physical examination (height, weight, waist circumference, hip circumference), and biochemical examination (liver function, renal function, blood lipids). HbA1C was determined by high-performance liquid chromatography using a D-10 HbA1C analyser (Bio-Rad, USA); GA was measured using a liquid enzymatic method with a Glamour 2000 biochemical automatic analyser (Lucica GA-L, Tokyo, Japan); Serum insulin levels were determined by electrochemiluminescence immunoassay using a Gobas e411 analyser (Roche Diagnostics GmbH, Mannheim, Germany). The plasma concentration of FPG, 2hPG, TC, TG, HDL-c, LDL-c, BUN, SCr and SUA levels were all measured. The calculation of the eGFR was based on a revised formula for Chinese subjects [15]. We collected blood samples from patients after overnight fasting, which were stored at − 80 °C prior to the further measurement of 1, 5-AG. Serum 1, 5-AG levels were measured with a GlycoMark assay using a Hitachi 917 analyser (Roche Diagnostics, USA).
Definition of β-cell function
Β-cell function was assessed by the calculation of insulin resistance index and secretion index. The homeostasis model assessment for insulin resistance (HOMA-IR) was calculated as follows: HOMA-IR = FINS (in mU/L) * FPG (in mmol/L)/22.5. The homeostasis model assessment for β-cell function (HOMA-β) was calculated as follows: HOMA-β = 20 *FINS [mU/L]/(FPG [mmol/L] – 3.5) [16].
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics 22.0 (International Business Machines Corporation, USA). With the normality test, all variables were divided into normally distributed data (presented as mean ± SD) and skewed data (presented as quartile P50 [P25, P75]). A one-way ANOVA was used to analyse differences among the three groups of the study. A Pearson or Spearman's simple correlation analysis was used to analyse the correlations between 1, 5-AG and the other indicators. A partial correlation analysis was used to adjust the other confounding variables. Finally, a stepwise linear regression analysis was used to find the independent factors for HOMA-β. A two-tailed p value of < 0.05 was considered to be statistically significant.
Results
Clinical characteristics of the three groups
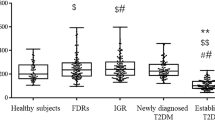
The 2184 subjects in the study were divided into three groups according to the WHO diabetes diagnostic criteria from 1999 [17]. 307 subjects were diagnosed as belonging in the T2DM group, 685 in the IGR group, and 1192 in the NGT group, accounting for 14.06%, 31.36%, and 54.58%, respectively. 1, 5-AG levels gradually decreased from the NGT group to the IGR group to the T2DM group (26.99 ± 7.23 μg/mL, 21.58 ± 8.29 μg/mL, and 15.74 ± 9.6 μg/mL, respectively, all p < 0.001). HOMA-IR gradually increased (all p < 0.001) and the T2DM group showed the most serious insulin resistance. HOMA-β was the highest in NGT group and the lowest in T2DM groupT2DM (all p < 0.001), so insulin secretion function was the worst in the T2DM group. The other indicators, including gender, age, glycaemic indexes (FPG, 2hPG, HbA1c, GA), blood pressure, lipid profile, blood urea nitrogen (BUN), serum uric acid (SUA), BMI, and W/P, had significant differences among the three groups. SCr and eGFR had no significant differences among the groups, as shown in Table 1. T2DM.
The correlations between 1, 5-AG and both HOMA-IR and HOMA-β in the three groups and their interference factors
A Spearman's simple correlation analysis was used to find the correlations between 1, 5-AG and both HOMA-IR and HOMA-β in the three groups. A significant positive correlation was shown between 1, 5-AG and HOMA-β in the T2DM group (r = 0.265, p < 0.001), which still existed after adjusting for gender, age, blood pressure, BMI, W/P, blood lipid profile (HDL, LDL, TG, TC), glycaemic indexes (HbA1c, GA, FPG, 2hPG), as show in Table 2. Meanwhile, 1, 5-AG and HOMA-IR showed a weak negative correlation in the T2DM group (r = -0.119, p = 0.038), but this negative correlation was lost after adjusting for other factors. In addition, we did not find a significant correlation between 1, 5-AG and HOMA-IR or HOMA-β in the NGT and IGR groups. Table 3 presents partial correlation analyses of factors correlated with the association between serum 1, 5-AG and HOMA-β, HOMA-IR in the T2DM group. In Table 3, Mode 1 indicates adjustment for age and gender. Model 2 represents model 1 plus SBP, DBP, BMI, W/P, HDL, LDL, TC and TG. Model 3 represents model 2 plus HbA1c, GA, FPG and 2H PG. Model 4 represents model 3 plus eGFR, Cr and BUN. Model 5 means model 4 plus UA.
Independent factors for HOMA-β for different SUA levels in the T2DM group
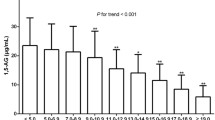
In the T2DM group, a multiple stepwise linear regression analysis was used by employing HOMA-β as a dependent variable. We found that both 1, 5-AG and HbA1c were independent factors for HOMA-β (1, 5-AG: β = 0.772, p = 0.023; HbA1c: β = -7.52, p = 0.003) after adjusting for age, blood pressure, BMI, W/P, lipid profile (TG, TC, LDL, HDL), glycaemic indexes (FPG, 2hPG, HbA1c, GA, 1, 5-AG), and renal function indicators (BUN, Cr, SUA, eGFR). As shown by the above results, SUA may be considered an interference factor between 1, 5-AG and HOMA-β. To study this further, the T2DM group was further divided into a NUA group and HUA (SUA male ≥ 420 μmol/L, female ≥ 360 μmol/L) [18, 19] group. In sub-group analysis, 1, 5-AG was observed to be an independent predictor in NUA group(β = 0.677, p = 0.017) but not HUA group. Furthermore, we found that there was no positive correlation between 1, 5-AG and HOMA-β in the HUA group. However, there was still a significant positive correlation between 1, 5-AG and HOMA-β in the NUA group, which was stronger in males than females. Figure 1 shows pearson's correlation coefficient between levels of serum 1, 5-AG and HOMA-β.
Discussion
Maintaining β-cell function and improving insulin resistance are considered to be the most effective measures for preventing diabetes and its progression [20] as they are both part of the pathological mechanisms of the development of T2DM. Studies have shown that once hyperglycaemia has obviously occurred, islet β-cell dysfunction is clearly manifest [21, 22]. Even in high-risk populations still in the NGT stage, β-cell function has been impaired [23, 24]. Therefore, the early evaluation and protection of β-cell function plays a key role in the prevention and treatment of diabetes. In addition to the invasive glucose clamp test, the monitoring of previous β-cell function can also be indirectly estimated by an insulin release test and some glycaemic indicators such as HbA1c, GA, and GA/A1c [25].
Serum 1, 5-AG, a new glycaemic marker, has received increasing attention since it was first described in 1972 [26]. It is a naturally occurring 1-deoxy form of glucose that undergoes glomerular filtration and tubular reabsorption and has a closed pyran ring structure that confers metabolic stability [27]. When glucose fluctuates beyond the renal threshold of glucosuria, the reabsorption of 1, 5-AG in the renal tubules is competitively inhibited by high levels of glucose, resulting in a decrease in serum 1, 5-AG levels [28]. Previous articles have confirmed that 1, 5-AG have a good correlation with 2hPG even in prediabetes [29]. Moreover, one study in China has confirmed that on the cellular level, 1, 5-AG is a good glycaemic marker [30]. In that study, the Michaelis constant and maximum velocity were determined to measure the affinity of glucose oxidase and hexokinase for 1, 5-AG and glucose. The authors concluded that 1, 5-AG is difficult to metabolize in vivo, and its transport is influenced by an acute glucose load in the hepatocytes. There have been few recent studies on the relationship between 1, 5-AG and β-cell function. A Korean study of a small sample in 2009 showed that low levels of 1, 5-AG were closely related to an elevation of 2hPG and a decline in islet secretion function in subjects with pre-diabetes and well-controlled T2DM [31]. In addition, a report on a small sample in China from 2015 showed that 1, 5-AG was closely associated with early-phase insulin secretion in newly diagnosed T2DM patients [32]. Our study took a natural population from Jiangsu Province, China that had no prior history of diabetes as a subject of research and explored for the first time the relationship between 1, 5-AG and β-cell function in people with different glucose metabolism statuses [33].
In the natural population of Jiangsu Province, 1, 5-AG, HOMA-IR, and HOMA-β had significant differences among the NGT, IGR, and T2DM groups [34]. In addition, 1, 5-AG was able to reflect different glucose metabolism statuses. With the gradual aggravation of glucose metabolism disorders, 1, 5-AG and HOMA-β showed gradually decreasing trends while HOMA-IR showed the opposite [35, 36]. It can be found that 1, 5-AG and HOMA-β maintained good positive correlations in the T2DM group as a whole and in the NUA group within the T2DM group (r = 0.265, 0.322, p < 0.001). Meanwhile, 1, 5-AG was an independent factor for HOMA-β (T2DM: β = 0.882, p = 0.023; NUA: β = 0.677, p = 0.017), proving that 1, 5-AG can reflect the secretory function of β cells. However, this correlation was lost in the HUA group. In the NUA group, 1, 5-AG could be used as an auxiliary observation index reflecting the secretory function of β cells.
SUA is a product of the metabolic breakdown of purine nucleotides and is excreted and absorbed via the kidney. Under physiological conditions, SUA levels in males are usually higher than in females [37]. In the three of groups of our study, men had higher SUA levels than women, and an independent sample t-test also proved that there were significant differences between males and females (all p < 0.001), which was consistent with previous observations. One possible explanation for this result is that oestrogens may promote renal clearance of SUA by inhibiting the active reabsorption of uric acid [38]. In recent years, the relationship between uric acid and 1, 5-AG has attracted much attention. In 2009, Koga et al. studied 158 male subjects with normal glucose tolerance and found that SUA was positively correlated with 1, 5-AG concentration. 1, 5-AG and SUA may share a common renal tubular transport system [39]. Another study in 2013 found that there was an independent positive correlation between 1, 5-AG and SUA in both T2DM and non-DM subjects [40]. In our study, we indeed found a positive relation between 1, 5-AG and SUA in the NGT, IGR, and T2DM groups (r = 0.168, r = 0.125, r = 0.14, respectively, p < 0.05). We suspect that 1, 5-AG and SUA share a common renal tubular transport system, causing 1, 5-AG to interfere with hyperuricemia in response to islet β-cell function in the HUA population of the T2DM group.
Interestingly, recent studies have shown that uric acid levels can also affect islet β-cell secretion, whether in diabetic or nondiabetic subjects [41]. A cross-sectional study was designed and performed on a total of 403 newly diagnosed T2DM patients and finally concluded that SUA may be considered as a predictor for β-cell function in clinical practice [42, 43]. Other studies have also supported this finding [44,45,46]. Our study also found that there was a positive correlation between SUA and HOMA-β in the T2DM group (r = 0.148, p = 0.009). However, the mechanism that causes SUA to interfere with a positive correlation between 1, 5-AG and HOMA-β needs further exploration.
The mechanism of the positive correlation between 1,5-AG and islet β-cell secretion has also been deeply studied recently. One study confirmed that 1,5-AG could stimulate insulin release in a dose-dependent manner at a cytological level [18]. Another study suggested that 1,5-AG appeared to inhibit the activity of disaccharidase to inhibit postprandial blood glucose and insulin secretion [19], however, the exact mechanism still needs further exploration.
The current study has some limitations. First, it would be better to use more prospective or cohort studies to enhance the efficacy of the cross-sectional research. On this point, our team is currently involved and working hard on achieving this goal. Second, it would be more accurate to use the internationally recognized gold standard glucose clamp test. By taking into account of manoeuvrability, a more suitable method approved by domestic and foreign experts is applied in our study.
In general, this study is based on the findings from a large-scale and multicentric adult population in Jiangsu, China. As a result of this study, we make the following conclusions: 1. In the natural adult population of Jiangsu, China, 1,5-AG can reflect different glucose metabolism statuses. 2. 1,5-AG, HOMA-IR, and HOMA-β show significant differences among NGT, IGR, and T2DM groups. In the T2DM group, 1,5-AG was positively correlated with HOMA-β. For those in the NUA group within the T2DM group, 1,5-AG could be used as an auxiliary observation index reflecting the secretory function of β cells.
Conclusions
In this paper, we investigate the relationship between serum 1, 5-anhydroglucitol and β-cell function in Chinese adults with different glucose metabolism statuses. In a natural population from Jiangsu Province, China with no previous history of diabetes, there were significant differences in 1, 5-AG, HOMA-IR, and HOMA-β among the NGT, IGR, and T2DM groups. Therefore, 1, 5-AG can reflect different glucose metabolism statuses. In the T2DM group, 1, 5-AG was positively correlated with HOMA-β, and 1, 5-AG could also be used as an auxiliary observation index reflecting the secretory function of β cells for those in the NUA group within the T2DM group. From the results, we can obtain that 1, 5-AG can reflect different glucose metabolism statuses in the natural adult population of Jiangsu, China. We did not find a significant correlation between 1, 5-AG and HOMA-IR or HOMA-β in the NGT and IGR groups, so 1,5-AG may not be a predictor of β-cell function until a certain level of blood glucose elevation. In addition, 1, 5-AG, HOMA-IR, and HOMA-β show significant differences among NGT, IGR, and T2DM groups. In the T2DM group, 1, 5-AG was positively correlated with HOMA-β. For those in the NUA group within the T2DM group, 1, 5-AG could be applied as an auxiliary observation index reflecting the secretory function of β cells.
Data availability
All the data supporting the results are shown in the paper and can be applicable from the corresponding author.
Abbreviations
- 1, 5-AG:
-
Serum 1, 5-anhydroglucitol
- OGTT:
-
Oral glucose tolerance test
- HOMA-IR:
-
Homeostasis model assessments for insulin resistance
- HOMA-β:
-
Homeostasis model assessments for β cells
- NUA:
-
Normal serum uric
- T2DM:
-
Type 2 diabetes mellitus
- OGIRT:
-
Oral glucose insulin release test
- HbA1c:
-
Glycosylated hemoglobin
- GA:
-
Glycated albumin
- FPG:
-
Fasting blood glucose
- 2hPG:
-
2-Hour postprandial plasma glucose
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- HDL-c:
-
High-density lipoprotein cholesterol
- LDL-c:
-
Low-density lipoprotein cholesterol
- BUN:
-
Blood urea nitrogen
- SCr:
-
Serum creatinine
- SUA:
-
Serum uric acid
- eGFR:
-
Estimated glomerular filtration rate
- NGT:
-
Normal glucose tolerance
- IGR:
-
Impaired glucose regulation
- HUA:
-
High uric acid
References
Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119.
Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29:1130–9.
Hong J, Gu WQ, Zhang YF, et al. The interplay of insulin resistance and beta-cell dysfunction involves the development of type 2 diabetes in Chinese obeses. Endocrine. 2007;31:93–9.
Pitkanen E. Serum 1, 5-Anhydroglucitol in Normal Subjects and in Patients with Insulin-Dependent Diabetes-Mellitus. Scand J Clin Lab Inv. 1982;42:445–8.
Ying L, Ma X, Yin J, et al. The metabolism and transport of 1, 5-anhydroglucitol in cells. Acta Diabetol. 2018;55:279–86.
Ma C, Sheng J, Liu Z, Guo M. Excretion rates of 1, 5-anhydro-D-glucitol, uric acid and microalbuminuria as glycemic control indexes in patients with type 2 diabetes. Sci Rep. 2017;7:44291.
Kim WJ, Park CY. 1, 5-Anhydroglucitol in diabetes mellitus. Endocrine. 2013;43:33–40.
Seok H, Huh JH, Kim HM, et al. 1, 5-anhydroglucitol as a useful marker for assessing short-term glycemic excursions in type 1 diabetes. Diabetes Metab J. 2015;39:164–70.
Kulozik F, Hasslacher C. 1, 5-Anhydroglucitol as a Marker for Short-Term Glycaemic Excursions. Diabetes Stoffwech H. 2015;24:89–94.
Mook-Kanamori DO, El-Din SMM, Takiddin AH, et al. 1, 5-Anhydroglucitol in Saliva Is a Noninvasive Marker of Short-Term Glycemic Control. J Clin Endocr Metab. 2017;102:3867–3867.
Kim MJ, Jung HS, Hwang-Bo Y, et al. Evaluation of 1, 5-anhydroglucitol as a marker for glycemic variability in patients with type 2 diabetes mellitus. Acta Diabetol. 2013;50:505–10.
Januszewski AS, Karschimkus C, Davis KE, et al. Plasma 1, 5 anhydroglucitol levels, a measure of short-term glycaemia: assay assessment and lower levels in diabetic vs. non-diabetic subjects. Diabetes Res Clin Pract. 2012;95:17–9.
Won JC, Park CY, Park HS, et al. 1, 5-Anhydroglucitol reflects postprandial hyperglycemia and a decreased insulinogenic index, even in subjects with prediabetes and well-controlled type 2 diabetes. Diabetes Res Clin Pract. 2009;84:51–7.
Ma X, Hao Y, Hu X, et al. 1, 5-anhydroglucitol is associated with early-phase insulin secretion in chinese patients with newly diagnosed type 2 diabetes mellitus. Diabetes Technol Ther. 2015;17:320–6.
Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937–44.
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment, pp. insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Med. 1998;15:539–53.
Yamanouchi T, Inoue T, Ichiyanagi K, et al. 1, 5-Anhydroglucitol stimulates insulin release in insulinoma cell lines. Biochim Biophys Acta. 2003;1623:82–7.
Nakamura S, Tanabe K, Yoshinaga K, et al. Effects of 1, 5-anhydroglucitol on postprandial blood glucose and insulin levels and hydrogen excretion in rats and healthy humans. Br J Nutr. 2017;118:81–91.
Chizynski K, Rozycka M. Hyperuricemia. Pol Merkur Lekarski. 2005;19:693–6.
DeFronzo RA, Abdul-Ghani MA. Preservation of beta-Cell Function: The Key to Diabetes Prevention. J Clin Endocr Metab. 2011;96:2354–66.
Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003;46:3–19.
Perley MJ, Kipnis DM. Plasma Insulin Responses to Oral and Intravenous Glucose - Studies in Normal and Diabetic Subjects. J Clin Invest. 1967;46:1954–8.
Brunzell JD, Robertson RP, Lerner RL, et al. Relationships between Fasting Plasma Glucose Levels and Insulin-Secretion during Intravenous Glucose-Tolerance Tests. J Clin Endocr Metab. 1976;42:222–9.
Ehrmann DA, Sturis J, Byrne MM, et al. Insulin Secretory Defects in Polycystic-Ovary-Syndrome - Relationship to Insulin Sensitivity and Family History of Non-Insulin-Dependent Diabetes-Mellitus. J Clin Invest. 1995;96:520–7.
Ward WK, Johnston C, Beard JC, et al. Insulin Resistance and Impaired Insulin-Secretion in Subjects with Histories of Gestational Diabetes-Mellitus. Diabetes. 1985;34:861–9.
Ryan EA, Imes S, Liu DT, et al. Defects in Insulin-Secretion and Action in Women with a History of Gestational Diabetes. Diabetes. 1995;44:506–12.
Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing - Comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–70.
Koga M, Murai J, Saito H, Kasayama S. Glycated Albumin and Glycated Hemoglobin Are Influenced Differently by Endogenous Insulin Secretion in Patients With Type 2 Diabetes. Diabetes Care. 2010;33:270–7.
Kim D, Kim KJ, Huh JH, et al. The ratio of glycated albumin to glycated haemoglobin correlates with insulin secretory function. Clin Endocrinol (Oxf). 2012;77:679–83.
Pitkanen E. Occurrence of 1, 5-Anhydroglucitol in Human Cerebrospinal-Fluid. Clin Chim Acta. 1973;48:159–66.
Yamanouchi T, Tachibana Y, Akanuma H, et al. Origin and disposal of 1, 5-anhydroglucitol, a major polyol in the human body. Am J Physiol. 1992;263:268–73.
Wang Y, Yuan Y, Zhang Y, et al. Serum 1, 5-anhydroglucitol level as a screening tool for diabetes mellitus in a community-based population at high risk of diabetes. Acta Diabetol. 2017;54:425–31.
Al-Masri AA, Eter EE, Tayel S, et al. Differential associations of circulating peroxiredoxins levels with indicators of glycemic control in type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci. 2014;18(5):710–6.
Christensen BL, Williams M. Assessing postprandial glucose using 1, 5-anhydroglucitol, pp. An integrative literature review. J Am Acad Nurse Pract. 2009;21:542–8.
Schindhelm RK, Diamant M, Bilo HJ, Slingerland RJ. Association of 1, 5-anhydroglucitol and 2-h postprandial blood glucose in type 2 diabetic patients. Diabetes Care. 2009;32:207–207.
González C, Alonso A, Grueso NA, et al. Effect of treatment with different doses of 17-beta-estradiol on the insulin receptor. Life Sci. 2019;70(14):1621–30.
Puig JG, Mateos FA, Miranda ME, et al. Purine Metabolism in Women with Primary Gout. Am J Med. 1994;97:332–8.
Hak AE, Choi HK. Menopause, postmenopausal hormone use and serum uric acid levels in US women - The Third National Health and Nutrition Examination Survey. Arthritis Res Ther. 2008;10:1–11.
Sumino H, Ichikawa S, Kanda T, et al. Reduction of serum uric acid by hormone replacement therapy in postmenopausal women with hyperuricaemia. Lancet. 1999;354:650–650.
Koga M, Murai J, Saito H, et al. Close relationship between serum concentrations of 1, 5-anhydroglucitol and uric acid in non-diabetic male subjects implies common renal transport system. Clin Chim Acta. 2009;410:70–3.
Ouchi M, Oba K, Aoyama J, et al. Serum uric acid in relation to serum 1, 5-anhydroglucitol levels in patients with and without type 2 diabetes mellitus. Clin Biochem. 2013;46:1436–41.
Hu YM, Liu J, Li HQ, et al. The association between elevated serum uric acid levels and islet β-cell function indexes in newly diagnosed type 2 diabetes mellitus: a cross-sectional study. PeerJ. 2018;6(7):4515–8.
Zhong X, Zhang D, Yang L. The relationship between serum uric acid within the normal range and beta-cell function in Chinese patients with type 2 diabetes: differences by body mass index and gender. PeerJ. 2019;7:6666.
Tang W, Fu Q, Zhang Q, et al. The association between serum uric acid and residual beta -cell function in type 2 diabetes. J Diabetes Res. 2014;2014: 709691.
Ling Y, Li XM, Gu Q. Correlation of serum uric acid and islet beta cell functions in female type 2 diabetics. Nat Med J China. 2012;92:541–5.
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was approved in 2017 by the Clinical Ethics Committee of Zhongda Hospital, an affiliate of Southeast University (No.2017ZDSYLL006-P01), and all subjects signed informed consent forms before registration. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. This study was approved by the Clinical Ethics Committee of our hospital and all subjects signed informed consent forms before registration.
Conflicts of interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yuan, Y., Tan, Y., Wang, Y. et al. The correlation between serum 1, 5-anhydroglucitol and β-cell function in Chinese adults with different glucose metabolism statuses. Int J Diabetes Dev Ctries (2023). https://doi.org/10.1007/s13410-023-01295-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13410-023-01295-0